- 1Rheumatology Department, Great Ormond Street Hospital for Children, London, United Kingdom
- 2Biomedical Research Centre, Great Ormond Street Hospital for Children, London, United Kingdom
- 3University College London (UCL) Great Ormond Street Institute of Child Health, London, United Kingdom
The challenges of childhood uveitis lie in the varied spectrum of its clinical presentation, the often asymptomatic nature of disease, and the evolving nature of the phenotype alongside normal physiological development. These issues can lead to delayed diagnosis which can cause significant morbidity and severe visual impairment. The most common ocular complications include cataracts, band keratopathy, glaucoma, and macular oedema, and the various associated systemic disorders can also result in extra-ophthalmic morbidity. Pediatricians have an important role to play. Their awareness of the various presentations and etiologies of uveitis in children afford the opportunity of prompt diagnosis before complications arise. Juvenile Idiopathic Arthritis (JIA) is one of the most common associated disorders seen in childhood uveitis, but there is a need to recognize other causes. In this review, different causes of uveitis are explored, including infections, autoimmune and autoinflammatory disease. As treatment is often informed by etiology, pediatricians can ensure early ophthalmological referral for children with inflammatory disease at risk of uveitis and can support management decisions for children with uveitis and possible underling multi-system inflammatory disease, thus reducing the risk of the development of irreversible sequelae.
Introduction
Pediatric uveitis is rare condition, which accounts for 5–10% of all-age uveitis (1). Although variable within and across different countries, the estimated incidence of childhood uveitis in industrialized nations is ~4.3 per 100,000 with a prevalence of 27.9 per 100,000 (2).
Diagnosis of pediatric uveitis can be challenging due to insidious (chronic, persistent and recurrent) presentation, lack of symptom reporting in early childhood, and difficulties in examining young children. Pediatric uveitis is also characterized by frequent complications and comorbidities such as cataract, glaucoma or amblyopia (potentially irreversible failure to develop good vision).
The focus of this review is the role of the pediatrician in all stages, including recognition of the signs or symptoms (or lack thereof) of uveitis in the child at risk due to their rheumatological condition; advising on possible etiology; and co-managing systemic therapy in the child with uveitis and systemic disorders.
Eye anatomy
The parts of the normal globe (anterior to posterior) are the cornea, anterior chamber, iris (anterior segment), lens, ciliary body, vitreous humor (intermediate structures), retina, choroid, and sclera (posterior segment). Uveitis can be described using this anatomy: anterior uveitis refers to inflammation of focused on anterior chamber; intermediate uveitis involves the vitreous cavity; posterior uveitis affects the retina and choroid; and panuveitis is characterized by the involvement of all layers of the eye (Figure 1) (4).
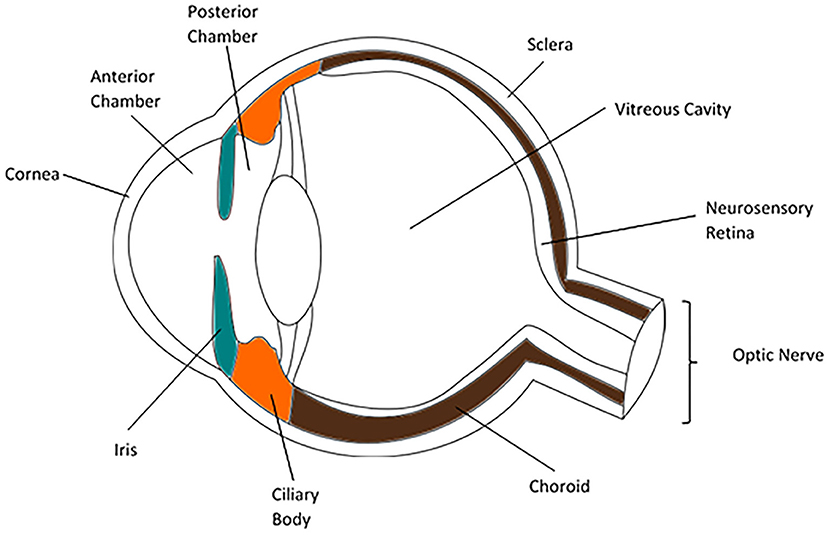
Figure 1. Anatomy of the human eye (3).
Detecting uveitis in the pediatric clinic—The challenge of the red eye
The most common causes of red eye in childhood are ocular surface conditions such viral, bacterial or allergic conjunctivitis, but other causes include ocular inflammatory conditions such as uveitis or inflammation of the sclera, scleritis. It is useful for the pediatrician to understand the “red flags” which warrant timely referral to the ophthalmologist. These comprise photophobia (adverse response to light stimulation) or significant pain, disturbed or reduced vision, and pupil or other structural abnormalities (Table 1). It should be noted that some forms of anterior uveitis are asymptomatic, that unilateral visual loss may not be noted unless vision is checked in each eye separately, and that younger children may struggle to articulate eye symptoms (6, 7). The red eye may be challenging by its absence: “silent” disease in a white (i.e., no redness) eye, which nevertheless has ongoing undetected anterior inflammation, will result in irreversible visual loss, and is unfortunately a common manifestation of JIA associated uveitis.
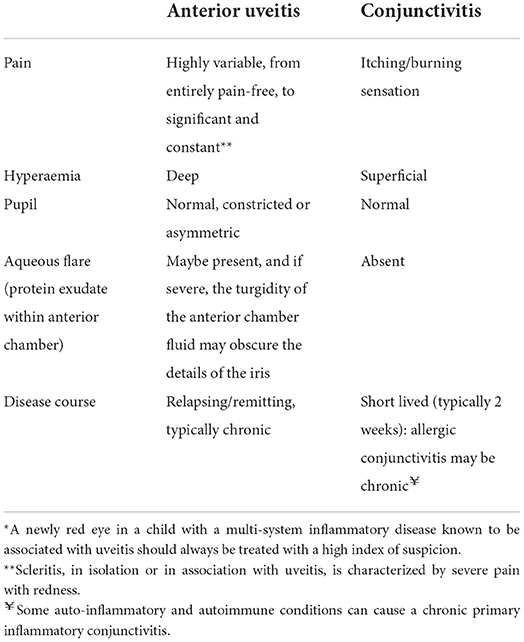
Table 1. Differentiating two key causes of the pediatric red eye* adapted from Gilani et al. (5).
Making the diagnosis
Uveitis is a descriptive term rather than a diagnosis and may have an infective or non-infective cause. In the following section, we describe the infective causes of uveitis. Note that since there is limited data published on pediatric infective uveitis, some references have been taken from the adult literature.
Infectious uveitis
Infectious uveitis should be excluded as the cause for childhood uveitis (particularly panuveitis) as the treatment differs significantly from that for non-infectious disease. The details of infections known to cause or be associated with uveitis are mentioned in Table 2. Recommended baseline evaluation for infectious and non-infectious uveitis is highlighted in Table 3.
Toxoplasmosis
Globally, Toxoplasmosis is a common parasitic cause of infectious uveitis, and can be either a congenital or acquired infection (the latter being a less common presentation). Eye inflammation can resolve without any sequalae, but often leaves a chorioretinal scaring. In 70–80%, this occurs as a unilateral focal chorioretinal lesion. Reactivation may cause eye pain, blurred vision and can lead to permanent eye damage and blindness. Although serological or intraocular diagnosis is useful, the initial suspicion is based on a typical chorioretinal lesion on eye examination. These lesions can be multiple, solitary or a satellite to an old chorioretinal scar (10–12).
Herpes viruses
Both herpes simplex and herpes zoster can cause keratouveitis. Unilateral eye disease is more common (Table 2). The presence of cutaneous vesicles, corneal lesions, reduced corneal sensation, and iris atrophy (which may cause unequal pupil shape or size) may be clues to the diagnosis. In acute infections, herpes usually causes non-granulomatous uveitis while in chronic cases granulomatous uveitis is more common (56), with the term granulomatous used to define a clinical phenotype (the presence of iris nodules and large, “mutton fat” inflammatory keratic precipitates) rather than being based on tissue histology. Both herpes simplex and herpes zoster can also cause a retinitis known as acute retinal necrosis. Although rare in children, ARN can cause devastating visual loss. The use of viral PCR on intraocular fluid samples (from anterior or posterior segment) and of long-term systemic antiviral maintenance treatment have improved visual outcomes (8, 9).
Cat-scratch disease
Bartonella henselae is the principal cause of cat-scratch disease. Although the presentation of ocular inflammation can vary, optic nerve inflammation and macular star (“neuroretinitis”) is characteristic, although the most common initial manifestation is a unilateral anterior uveitis. Exploration of a history of contacts with domestic pets is thus important in childhood uveitis (24, 25).
Tuberculosis
Ocular TB represents an extrapulmonary form of the disease which may occur in isolation, with no other evidence of pulmonary infection or active disease at any other site in the body (Table 2) (16).
One of the distinguishing features of the TB infection is the development of granulomatous uveitis with the incidence and prevalence depending on the region and endemic nature. Features that should raise suspicion are a history of TB exposure, pyrexia of unknown origin, night sweats, lymphadenopathy, or an immunosuppressed child, and granulomatous forms of ocular inflammation. Bilateral presentation and posterior uveitis are the most reported phenotypes. The Collaborative Ocular Tuberculosis (COTS−1) study reported that chest computed tomography (CT) was three times more sensitive than X ray in identifying old, inactive TB (17). Early anti-TB therapy reduces the rate of recurrences in patients with TB uveitis, whilst systemic corticosteroids reduce the inflammatory sequalae of active disease (18–22).
Syphilis
Syphilis can manifest in various forms, including posterior uveitis (retinitis, chorioretinitis or retinal vasculitis) and papillitis. The frequency of this uveitis is <1% of all uveitis (13). HIV positive patients had more commonly anterior uveitis as compared to panuveitis or posterior uveitis in the immunocompetent individuals. It is very important to identify syphilis because of its therapeutic implications (14, 15).
Lyme disease
Eye manifestations occur in 1–4% of infected patients, and whilst early disease can have transient non-specific conjunctivitis, later stages can preset with uveitis, typically intermediate (Table 2) Anterior and posterior uveitis can also occur, as well as keratitis, episcleritis (hyperaemia of the conjunctiva and sclera), exudative retinal detachment and neuro-ophthalmological disorders (40–42). Treatment usually is a combination of antibiotics (usually doxycycline) and steroids (topical, intravitreal, subconjunctival, oral, or intravenous). A history of tick bite may not be present in all patients.
Candida
Candida infection can cause chorioretinitis and endophthalmitis and may result in visual loss. Endogenous candida endophthalmitis is caused by involvement of the choroid and retina via small capillaries, leading to an endophthalmitis with typical “fluff balls” or vitreous abscesses. Other eye findings in endophthalmitis are hypopyon, optic nerve involvement or vitreous abscesses (43, 44). Fluconazale is usually the first line treatment, with new generation azoles such as Voriconazole used previously for resistant cases (45).
Infectious uveitis in immunosuppressed patients
It is particularly important to exclude infectious causes in the immunocompromised child. The most common infectious uveitides in this population comprise cytomegalovirus retinitis, followed by herpetic acute retinal necrosis, herpetic anterior uveitis, endogenous fungal endophthalmitis, toxoplasmic retinochoroiditis, and herpetic progressive outer retinal necrosis. Intraocular fluid samples are a vital diagnostic tool but visual outcome is usually poor (57).
Human immunodeficiency virus (HIV) may be associated with different types of ocular inflammation caused by various opportunistic or pathogenic viruses, parasites, or bacteria. A retrospective study from Congo reported a prevalence of 14.3% of uveitis in HIV patients. CMV is another frequently associated infection in this group of patients, causing posterior uveitis or retinitis which are considered as one of the most severe ocular complications of HIV associated with severe morbidity (53, 54). The location of ocular inflammation may be predictive of intraocular infection amongst immunocompromised patients as all patients with posterior and panuveitis, had intraocular infections. The most common (almost 50%) may have CMV infection whilst the others may have Toxoplasma gondii, Treponema pallidum, VZV, Aspergillus and Candida. Toxoplasma gondii was most frequently detected amongst non-HIV patients (58).
A simplified flowchart regarding the differential diagnosis of uveitis based on its location and etiology is shown in Figure 2.
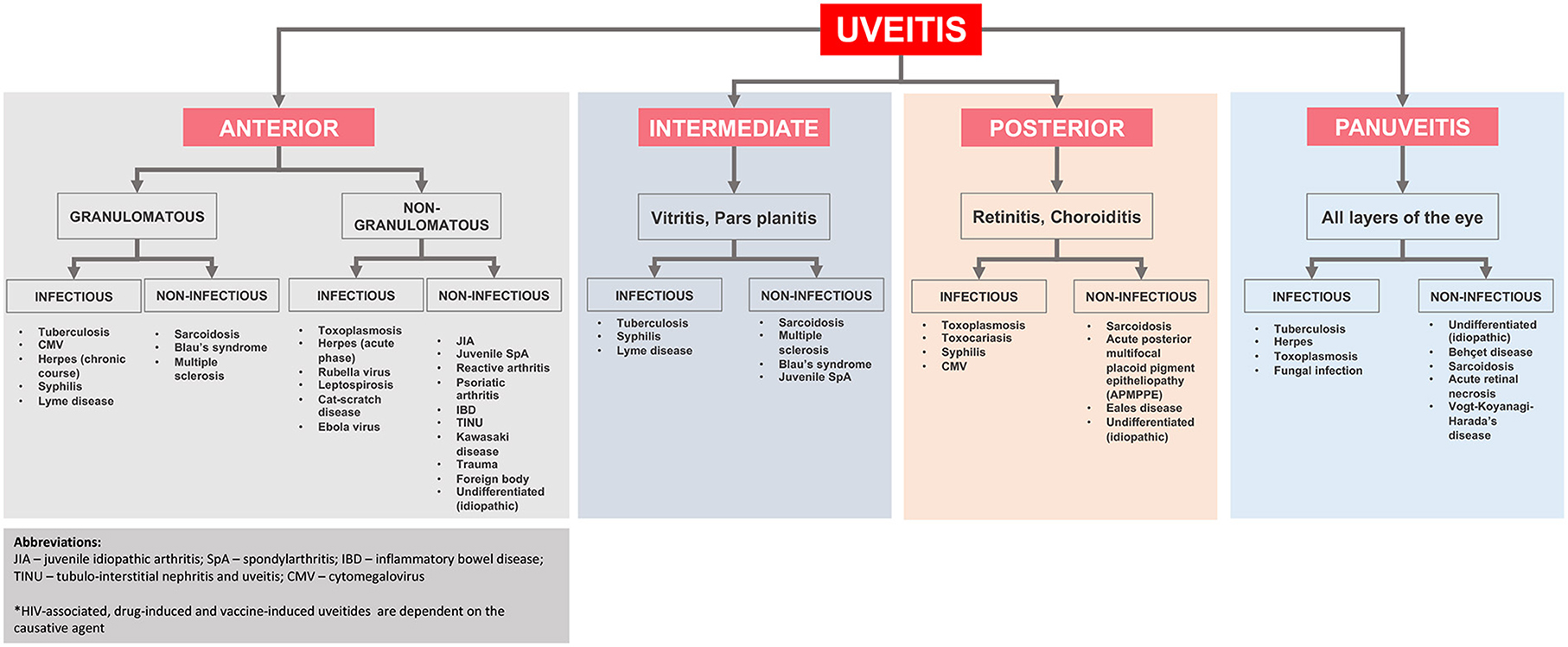
Figure 2. Anatomical classification and underlying diagnoses in pediatric uveitis adapted from Maleki et al. (2).
Non-infectious uveitis
Drug induced uveitis
Drug induced uveitis may be triggered by topical, systemic, or intraocular medications. It is important to take a detailed drug history in patients with unexplained uveitis (59). The onset of uveitis can be immediate or delayed up to several months.
Topical medications such as Brimonidine and Prostaglandin analogs and intraocular injections with Vancomycin, Triamcinolone acetonide or anti-vascular endothelial growth factor (anti VEGF) agents can cause uveitis. Common systemic drugs which may cause uveitis are Cidofovir, Rifabutin, Fluoroquinolones, bisphosphonates, immune checkpoint inhibitors, protein kinase inhibitors and sulfonamides. Mechanism may be toxic or immune mediated (59, 60).
Cidofovir-induced uveitis occurs mostly post-intravitreal injections. Non-granulomatous anterior uveitis and hypotony are two most common findings. Chances of uveitis are higher in patients on protease inhibitors, who have been previously treated for CMV retinitis, have recurrent retinitis, or immune recovery (61). Pathological impact of Rifabutin on the eye is dose dependent and may present as anterior uveitis (with or without hypopyon), panuveitis, intermediate uveitis or retinal vasculitis (62). Treatment with intravenous bisphosphonates (mainly Pamidronate) carries a risk of development of uveitis 1–6 days after administration (63). In such cases, the infusion should be discontinued, changed to a non-nitrogenated bisphosphonate and treatment with topical steroids may be initiated if required (64, 65). Sulfonamides (such as Sulfamethoxazole/Trimethoprim) may cause bilateral anterior uveitis which develops within a week of initiation of therapy (66).
Protein kinase inhibitors have been linked to all types of uveitis and are responsive to topical steroids. Drug-induced Vogt-Koyanagi-Harada syndrome has also been described in association with the use of some protein kinase inhibitors (60).
Antibiotic use and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) may cause Tubulointerstitial nephritis and uveitis (TINU) which is an immune mediated hypersensitivity reaction. The importance of NSAID as a risk factor varies greatly between the studies but may be present in up to 60–70% of the cases (67).
Vaccine induced uveitis
There is emerging awareness of a potential link between vaccines and uveitis onset. It is possible that this is not causative, and simply an ascertainment bias with associations only posited because of an incidental temporal association. Post-vaccine uveitis has been described as a mild course of anterior uveitis which are usually responsive to topical steroids. The most common associations are with the Hepatitis B vaccine, but also HPV vaccine, influenza vaccine, BCG, MMR, and varicella vaccine (68–70). Recently, transient uveitis has been described following COVID-19 vaccination with no significant impact on visual outcome (71). A causal link is still debated, and these side-effects of vaccines should not modify the general vaccine calendar.
Uveitis and systemic diseases (autoimmune and autoinflammatory diseases)
Non-infectious uveitis causes or associated disorders span a wide range of conditions, the most significant of which are discussed here.
JIA associated uveitis
JIA-associated uveitis, with a pooled prevalence of 11.8% (95%CI: 11.2–12.4%) amongst JIA patients, is the most common extra-articular manifestation of disease. In comparison, the prevalence uveitis in pediatric SLE is <1% (72). Uveitis prevalence in pediatric Behcets is 18%, but as JIA is the most common rheumatological condition in childhood, JIA is the most common extra-ocular disorder seen in childhood uveitis clinics.
The seven subtypes of Juvenile idiopathic arthritis (JIA) can be divided into JIA with low risk of uveitis (systemic arthritis and other unclassified arthritis), moderate risk (polyarthritis rheumatoid factor, RF, positive) and higher risk of uveitis (oligoarthritis, persistent or extended; polyarthritis RF negative; enthesitis related arthritis or ERA, juvenile psoriatic arthritis or JpSA) (73, 74). Chronic silent anterior uveitis is usually non-granulomatous in oligo or polyarticular JIA, as is acute anterior uveitis of ERA.
Chronic anterior uveitis is the most common form seen in JIA associated uveitis, and in most cases, JIA precedes the onset of uveitis. Children are often asymptomatic at onset and can develop irreversible complications by the time of eventual diagnosis. Complications at diagnosis are highly predictive of later permanent loss of vision, and this underpins the importance of early detection and treatment initiation for JIA-associated uveitis. In the UK and USA, commencement of regular eye examinations is generally recommended to start as soon as possible after JIA is suspected for those at moderate or high risk, and to continue every 4 months until the age of 11–12 years, with the other key time for surveillance being the first few months after stopping systemic JIA treatment.
The risk of developing uveitis appears to be highest in young females with oligoarticular JIA who are ANA antibody positive. As well as differing risk, clinical characteristics differ between JIA subtypes. In sharp contrast to the “silent uveitis” classically seen in early onset oligoarticular JIA, uveitis onset and recurrences in ERA and JPsA JIA can be acutely symptomatic (the “painful red eye”) (75).
Children with juvenile psoriatic arthritis (JPsA, i.e., oligoarthritis, polyarthritis or axial disease with the onset of >4 years of age, and with high prevalence of enthesitis) (76, 77) can also develop an inflammatory conjunctivitis: however, managing pediatricians should have a high index of suspicion for uveitis: 33% of patients with axial involvement were diagnosed with anterior uveitis vs. 6% of the patients with no axial disease (78), and children with JPsA had a worse visual outcome when compared to those with other forms of JIA (79, 80).
Predictors of uveitis incidence in children with JIA include clinical subtype, as above, inflammatory markers, autoantibodies and genetic susceptibility. Several disease activity biomarkers are used routinely (e.g., ESR in JsPA), but there are also emerging biomarkers which are still undergoing validation, including S100A12, S100A8/A9 (also known as MRP8/14) and soluble IL-2 (also known as soluble CD25). The main genetic factor identified in JIA is MHC class II HLA-DRB1 that is involved in antigen presentation being a predictor of JIA or, in some cases, JIA associated uveitis (81).
Sarcoidosis
Sarcoidosis can affect almost any portion of the eye or surrounding tissue.
Sarcoidosis associated uveitis can present as anterior, intermediate, posterior or panuveitis. Anterior uveitis may be asymptomatic or painful, but presentation may be with visual symptoms, dependent on the extent, severity and location of ocular inflammation (82). The manifold other examples of ocular disease include dry eye (aqueous deficiency due to infiltration of the lacrimal gland), conjunctival granuloma, scleritis, and optic neuritis.
As well as bilateral and chronic, the uveitis associated with sarcoid is usually clinically granulomatous: large “greasy” keratic precipitates, iris nodules, choroidal granuloma, which may encircle the optic nerve, and/or various degree of vitreous opacities, snowballs and snowbanks in the vitreous cavity (83–85).
Sarcoidosis can be included on the differential diagnosis of almost any presentation of uveitis. Diagnostic criteria for sarcoidosis associated uveitis have been proposed at first International Workshop on Ocular Sarcoidosis (IWOS) in 2009. To make a diagnosis of ocular sarcoidosis, 3 conditions must be met—First, patient must have clinical findings consistent with sarcoidosis by IWOS criteria. Second, evidence of non-caseating granulomas must be demonstrated histologically on a tissue biopsy. Third, other causes of granulomatous inflammation must be excluded such as tuberculosis or syphilis. Many patients with clinical features of ocular sarcoidosis are unable to undergo biopsy or do not have an appropriate site for biopsy. In these cases, a diagnosis of probable or presumed ocular sarcoidosis is made based on clinical features, laboratory findings and chest imaging results according to the IWOS criteria. The IWOS criteria have been recently validated in several countries and shown high reliability for the clinical diagnosis of ocular sarcoidosis (83).
Recommended laboratory investigations in suspected ocular sarcoidosis include chest x-ray to look for bilateral hilar lymphadenopathy, liver function tests, Angiotensin converting enzyme and other sarcoidosis biomarkers in the blood such as lysozyme, neopterin levels and sCD163 serum levels (86).
Tubulointerstitial nephritis and uveitis
TINU is a rare benign autoimmune condition of unknown etiology characterized by combined ocular and renal damage. Usually, the eye inflammation precedes or is diagnosed soon after the accidental finding of renal abnormalities and associated systemic features (87–89).
TINU is diagnosed in ~1–2% of pediatric and adolescent patients attending uveitis clinics (90). A Japanese study reported the incidence of TINU at 0.4% among 2556 patients with uveitis (91). Therefore, due to the rarity, most pediatricians, nephrologists and ophthalmologists are unfamiliar with the condition so TINU is often underdiagnosed (89).
Main clinical and laboratory features of TINU include sudden onset of bilateral non-granulomatous anterior uveitis (rare posterior uveitis or panuveitis) associated with typical anterior uveitis symptoms (eye pain, redness, decreased vision, and photophobia) and mildly to moderately abnormal renal function, in particular elevated Creatinine with or without raised urea, high inflammatory markers (ESR, CRP), abnormal urinalysis (low-grade proteinuria, normoglycemic glucosuria, microscopic haematuria and elevated β2-microglobulin). To confirm the renal involvement, kidney biopsy can be considered demonstrating tubulointerstitial inflammatory infiltrations containing lymphocytes and non-specific histiocytes with vessels and glomeruli being typically spared (87, 90, 92). Apart from the mentioned above clinical and laboratory changes, most of the patients with TINU have systemic features, including fever, weight loss, fatigue, and abdominal/flank pain.
Response to treatment is usually good although patients may develop chronic relapsing course of uveitis. Escalation of treatment should be considered without delays to avoid irreversible ocular damage, including, cataract and loss of vision.
Vasculitis
Uveitis can be associated with systemic vasculitis. Broadly, pediatric vasculitis is classified as large, medium, small, or variable vessel vasculitis.
Behcet's disease
BD comes under an overlapping category of autoimmune and autoinflammatory diseases as both adaptive and innate immune systems play a role in its pathogenesis. The prevalence of BD is exclusively high in countries across the Old “Silk Road”, the area between the Mediterranean and East Asia. The highest prevalence is reported in Turkey, Iran, Israel, Northern Jordan, Korea, Northern China and Japan (93, 94). The most frequent types of ocular involvement in both adults and children are bilateral anterior uveitis with hypopyon, panuveitis, retinitis with retinal infiltrates and occlusive retinal vasculitis with high risk of severe course of ocular inflammation in children (95). The eye is the most frequently involved organ, in up to 60–80% of cases with manifestation within 2–3 years after the onset of BD (94, 96), and in several cases the ocular disease occurs first. This, pediatricians must be aware of the possibility of disease in children with uveitis but must also be aware of the possibility of eye disease in children with BD. Various ocular symptoms may include red eye, floaters, photophobia, and varying degrees of visual loss ranging from mild to severe. The ocular involvement can be recurrent leading to poor visual outcome, so early identification and early and aggressive immunomodulatory treatment approach is essential (97–99).
Takayasu arteritis
A type of large vessel vasculitis and defined as panarteritis involving all layers of large vessels of the neck, thorax and abdomen, Takayasu arteritis frequently leads to ischaemic events. The incidence of ocular involvement is rare and includes retinopathy, ischaemic optic neuropathy and much less frequently anterior uveitis. It usually occurs late in the disease course and may be associated with severe involvement of the aortic arch and carotid arteries (100–102).
Kawasaki disease
A medium vessel vasculitis, KD is typically associated with bilateral non-exudative bulbar conjunctivitis sparing the limbus and seen in ~85% of children but can also manifest as anterior uveitis in up to 66% of children. Uveitis is usually mild, resolving within 2–8 weeks and maybe associated with keratic precipitates (103, 104).
Henoch schonlein purpura
HSP typically presents with a triad of symptoms including palpable purpura, abdominal pain and arthritis. Uveitis is a rare complication of HSP that can be associated with the development of bilateral granulomatous anterior uveitis and keratitis, commonly reported in adult patients or those with HSP-related nephritis (105). The eye disease may be insidious in onset with an aggressive clinical course (106).
Granulomatosis with polyangiitis
Orbital involvement is one of the most frequently diagnosed types of ocular manifestations occurring in up to two third of patients with GPA, and in some cases can be the initial presentation of the disease. More broadly, disease tends to affect the respiratory tract and kidneys and is characterized by necrotizing vasculitis of small arteries and veins. A small number of cases of ocular inflammation in GPA can also be associated with uveitis that can be unilateral or bilateral, anterior, intermediate or posterior, with or without vitritis. Granulomatous panuveitis has also been described (107–110).
Inflammatory bowel disease
IBD is characterized by chronic remitting-relapsing inflammatory disease of gastro-intestinal tract (GIT) and refers to as Crohn's disease (CD) and ulcerative colitis (UC), where CD is characterized by non-contiguous disturbed, granulomatous inflammation of any part of GIT, while UC is a diffuse, non-specific inflammation of the colonic mucosa, mainly the end part of the GIT, resulting in erosions and ulcer formation (111). The etiological factors are still unknown; however, pathophysiological mechanisms include abnormal response of the host immune system, presence of susceptibility genes and environmental factors (111, 112).
Both, CD and UC have recognized extra-intestinal manifestations (EIMs) that include skin involvement, arthritis (in particular spondylarthritis) and development of ocular inflammation. The muscular-skeletal manifestations may be associated with concomitant eye involvement. The prevalence of eye inflammation is higher in patients with CD than in patients with UC and ranges from 0.6 to 1.8% of all EIMs in the pediatric population (113–115).
Ocular inflammation usually manifests as episcleritis, scleritis but children can also present with a bilateral acute or chronic anterior uveitis. The uveitis can be primary, i.e., manifests before the intestinal symptoms, or secondary (112).
Systemic autoinflammatory diseases
SAIDs are a broad spectrum of rare inherited disorders caused by genetic mutations leading to upregulation of key innate immune mediators and characterized by recurrent episodes of self-limiting inflammation without evidence of infection and autoimmunity (i.e., absence of autoantibodies and/or autoreactive effector T cells). SAIDs share common features, particularly recurrent fevers, typical skin rash and multisystemic involvement affecting almost all organ systems depending on the subtype of SAID (116–118).
Depending on the underlying immune mechanisms, SAIDs can be categorized into inflammasomopathies and interferonopathies. Both categories are associated with uveitis, but the most commonly associated SAIDs comprise cryopyrin-associated periodic syndromes (CAPS), familial Mediterranean fever (FMF), TNF receptor associated periodic syndrome (TRAPS), mevalonate kinase deficiency (MVKD), Blau's syndrome (BS), deficiency of adenosine deaminase 2 (DADA2) and haploinsufficiency of A20 (HA20), Aicardi-Goutieres syndrome (AGS), and chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) (116, 119).
According to the literature reports, nearly all SAIDs are associated with transient or chronic recurrent ocular inflammation of varying severity and diversity and in some cases leading to severe ocular impairment. CAPS and Blau's syndrome are more frequently associated with moderate to severe eye inflammation (116).
CAPS is associated with a gain-of-function missense mutation of the NLRP3 gene and include three overlapping clinical phenotypes: familial cold autoinflammatory syndrome (FCAS, mild CAPS phenotype), Muckle-Wells syndrome (MWS, moderate) and neonatal-onset multisystem inflammatory disease (NOMID, severe). Ocular involvement is reported in all phenotypes with conjunctivitis being the most frequently reported, typically occurring during flares. However, a chronic anterior non-granulomatous uveitis is seen roughly in half of CAPS patients, and other uveitis types may develop. Patients with NOMID may also develop papilledema related to high intracranial pressure secondary to CNS involvement (116, 119, 120).
Blau's Syndrome, NFkB activation disorders caused by mutation in NOD2/CARD15 gene, share common clinical features with early-onset sarcoidosis, including recurrent fever, granulomatous dermatitis, sterile arthritis, and ocular inflammation, particularly granulomatours anterior and panuveitis (116, 118, 120).
Ocular inflammation is a recognized feature of the other SAIDS, but occur at a much lower frequency (i.e., found in <1% of the FMF and TRAPS belong to the group of inflammasomopathies that are associated with the mutation in MEFV and TNFRSF1A genes, respectively). The clinical presentation of FMF and TRAPS is similar and characterized by recurrent fever, erysipelas-like or erythematous skin rash, serositis, arthralgia, myalgia, and lymphadenopathy. The most frequent ocular findings are choroidal thickening, retinal vasculitis and anterior uveitis (118, 121–126).
- MVKD is determined by mutation of MVK gene resulting in metabolic defect leading to recurrent fever, rash, painful adenopathy, arthralgia, mouth and genital ulcerations, hepatosplenomegaly, and abdominal pain. MVKD ranges from a milder form known as Hyper IgD syndrome (HIDS), to the most severe phenotype, mevalonic aciduria (MA). The ocular manifestation is less frequently reported with anterior uveitis being the dominant ocular finding, followed by retinal dystrophy and cataract formation in severe cases. The latter is explained by a direct toxicity of mevalonic acid on the retina and lens (116, 118).
- Other rare inflammasomopathies, such as deficiency in ADA2 and interferonopathies, including HA20 haploinsufficiency, Aicardi-Gouttieres syndrome (AGS) and CANDLE can also present with ocular inflammation. This can be associated with severe involvement of choroid, retina, and cornea with high intraocular pressure in some cases (116). Interferon signature is present (upregulation of type-1 interferon regulated genes) and is key in the diagnosis of interferonopathies (127). However, this test is not yet routinely available so clinical features and when possible, molecular diagnosis of SAIDs is most important in suspecting the diagnosis.
Reactive arthritis
ReA may also be associated with a various ocular involvement with mild bilateral conjunctivitis being the most frequently diagnosed in the affected patients. The symptoms of conjunctivitis may vary from mild and painless with spontaneous recovery, to severe inflammation including blepharospasm and photophobia requiring topical and sometimes systemic treatment (128). The second most common ocular finding is non-granulomatous anterior uveitis that may occur in up to a third of patients with ReA and more frequently is described in patients carrying HLA B27 and those with sacroiliitis (129).
Systemic lupus erythematosus
Ocular manifestation has been described in a third of patients with Juvenile SLE affecting various parts of the eye (130). The most frequently described ocular inflammation include surface epitheliopathy (lacrimal gland involvement leading to keratoconjunctivitis sicca), corneal involvement, episcleritis, scleritis, retinopathy and non-granulomatous anterior uveitis that may lead to blindness. Posterior segment involvement is rare but a reflection of severe systemic inflammation (131, 132).
Vogt-Koyanagi-Harada disease
VKHD is described as multisystemic autoimmune sight-threatening disease and is characterized by granulomatous ocular inflammation related to T-cell mediated dysregulation targeting melanocyte-containing tissue in the eye, the CNS, the inner ear, and the skin. The incidence of VKH in pediatric population is rare and race-dependent varying between 0.5 and 3% of all pediatric uveitides (133–136). The challenge in pediatric population is the delayed diagnosis leading to worse visual outcome compared to adults (137).
The etiological factors are still unknown; however viral infection combined with the presence of HLA DRB1*0405 may play a role. The high prevalence of VKHD is seen among Hispanic and Asian population with young females being more affected and relatively rare in pediatric cohort (138).
According to the revised criteria proposed by American Uveitis Society (AUS) in 2001, VKHD may present as complete, incomplete, or probable. In its complete form, VKHD is defined as a non-traumatic bilateral panuveitis and is associated with integumentary, neurological/auditory signs. Patients with the incomplete form of the disease usually present with bilateral ocular involvement with either integumentary features or neurologic/auditory manifestations. Probable (ocular) VKHD is characterized by the presence of ocular features with the absence of extraocular manifestations. In all forms of the disease, a history of ocular surgery and clinical or laboratory evidence suggestive of other ocular diseases make the diagnosis of VKH unlikely (139).
Therefore, VKH should be considered if, there is no history of ocular surgery and no clinical or laboratory evidence suggestive of other ocular diseases having similar disease course. There is no single pathognomonic sign or highly specific laboratory tests that could reliably help with the diagnosis of VKHD. Initial step toward the diagnosis of VKHD is a thorough medical history, including history of eye trauma or intraocular surgery, family history of uveitis, detailed systems and medication history.
Multiple sclerosis
Multiple Sclerosis (MS) is a chronic inflammatory CNS disorder of the white matter. The reported frequency of MS following uveitis diagnosis ranges from 0.4 to 26.9%. Intermediate uveitis and retinal vasculitis are the most common ocular manifestations, usually occurring bilaterally and most frequently affecting females (140, 141).
Pars planitis
Pars planitis is a form of idiopathic intermediate uveitis that primarily affects children. The incidence of pars planitis has been reported to be 1.5–2 cases per 100,000 persons (142) accounting for 5.6–24.0% of pediatric uveitis diagnoses (134). Most cases present bilaterally with floaters and decreased vision. Snowbanks over the pars plana or snowballs in the anterior vitreous are characteristic in the absence of infection or systemic disease (142).
Cogan's syndrome
CS is a rare autoimmune vasculitis of unknown etiology that is classically presented with interstitial keratitis and sensorineural hearing loss with tinnitus, vertigo and constitutional symptoms (143). Development of ocular and audiovestibulary manifestations can occur within days or weeks to months. CS can be classified as “typical” characterized by the presence of iritis and conjunctival hemorrhage, and “atypical” in which chronic or recurrent conjunctivitis, scleritis, uveitis, optic disc oedema, and retinal vasculitis are present in addition to or other than interstitial keratitis.
Treatment of CS includes corticosteroids and immunosuppressive agents. It has been proposed recently that early treatment with biologic DMARDs, including TNFα blockers such as Adalimumab and Infliximab or anti-CD20 monoclonal antibody Rituximab, may improve hearing function and significantly reduce eye inflammation (144, 145).
Susac's syndrome
SS is a rare self-limiting autoimmune endotheliopathy characterized by the clinical triad: CNS dysfunction, sensorineural hearing loss and retinal vaso-occlusive disease. Branched retinal artery occlusion (BRAO) is the classical ocular involvement in patients with SS resulting in the irreversible visual loss by the occlusions of arterioles supplying the retina. The process can be unilateral or bilateral, characterized by altitudinal defects or central/paracentral scotomas; however, patients may remain asymptomatic if the occlusions are in the peripheral retina (146, 147). Blurred vision and photopsia are most frequently reported features; however, vitreous hemorrhage and neo-vascularisation may also be seen (148).
High dose of corticosteroids and intravenous immunoglobulins (IVIG) are the mainstay of the treatment of SS and have shown high effectiveness. Other treatment options including plasma exchange may be considered as alternative treatment in corticosteroid-resistant cases (149).
Management
Role of the pediatrician in the management and monitoring of pediatric uveitis
Although management of uveitis is led by ophthalmologists, multi-disciplinary care teams which involve pediatricians are needed for holistic care of these inflammatory, chronic, relapsing and remitting disorders (150, 151). They are particularly involved in treatment. The main goals of treatment of chronic non-infectious uveitis are to resolve the intraocular inflammation, to achieve remission and prevent recurrences, to preserve vision and prevent complications (152). Complications include band keratopathy (15.7–29% of all pediatric chronic anterior uveitis), cataract (8–31%), macular edema (6–25%), ocular hypertension/glaucoma (8–19%), ocular hypotension, and macular fibrosis (4%). A systematic review identified that complications of uveitis developed overall in up to 67% of children and one-third of them were present at diagnosis (2, 72).
First line of treatment of anterior chamber involvement is topical steroids, but its use is associated with a risk of ocular complication such as raised intraocular pressure, glaucoma and cataract (153, 154). Long-term treatment is not based on corticosteroids but on disease-modifying antirheumatic drugs (DMARDs) such as Methotrexate as a first line agent. Biologic agents are important for cases refractory to DMARDs.
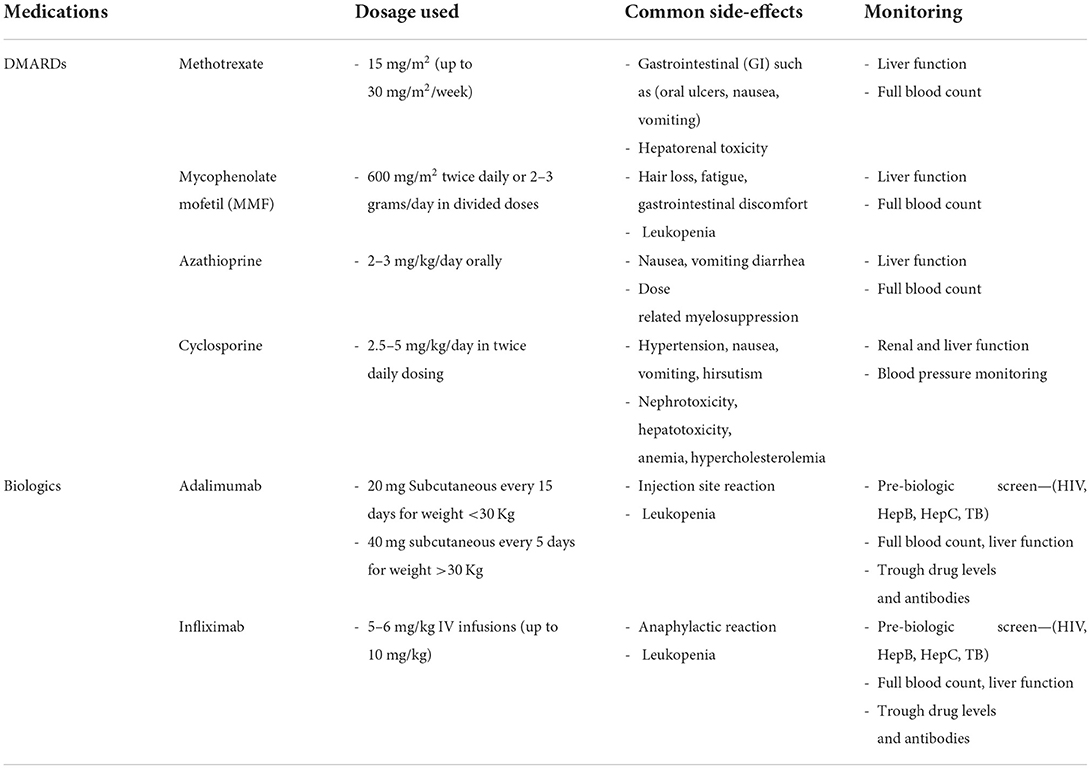
Pediatric investigations prior to commencement of biologic DMARDs treatment should include work up to rule out tuberculosis (Quantiferon/T-spot, chest x-ray), serology for varicella zoster virus (VZV), hepatitis B, and hepatitis C (and HIV when appropriate). Immunizations should be updated prior to starting immunosuppression, according to national recommendations including live attenuated vaccines such as MMR and varicella vaccine when patient is seronegative (155, 156). Monitoring of compliance with medications and topical treatment is particularly important in children and adolescent patients. Patient with non-infectious uveitis requiring immunosuppression with poor compliance were shown to achieve less steroid free remission (150, 157). Dosing, side effects and monitoring of all these medications are summarized in Table 4.
VZV non-immune children who are exposed to chickenpox and not eligible for vaccine should be offered post-exposure prophylaxis (PEP). Although there is no standardized PEP, anti-VZV immunoglobulins (V-ZIG) and oral acyclovir are both used. Varicella immunization of seronegative family members is recommended (158–160). Most immunocompromised patients who develop chickenpox may be admitted for IV acyclovir treatment although data from a single center study showed mostly uncomplicated varicella. However, fever and delay to treatment are predictors of complications (161).
Although there is little evidence in pediatric uveitis, immunizations are safe and effective in children with Rheumatic diseases on immunosuppression but in one third of the patients, response to vaccine may be decreased and this can be confirmed by measuring antibody levels so timing to boosters can be adjusted (162, 163). It is recommended that household contacts of children on immunosuppression are to up to date with immunizations. Children on immunosuppression should follow their normal national immunization schedule for non-live/inactivated vaccines and receive annual influenza vaccine (156). For live attenuated vaccines, in particular varicella and MMR, data suggest these vaccines may still be safe and effective but are usually not recommended in children on biologic DMARDs and systemic corticosteroids (164, 165). Increased disease activity of the underlying disease such as JIA in children on non-biologic DMARDs does not seem to be associated with MMR booster vaccination (164, 166).
Most patients with non-JIA-associated uveitis will have a baseline work-up to identify extra-ocular involvement. The identification of an underlying autoimmune or autoinflammatory disease may tailor management. Extra-ocular involvement may need monitoring that can be undertaken by the pediatrician alongside the tertiary care center. In the absence of extra-ocular involvement at presentation, pediatricians may monitor extra-ocular involvement with history taking, clinical examination, and further investigations as indicated (Table 5). In two recent studies that included children, monitoring identified an underlying disease in 19–29% of the patients monitored for at least 1 year. The most common diagnosis was sarcoidosis and other inflammatory conditions (including JIA, TINU, BD, MS, or Crohn's disease) but also lymphoma and infectious causes (such as tuberculosis, HSV, syphilis or toxoplasmosis). This supports the need for a monitoring based on uveitis classification and evidence (192–194).
Close monitoring of children with uveitis is recommended to prevent complications due to uncontrolled inflammation or long-term medication use such as steroids. For JIA-associated uveitis patients, the frequency of screening should be based on individual risk factors (ANA positivity, early age, oligoarticular course) and current immunosuppressive treatment even when the patient is in remission of uveitis (195–197). Similarly for non-JIA associated uveitis, the uveitis screening should continue when remission is achieved although there is no consensus guideline. In most cases monitoring should be done at 2–6-week intervals tailored based on the frequency of topical steroid use, degree of intraocular inflammation, intraocular pressure and presence of complications as agreed by the treating team (195, 198).
Corticosteroids
Corticosteroids are useful in acute inflammation for non-infectious ocular involvement (199).
Their mechanism of action is by modulating the gene expression of pro-inflammatory cytokines. This anti-inflammatory action of steroids can be achieved either through topical or systemic administration of steroids depending on the location of ocular inflammation. Local treatments include topical steroid drops or periocular, intraocular injections or implants.
Topical corticosteroids
Topical corticosteroids are usually the first-line treatment in anterior segment ocular inflammation and were used in about 90% of patients with idiopathic uveitis enrolled in the CARRA Registry. (200). Dexamethasone and prednisolone acetate are the most used topical steroids, the frequency of which is decided by the severity of inflammation. Long-term use of topical corticosteroids (especially difluprednate 0.05%) is associated with cataracts and steroid induced ocular hypertension (201, 202). Literature suggests that the need for >2 drops per day leads to higher ocular complications.
Local injection of corticosteroids is a very useful modality for treating uveitis especially intermediate and posterior uveitis. Regional delivery of steroids is used to minimize systemic side-effects maximizing drug delivery to the target area. This can be done by either periocular (around the eye) or intravitreal (inside the eye) steroid injections or by using intraocular steroid implants.
Periocular and intraocular steroid injections
Periocular and intraocular steroid injections are most likely useful for severe presentations or flare up of remitting types of ocular inflammation and for non-chronic uveitis macular edema (203). Ocular side-effects such as glaucoma, ocular hypertension and need for cataract surgery were lesser as compared to intravitreal steroid injections. Intravitreal steroids, used for similar indications as periocular steroids but risks include vitreous hemorrhage, retinal detachment and endophthalmitis.
Steroid implants
Steroid implants were created to be used when long-term control of inflammation is desired with minimal relapses. These are approved for treatment of macular edema due to retinal vein occlusion and for treatment of non-infectious posterior uveitis. More studies are required to assess the long-term efficacy and safety profile of steroid implants in patients (204), but they have been used to control posterior disease or as a pre-surgery treatment to lessen the risk of post-cataract surgery macular oedema.
Systemic corticosteroids
Systemic Corticosteroids are very useful in children with intermediate, posterior or panuveitis, for urgent control of inflammation. According to the CARRA registry, roughly 94% of children with idiopathic uveitis were given systemic steroids during the disease course (200). Based on severity of inflammation, either oral or IV steroids can be used. Oral steroids are used at a dose of 1–2 mg/kg/day and tapered according to response, while IV steroid boluses are used in more severe cases in higher doses. Recurrences or severe initial presentation may need additional non-biologic or biologic Disease Modifying Anti-Rheumatic Drugs (DMARDs) to limit prolonged steroid associated side effects.
Ocular complications of corticosteroids
Ocular complications of corticosteroids are mainly ocular hypertension/glaucoma and cataract. Ocular hypertension and secondary glaucoma have been reported with the use of systemic and topical steroids causing high incidence of blindness at 5 years. Early aggressive immunosuppression with DMARDs to control ocular inflammation may help prevent these ocular complications (205).
Topical Dexamethasone (eye drops) and intravitreal implant (dexamethasone) are both associated with cataract formation and increased intraocular pressure (206, 207). Although there is little data on systemic effects of steroid eye drops in children with uveitis, there are data suggesting that even inhaled steroids affect growth and bone mineral density (208, 209). Systemic corticosteroids have wider side-effects: hypertension, osteoporosis, cataract, gastro-intestinal complications, and metabolic issues (weight gain, diabetes, growth delay). Prevention of weight gain and diabetes may require diet adaptation with limited sugars as well as strict parental control of caloric input. Complications can also have severe long-term adverse events such as peptic ulcers or myocardial infarction (210).
In JIA, growth is known to be affected by disease activity and prolonged high dose systemic corticosteroid use (211, 212). Growth is improved with the use of anti-TNF as it has been shown in several conditions such as JIA or IBD (213, 214). Monitoring of growth is key in patient with non-infectious uveitis as it is likely to be associated with disease activity and the use of steroids. Decreased growth velocity and short stature may play a role in the decision to escalate treatment and even in some selected children to discuss growth hormone therapy, so patient may achieve an acceptable final height.
Conventional DMARDs
Medicines for long-term immunosuppression are Methotrexate (MTX) (first-line conventional immunotherapy), and less commonly, mycophenolate mofetil (MMF), Azathioprine and cyclosporine A. MTX has a folic-acid analog mechanism of action inhibiting DNA replication and RNA transcription in B and T lymphocytes (199). Folic acid should be used as supplementation to prevent methotrexate related side effects, including anemia, abdominal pain, nausea, vomiting, hair loss. MMF can also be used in refractory uveitis; however, in patients with musculoskeletal involvement, this medication is not known to be effective. Azathioprine and Cyclosporine A are other less commonly used DMARDs used for childhood uveitis and can also be considered in refractory uveitis.
It is well known that withdrawal of Methotrexate may lead to flare of inactive JIA-associated uveitis. A longer period of uveitis inactivity prior to withdrawal may decrease the risk of flare (215).
Biologic DMARDs
Since pro-inflammatory cytokines, such as TNFα, interferons and Interleukins (IL) are consistently elevated in aqueous fluid and serum of children with intraocular inflammation, targeting these molecules is an attractive and less toxic approach for children with uveitis refractory to steroids and conventional DMARDs. Targeted blocking these pro-inflammatory cytokines can represent a decent steroid-sparing alternative to treat uncontrolled ocular inflammation (216).
Anti TNF alpha agents
Adalimumab, Infliximab, Golimumab and Certolizumab are widely used in patients with non-infectious uveitis (217). The effectiveness of Anti-TNF-alpha for treating refractory uveitis has been demonstrated in clinical trials and case series (218–221). Publications show not only a reduction of inflammatory signs and relapses, but also a preserved visual acuity (more than 90% in some cases) along with the quality of life of patients with uveitis (222–224). Early use of anti-TNF is recommended if uveitis is not or is partially responsive to the initial treatment associating systemic corticosteroids and a conventional DMARD to prevent visual loss especially if patient is in a sight-threatening situation. There are also cases when patient is intolerant to DMARDs or is developing ocular and/or systemic side-effects (225, 226). Two main phase III randomized controlled clinical trials (SYCAMORE and ADJUVITE) reporting the use of adalimumab in JIA-associated uveitis have been published in 2017 and 2018. Although using a different design, they both concluded that adalimumab is safe and efficacious in controlling chronic anterior uveitis in patients above 2 years of age who have failed topical steroids and Methotrexate. However, it was shown that the adalimumab group had more side-effect than the placebo group, mainly respiratory infections (220, 221). Etanercept is not efficacious in preventing the occurrence of uveitis and in controlling ocular inflammation in JIA-associated uveitis and is therefore not used in clinical practice for uveitis treatment (217, 227). A small pilot clinical trial did not identify any difference in JIA-associated uveitis disease activity (anterior chamber) in the etanercept group and the placebo group (228).
Although there is limited evidence mostly in JIA associated uveitis, Golimumab and Certolizumab appears to be off-label medications of interest (217, 229–231).
Two systematic reviews identified that adalimumab is better at controlling intra-ocular inflammation in JIA-associated uveitis than infliximab (217, 232). The data from the BiKeR JIA registry showed that there was a tendency for more occurrence of JIA-associated uveitis in the Etanercept group than in the biologic naïve group although the difference was not significant (1.9/100 PY vs. 1.4/100 PY, p = 0.09) (233). Similarly, a recent article reporting data from 3 registries, showed that patients with JIA on Methotrexate but also on biologics continues to develop uveitis although at a lower rate suggesting that the use of conventional and biologic DMARDs reduce the incidence of uveitis in JIA (234). Early MTX use and the combination of MTX with an anti-TNF have the highest protective effect (235).
Although limited to few case series, adalimumab and infliximab have been reported to be safe and efficacious in uveitis of several underlying causes including Behcet's disease, Blau's syndrome and sarcoidosis (236–242).
Other biologic therapies
Abatacept (CTLA4-Ig), a novel fusion protein that modulates the T cell co-stimulatory signal mediated through the CD28-CD80/86 pathway, Tocilizumab (IL-6 blockade), Rituximab (B cell blockade), Secukinumab (anti IL-17 A monoclonal antibody) are some other off-label agents used in uveitis refractory to the above mentioned therapies (150, 243). Although most of the evidence is in JIA-associated uveitis, it is worth mentioning the use of Tocilizumab with success in non-infectious uveitis with macular oedema (244, 245) and in Behcet's disease as an alternative to anti-TNF agents (246). APTITUDE, a multicentre, single-arm phase II trial, recently assessed the safety and efficacy of tocilizumab in children with JIA-associated uveitis refractory to both MTX and adalimumab. The primary endpoint of the trial was not met but the authors considered that tocilizumab was beneficial in a subset of patients with macular oedema (245).
Therapeutic drug monitoring
TDM is not very much used in routine practice for the monitoring of conventional DMARDs such as Methotrexate, Azathioprine or MMF. It is becoming routine practice in the monitoring of biologic DMARDs, particularly anti-TNF. Adalimumab and infliximab are well known to be immunogenic in particular if they are not used in association with a conventional DMARD and therefore monitoring is recommended (247, 248). Therapeutic drug monitoring includes proactive and reactive monitoring. Reactive monitoring is performed when the patient flares so medication dose may be adjusted to achieve clinical remission especially if the level is low and/or if the patient has developed anti-drug antibodies. Proactive monitoring will ensure that the patient's drug level is within the target drug concentration range to allow optimal response. There is a correlation between the drug level and the outcome in the presence of anti-drug antibodies. The presence of anti-drug antibodies may negatively influence the outcome with flares and secondary drug failure. Recent studies were conducted in pediatric inflammatory diseases (247, 249–251). Data suggest that optimisation of anti-TNF levels from initiation of treatment is safe and efficacious in preventing secondary failure in JIA. Using higher dose of a biologic DMARD may prevent the patient from switching to another biologic agent (249, 252). Recent data are also available in JIA-associated uveitis. Anti-adalimumab antibodies are associated with lower drug levels and more active uveitis. The use of concomitant MTX decreased the risk of anti-drug antibodies. In the absence of anti-drug antibodies, a higher drug level was not always associated with disease control (253, 254). A recent clinical trial conducted in adults with inflammatory conditions identified that proactive infliximab monitoring (drug and anti-drug antibody levels) with dose and interval adjustment is significantly associated with sustained disease control with no increased paradoxical adverse events (255). This suggests that TDM is an important tool to achieve optimal disease control.
Janus kinase inhibitors
There are limited data on the use of JAKi in the management of uveitis. It has been to date only used to rescue patients who failed biologic DMARDs in particular anti-TNF with improvement of the ocular inflammation and with no side-effects (256). A clinical trial is on-going in JIA-associated uveitis and chronic anterior ANA-positive uveitis (257). However, very promising data have been published in other off-labels indications such as Juvenile Dermatomyositis and monogenic IFN-mediated autoinflammatory diseases (258, 259). Tofacitinib has recently been approved in polyarticular JIA following a phase III randomized controlled trial (260).
Treatment withdrawal
ACR (American College of Rheumatology) and SHARE initiative both recommend at least 2 years of uveitis remission on slit-lamp examination before tapering treatment intensity when uveitis is well controlled on DMARD and biologic therapy only (195, 197). Earlier treatment withdrawal or treatment intensity reduction is at high risk of uveitis flare. The on-going randomized, controlled ADJUST trial's goal is to assess the efficacy of discontinuing adalimumab after achieving JIA-associated uveitis control for at least 12 months (261).
Impact on quality of life
Visual impairment is an important component of quality of life in children with uveitis and compounds the negative impact of reduced physical function due to associated rheumatological disease (149). Even amongst those with good vision, the fear of blindness is a major stressor for children and their families. Medical interventions including medication side-effects and frequent hospital attendance also contribute to the psychosocial burden of uveitis, as shown by studies involving a patient reported measure (the EYE-Q) developed specifically for children with uveitis. This tool is associated with pedQL and visual acuity (262). EYE-Q scores correlate with extend of uveitis and of visual impairment (263). Early recognition of the psychosocial impact of uveitis and appropriate referral may help families and support patient achieve the best possible outcome (264). It should be remembered that children with uveitis will mature into adults with childhood onset uveitis and it has been shown that on-going active uveitis in adulthood is associated with depression and anxiety (158–160). The number of uveitis related admission in the recent years has significantly decreased likely due to the use of immunosuppressive medications (265). It may be that there is a corresponding reduction in psychosocial burden (266).
Conclusion
This review on pediatric uveitis presents the case for the multi-disciplinary management of this complex childhood ocular inflammatory disease, in order to improve patient outcomes, patient experience and quality of life. Early recognition of ocular manifestations of established inflammatory disorders reduces the risk of sight loss. In the child who first presents with uveitis, prompt diagnosis of associated systemic conditions is supported by an understanding of the different uveitis types, and in turn supports the individualization of care and early escalation of treatment to reduce morbidity. Management of uveitis, whether it is isolated ocular disease treated with DMARDs, or as part of multisystem disease, involves ongoing surveillance for evolving extra-ocular disease, therapeutic drug monitoring, the management of side-effects of treatment and the psychosocial aspect of loss of vision and the underlying chronic disease.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by Biomedical Research Center, Great Ormond Street Hospital for Children, London, UK.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Edelsten C, Lee V, Bentley CR, Kanski JJ, Graham EM. An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol. (2002) 86:51–6. doi: 10.1136/bjo.86.1.51
2. Maleki A, Anesi SD, Look-Why S, Manhapra A, Foster CS. Pediatric uveitis: a comprehensive review. Surv Ophthalmol. (2022) 67:510–29. doi: 10.1016/j.survophthal.2021.06.006
3. Reekie IR, Sharma S, Foers A, Sherlock J, Coles MC, Dick AD, et al. The cellular composition of the uveal immune environment. Front Med. (2021) 8:721953. doi: 10.3389/fmed.2021.721953
4. Harman LE, Margo CE, Roetzheim RG. Uveitis: the collaborative diagnostic evaluation. Am Fam Physician. (2014) 90:711–6.
5. Gilani CJ, Yang A, Yonkers M, Boysen-Osborn M. Differentiating urgent and emergent causes of acute red eye for the emergency physician. West J Emerg Med. (2017) 18:509–17. doi: 10.5811/westjem.2016.12.31798
6. Tarff A, Behrens A. Ocular emergencies: red eye. Med Clin North Am. (2017) 101:615–39. doi: 10.1016/j.mcna.2016.12.013
7. Cronau H, Kankanala RR, Mauger T. Diagnosis and management of red eye in primary care. Am Fam Physician. (2010) 81:137–44.
8. Mets MB, Chhabra MS. Eye manifestations of intrauterine infections and their impact on childhood blindness. Surv Ophthalmol. (2008) 53:95–111. doi: 10.1016/j.survophthal.2007.12.003
9. Valerio GS, Lin CC. Ocular manifestations of herpes simplex virus. Curr Opin Ophthalmol. (2019) 30:525–31. doi: 10.1097/ICU.0000000000000618
10. Bosch-Driessen LE, Berendschot TT, Ongkosuwito JV, Rothova A. Ocular toxoplasmosis: clinical features and prognosis of 154 patients. Ophthalmology. (2002) 109:869–78. doi: 10.1016/S0161-6420(02)00990-9
11. Fabiani S, Caroselli C, Menchini M, Gabbriellini G, Falcone M, Bruschi F. Ocular toxoplasmosis, an overview focusing on clinical aspects. Acta Trop. (2022) 225:106180. doi: 10.1016/j.actatropica.2021.106180
12. Bonfioli AA, Orefice F. Toxoplasmosis. Semin Ophthalmol. (2005) 20:129–41. doi: 10.1080/08820530500231961
13. Jones NP. The manchester uveitis clinic: the first 3000 patients–epidemiology and casemix. Ocul Immunol Inflamm. (2015) 23:118–26. doi: 10.3109/09273948.2013.855799
14. Furtado JM, Simoes M, Vasconcelos-Santos D, Oliver GF, Tyagi M, Nascimento H, et al. Ocular syphilis. Surv Ophthalmol. (2022) 67:440–62. doi: 10.1016/j.survophthal.2021.06.003
15. Amaratunge BC, Camuglia JE, Hall AJ. Syphilitic uveitis: a review of clinical manifestations and treatment outcomes of syphilitic uveitis in human immunodeficiency virus-positive and negative patients. Clin Exp Ophthalmol. (2010) 38:68–74. doi: 10.1111/j.1442-9071.2010.02203.x
16. Agrawal R, Gunasekeran DV, Grant R, Agarwal A, Kon OM, Nguyen QD, et al. Clinical features and outcomes of patients with tubercular uveitis treated with antitubercular therapy in the collaborative ocular tuberculosis study (COTS)-1. JAMA Ophthalmol. (2017) 135:1318–27. doi: 10.1001/jamaophthalmol.2017.4485
17. Testi I, Agrawal R, Mahajan S, Agarwal A, Gunasekeran DV, Raje D, et al. Tubercular uveitis: nuggets from collaborative ocular tuberculosis study (COTS)-1. Ocul Immunol Inflamm. (2019) 1–9. doi: 10.1080/09273948.2019.1646774
18. Cunningham ET Jr., Rathinam SR, Albini TA, Chee SP, Zierhut M. Tuberculous uveitis. Ocul Immunol Inflamm. (2015) 23:2–6. doi: 10.3109/09273948.2014.1004016
19. Ang M, Hedayatfar A, Zhang R, Chee SP. Clinical signs of uveitis associated with latent tuberculosis. Clin Exp Ophthalmol. (2012) 40:689–96. doi: 10.1111/j.1442-9071.2012.02766.x
20. Gupta A, Bansal R, Gupta V, Sharma A, Bambery P. Ocular signs predictive of tubercular uveitis. Am J Ophthalmol. (2010) 149:562–70. doi: 10.1016/j.ajo.2009.11.020
21. Agrawal RMF, Betzler BM, Testi IM, Mahajan SM, Agarwal AM, Gunasekeran DM, et al. The collaborative ocular tuberculosis study (COTS)-1: a multinational review of 165 patients with tubercular anterior uveitis. Ocul Immunol Inflamm. (2020) 1–10. doi: 10.1080/09273948.2020.1761400
22. Agrawal R, Testi I, Mahajan S, Yuen YS, Agarwal A, Kon OM, et al. Collaborative ocular tuberculosis study consensus guidelines on the management of tubercular uveitis-report 1: guidelines for initiating antitubercular therapy in tubercular choroiditis. Ophthalmology. (2021) 128:266–76. doi: 10.1016/j.ophtha.2021.08.008
23. Agrawal R, Testi I, Bodaghi B, Barisani-Asenbauer T, McCluskey P, Agarwal A, et al. Collaborative ocular tuberculosis study consensus guidelines on the management of tubercular uveitis-report 2: guidelines for initiating antitubercular therapy in anterior uveitis, intermediate uveitis, panuveitis, and retinal vasculitis. Ophthalmology. (2021) 128:277–87. doi: 10.1016/j.ophtha.2021.03.024
24. Suhler EB, Lauer AK, Rosenbaum JT. Prevalence of serologic evidence of cat scratch disease in patients with neuroretinitis. Ophthalmology. (2000) 107:871–6. doi: 10.1016/S0161-6420(00)00002-6
25. Font RL, Del Valle M, Mitchell BM, Boniuk M. Cat-scratch uveitis confirmed by histological, serological, and molecular diagnoses. Cornea. (2011) 30:468–71. doi: 10.1097/ICO.0b013e3181fb7fe8
26. Sivakumar RR, Prajna L, Arya LK, Muraly P, Shukla J, Saxena D, et al. Molecular diagnosis and ocular imaging of West Nile virus retinitis and neuroretinitis. Ophthalmology. (2013) 120:1820–6. doi: 10.1016/j.ophtha.2013.02.006
27. Chan CK, Limstrom SA, Tarasewicz DG, Lin SG. Ocular features of west nile virus infection in North America: a study of 14 eyes. Ophthalmology. (2006) 113:1539–46. doi: 10.1016/j.ophtha.2006.04.021
28. Sivakumar R, Balakrishnan V, Gowri P, Visalakshi J. Leptospiral uveitis: usefulness of clinical signs as diagnostic predictors. Ocul Immunol Inflamm. (2018) 26:569–76. doi: 10.1080/09273948.2016.1217341
29. Davies O, Allen F, Gruener AM, Simons R, Graham EM, Larbalestier N. Uveitis secondary to leishmaniasis immune reconstitution syndrome in a HIV-positive patient. Int J STD AIDS. (2016) 27:598–600. doi: 10.1177/0956462415588444
30. Pigott DM, Bhatt S, Golding N, Duda KA, Battle KE, Brady OJ, et al. Global distribution maps of the leishmaniases. Elife. (2014) 3:e02851. doi: 10.7554/eLife.02851
31. Mattia JG, Vandy MJ, Chang JC, Platt DE, Dierberg K, Bausch DG, et al. Early clinical sequelae of Ebola virus disease in Sierra Leone: a cross-sectional study. Lancet Infect Dis. (2016) 16:331–8. doi: 10.1016/S1473-3099(15)00489-2
32. Oliver GF, Carr JM, Smith JR. Emerging infectious uveitis: Chikungunya, dengue, Zika and Ebola: a review. Clin Exp Ophthalmol. (2019) 47:372–80. doi: 10.1111/ceo.13450
33. Shantha JG, Crozier I, Yeh S. An update on ocular complications of Ebola virus disease. Curr Opin Ophthalmol. (2017) 28:600–6. doi: 10.1097/ICU.0000000000000426
34. Furtado JM, Esposito DL, Klein TM, Teixeira-Pinto T, da Fonseca BA. Uveitis associated with zika virus infection. N Engl J Med. (2016) 375:394–6. doi: 10.1056/NEJMc1603618
35. Kodati S, Palmore TN, Spellman FA, Cunningham D, Weistrop B, Sen HN. Bilateral posterior uveitis associated with Zika virus infection. Lancet. (2017) 389:125–6. doi: 10.1016/S0140-6736(16)32518-1
36. de Paula Freitas B, de Oliveira Dias JR, Prazeres J, Sacramento GA, Ko AI, Maia M, et al. Ocular findings in infants with microcephaly associated with presumed zika virus congenital infection in Salvador, Brazil. JAMA Ophthalmol. (2016) 134:529–35. doi: 10.1001/jamaophthalmol.2016.0267
37. Troumani Y, Touhami S, Jackson TL, Ventura CV, Stanescu-Segall DM, Errera MH, et al. Association of anterior uveitis with acute zika virus infection in adults. JAMA Ophthalmol. (2021) 139:95–102. doi: 10.1001/jamaophthalmol.2020.5131
38. Weinstein O, Shneck M, Levy J, Lifshitz T. Bilateral acute anterior uveitis as a presenting symptom of Mycoplasma pneumoniae infection. Can J Ophthalmol. (2006) 41:594–5. doi: 10.1016/S0008-4182(06)80028-1
39. Matsou A, Riga P, Samouilidou M, Dimitrakos S, Anastasopoulos E. Bilateral intermediate uveitis with appearance of frosted branch angiitis and association with Mycoplasma pneumoniae infection: case report and review of the literature. J AAPOS. (2016) 20:358–61. doi: 10.1016/j.jaapos.2016.02.005
40. Bernard A, Kodjikian L, Abukhashabh A, Roure-Sobas C, Boibieux A, Denis P, et al. Diagnosis of Lyme-associated uveitis: value of serological testing in a tertiary centre. Br J Ophthalmol. (2018) 102:369–72. doi: 10.1136/bjophthalmol-2017-310251
41. Bernard A, Seve P, Abukhashabh A, Roure-Sobas C, Boibieux A, Denis P, et al. Lyme-associated uveitis: clinical spectrum and review of literature. Eur J Ophthalmol. (2020) 30:874–85. doi: 10.1177/1120672119856943
42. Havuz E, Gudul Havuz S. Lyme disease atypically presenting with a singular symptom: unilateral chorioretinitis. Eur J Ophthalmol. (2021) 31:NP151–NP6. doi: 10.1177/1120672120962055
43. Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol. (2008) 92:466–8. doi: 10.1136/bjo.2007.133405
44. Amin RM, Hamdy I, Osman IM. Chronic candida endophthalmitis as a cause of intermediate uveitis. BMJ Case Rep. (2015) 2015:208847. doi: 10.1136/bcr-2014-208847
45. Oude Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, et al. Ocular manifestations of candidemia. Clin Infect Dis. (2011) 53:262–8. doi: 10.1093/cid/cir355
46. Gonzales JA, Hinterwirth A, Shantha J, Wang K, Zhong L, Cummings SL, et al. Association of ocular inflammation and rubella virus persistence. JAMA Ophthalmol. (2019) 137:435–8. doi: 10.1001/jamaophthalmol.2018.6185
47. de Visser L, Braakenburg A, Rothova A, de Boer JH. Rubella virus-associated uveitis: clinical manifestations and visual prognosis. Am J Ophthalmol. (2008) 146:292–7. doi: 10.1016/j.ajo.2008.04.011
48. Boonsopon S, Maghsoudlou A, Kombo NE, Foster CS. A therapeutic trial of valganciclovir in patients with uveitis and positive Epstein-Barr virus early antigen D IgG titers. Eur J Ophthalmol. (2016) 26:30–5. doi: 10.5301/ejo.5000673
49. Takahashi H, Takase H, Arai A, Mochizuki M, Ohno-Matsui K. Bilateral granulomatous panuveitis in two patients with T-cell type of chronic active Epstein-Barr virus infection. BMC Ophthalmol. (2019) 19:83. doi: 10.1186/s12886-019-1090-5
50. Xiao H, Hu B, Luo R, Hu H, Zhang J, Kuang W, et al. Chronic active Epstein-Barr virus infection manifesting as coronary artery aneurysm and uveitis. Virol J. (2020) 17:166. doi: 10.1186/s12985-020-01409-8
51. Verma S, Hughes JD, Mabey D, Graham EM. Symptomatic anterior uveitis in HIV-positive patients. Int J STD AIDS. (1999) 10:268–74. doi: 10.1258/0956462991913925
52. Kunavisarut P, Sirirungsi W, Pathanapitoon K, Rothova A. Clinical manifestations of human immunodeficiency virus-induced uveitis. Ophthalmology. (2012) 119:1455–9. doi: 10.1016/j.ophtha.2012.01.030
53. van Boxtel LA, van der Lelij A, van der Meer J, Los LI. Cytomegalovirus as a cause of anterior uveitis in immunocompetent patients. Ophthalmology. (2007) 114:1358–62. doi: 10.1016/j.ophtha.2006.09.035
54. Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. Am J Ophthalmol. (2008) 145:834–40. doi: 10.1016/j.ajo.2007.12.015
55. Rothova A, Hajjaj A, de Hoog J, Thiadens A, Dalm V. Uveitis causes according to immune status of patients. Acta Ophthalmol. (2019) 97:53–9. doi: 10.1111/aos.13877
56. Harthan JS, Opitz DL, Fromstein SR, Morettin CE. Diagnosis and treatment of anterior uveitis: optometric management. Clin Optom. (2016) 8:23–35. doi: 10.2147/OPTO.S72079
57. Svozilkova P, Rihova E, Brichova M, Havlikova A, Klimova A, Heissigerova J. Infectious uveitis in immunodeficient HIV-negative patients: a retrospective study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2020) 164:410–6. doi: 10.5507/bp.2019.036
58. Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol. (2007) 144:781–5. doi: 10.1016/j.ajo.2007.06.034
59. Moorthy RS, Moorthy MS, Cunningham ET Jr. Drug-induced uveitis. Curr Opin Ophthalmol. (2018) 29:588–603. doi: 10.1097/ICU.0000000000000530
60. Iqbal KM, Hay MW, Emami-Naeini P. Medication-induced uveitis: an update. J Ophthalmic Vis Res. (2021) 16:84–92. doi: 10.18502/jovr.v16i1.8254
61. Davis JL, Taskintuna I, Freeman WR, Weinberg DV, Feuer WJ, Leonard RE. Iritis and hypotony after treatment with intravenous cidofovir for cytomegalovirus retinitis. Arch Ophthalmol. (1997) 115:733–7. doi: 10.1001/archopht.1997.01100150735008
62. Saran BR, Maguire AM, Nichols C, Frank I, Hertle RW, Brucker AJ, et al. Hypopyon uveitis in patients with acquired immunodeficiency syndrome treated for systemic Mycobacterium avium complex infection with rifabutin. Arch Ophthalmol. (1994) 112:1159–65. doi: 10.1001/archopht.1994.01090210043015
63. Sauty A, Pecherstorfer M, Zimmer-Roth I, Fioroni P, Juillerat L, Markert M, et al. Interleukin-6 and tumor necrosis factor alpha levels after bisphosphonates treatment in vitro and in patients with malignancy. Bone. (1996) 18:133–9. doi: 10.1016/8756-3282(95)00448-3
64. Fraunfelder FW, Fraunfelder FT. Bisphosphonates and ocular inflammation. N Engl J Med. (2003) 348:1187–8. doi: 10.1056/NEJM200303203481225
65. Fraunfelder FW, Winthrop K, Suhler E, Choi D, Fraunfelder FT. Postmarketing surveillance rates of uveitis and scleritis with bisphosphonates among a national veteran cohort. Retina. (2009) 29:285–6; author reply 6–7. doi: 10.1097/IAE.0b013e318191df82
66. Kristinsson JK, Hannesson OB, Sveinsson O, Thorleifsson H. Bilateral anterior uveitis and retinal haemorrhages after administration of trimethoprim. Acta Ophthalmol Scand. (1997) 75:314–5. doi: 10.1111/j.1600-0420.1997.tb00783.x
67. Okafor LO, Hewins P, Murray PI, Denniston AK. Tubulointerstitial nephritis and uveitis (TINU) syndrome: a systematic review of its epidemiology, demographics and risk factors. Orphanet J Rare Dis. (2017) 12:128. doi: 10.1186/s13023-017-0677-2
69. Ferrini W, Aubert V, Balmer A, Munier FL, Abouzeid H. Anterior uveitis and cataract after rubella vaccination: a case report of a 12-month-old girl. Pediatrics. (2013) 132:e1035–8. doi: 10.1542/peds.2012-2930
70. Cunningham ET Jr., Moorthy RS, Fraunfelder FW, Zierhut M. Vaccine-associated uveitis. Ocul Immunol Inflamm. (2019) 27:517–20. doi: 10.1080/09273948.2019.1626188
71. Ferrand N, Accorinti M, Agarwal M, Spartalis C, Manni P, Stuebiger N, et al. COVID-19 vaccination and uveitis: epidemiology, clinical features and visual prognosis. Ocul Immunol Inflamm. (2022) 1–9. doi: 10.1080/09273948.2022.2058964
72. Jari M, Shiari R, Salehpour O, Rahmani K. Epidemiological and advanced therapeutic approaches to treatment of uveitis in pediatric rheumatic diseases: a systematic review and meta-analysis. Orphanet J Rare Dis. (2020) 15:41. doi: 10.1186/s13023-020-1324-x
73. Carlsson E, Beresford MW, Ramanan AV, Dick AD, Hedrich CM. Juvenile idiopathic arthritis associated uveitis. Children. (2021) 8:646. doi: 10.3390/children8080646
74. Angeles-Han ST, McCracken C, Yeh S, Jenkins K, Stryker D, Rouster-Stevens K, et al. Characteristics of a cohort of children with Juvenile Idiopathic Arthritis and JIA-associated Uveitis. Pediatr Rheumatol Online J. (2015) 13:19. doi: 10.1186/s12969-015-0018-8
75. Walscheid K, Glandorf K, Rothaus K, Niewerth M, Klotsche J, Minden K, et al. Enthesitis-related arthritis: prevalence and complications of associated uveitis in children and adolescents from a population-based nationwide study in Germany. J Rheumatol. (2021) 48:262–9. doi: 10.3899/jrheum.191085
76. Zisman D, Gladman DD, Stoll ML, Strand V, Lavi I, Hsu JJ, et al. The juvenile psoriatic arthritis cohort in the CARRA registry: clinical characteristics, classification, and outcomes. J Rheumatol. (2017) 44:342–51. doi: 10.3899/jrheum.160717
77. Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT. Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis. (2000) 59:67–70. doi: 10.1136/ard.59.1.67
78. Lambert JR, Wright V. Eye inflammation in psoriatic arthritis. Ann Rheum Dis. (1976) 35:354–6. doi: 10.1136/ard.35.4.354
79. Cabral DA, Petty RE, Malleson PN, Ensworth S, McCormick AQ, Shroeder ML. Visual prognosis in children with chronic anterior uveitis and arthritis. J Rheumatol. (1994) 21:2370–5.
80. Pagnini I, Savelli S, Matucci-Cerinic M, Fonda C, Cimaz R, Simonini G. Early predictors of juvenile sacroiliitis in enthesitis-related arthritis. J Rheumatol. (2010) 37:2395–401. doi: 10.3899/jrheum.100090
81. Haasnoot AJW, Kuiper JJW, de Boer JH. Predicting uveitis in juvenile idiopathic arthritis: from biomarkers to clinical practice. Expert Rev Clin Immunol. (2019) 15:657–66. doi: 10.1080/1744666X.2019.1593139
82. Radosavljevic A, Jaksic V, Pezo L, Kovacevic-Pavicevic D, Ilic A, Mihailovic Vucinic V. Clinical features of ocular sarcoidosis in patients with biopsy-proven pulmonary sarcoidosis in Serbia. Ocul Immunol Inflamm. (2017) 25:785–9. doi: 10.3109/09273948.2016.1167224
83. Agrawal R, Gonzalez-Lopez JJ, Meier F, Gupta B, Pavesio CE. Ocular and systemic features of sarcoidosis and correlation with the International Workshop for Ocular Sarcoidosis diagnostic criteria. Sarcoidosis Vasc Diffuse Lung Dis. (2015) 32:237–45.
84. Yang SJ, Salek S, Rosenbaum JT. Ocular sarcoidosis: new diagnostic modalities and treatment. Curr Opin Pulm Med. (2017) 23:458–67. doi: 10.1097/MCP.0000000000000409
85. Nathan N, Sileo C, Calender A, Pacheco Y, Rosental PA, Cavalin C, et al. Paediatric sarcoidosis. Paediatr Respir Rev. (2019) 29:53–9. doi: 10.1016/j.prrv.2018.05.003
86. Kraaijvanger R, Janssen Bonas M, Vorselaars ADM, Veltkamp M. Biomarkers in the diagnosis and prognosis of sarcoidosis: current use and future prospects. Front Immunol. (2020) 11:1443. doi: 10.3389/fimmu.2020.01443
87. Wente-Schulz S, Aksenova M, Awan A, Ambarsari CG, Becherucci F, Emma F, et al. Aetiology, course and treatment of acute tubulointerstitial nephritis in paediatric patients: a cross-sectional web-based survey. BMJ Open. (2021) 11:e047059. doi: 10.1136/bmjopen-2020-047059
88. Joyce E, Glasner P, Ranganathan S, Swiatecka-Urban A. Tubulointerstitial nephritis: diagnosis, treatment, and monitoring. Pediatr Nephrol. (2017) 32:577–87. doi: 10.1007/s00467-016-3394-5
89. Kanno H, Ishida K, Yamada W, Shiraki I, Murase H, Yamagishi Y, et al. Clinical and genetic features of tubulointerstitial nephritis and uveitis syndrome with long-term follow-up. J Ophthalmol. (2018) 2018:4586532. doi: 10.1155/2018/4586532
90. Pakzad-Vaezi K, Pepple KL. Tubulointerstitial nephritis and uveitis. Curr Opin Ophthalmol. (2017) 28:629–35. doi: 10.1097/ICU.0000000000000421
91. Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol. (2012) 56:432–5. doi: 10.1007/s10384-012-0158-z
92. Martinez Valenzuela L, Draibe J, Fulladosa X, Torras J. New biomarkers in acute tubulointerstitial nephritis: a novel approach to a classic condition. Int J Mol Sci. (2020) 21:690. doi: 10.3390/ijms21134690
93. Costagliola G, Cappelli S, Consolini R. Behcet's disease in children: diagnostic and management challenges. Ther Clin Risk Manag. (2020) 16:495–507. doi: 10.2147/TCRM.S232660
94. Yildiz M, Haslak F, Adrovic A, Sahin S, Koker O, Barut K, et al. Pediatric Behcet's disease. Front Med. (2021) 8:627192. doi: 10.3389/fmed.2021.627192
95. Kesen MR, Goldstein DA, Tessler HH. Uveitis associated with pediatric behçet disease in the american midwest. Am J Ophthalmol. (2008) 146:819–27.e2. doi: 10.1016/j.ajo.2008.05.043
96. Park UC, Kim TW, Yu HG. Immunopathogenesis of ocular Behcet's disease. J Immunol Res. (2014) 2014:653539. doi: 10.1155/2014/653539
97. Ksiaa I, Abroug N, Kechida M, Zina S, Jelliti B, Khochtali S, et al. Eye and Behcet's disease. J Fr Ophtalmol. (2019) 42:e133–e46. doi: 10.1016/j.jfo.2019.02.002
98. Ho M, Chen LJ, Sin HPY, Iu LPL, Brelen M, Ho ACH, et al. Experience of using adalimumab in treating sight-threatening paediatric or adolescent Behcet's disease-related uveitis. J Ophthalmic Inflamm Infect. (2019) 9:14. doi: 10.1186/s12348-019-0181-z
99. Hiyama T, Harada Y, Doi T, Kiuchi Y. Early administration of adalimumab for paediatric uveitis due to Behcet's disease. Pediatr Rheumatol Online J. (2019) 17:29. doi: 10.1186/s12969-019-0333-6
100. Kausman JY, Walker A, Piper S. Acute panuveitis and Takayasu's arteritis. Arch Dis Child. (2003) 88:938–9. doi: 10.1136/adc.88.10.938
101. Becker RW, Sohn RL, Poulik JM, Berguer R. Takayasu's arteritis presenting as uveitis in a 5-year-old girl. Ann Vasc Surg. (2005) 19:258–62. doi: 10.1007/s10016-004-0178-3
102. Kwon OC, Lee SW, Park YB, Oh JS, Lee SH, Hong S, et al. Extravascular manifestations of Takayasu arteritis: focusing on the features shared with spondyloarthritis. Arthritis Res Ther. (2018) 20:142. doi: 10.1186/s13075-018-1643-7
103. Madhusudan S, Singh S, Suri D, Gupta A, Gupta A. Acute anterior uveitis as the presenting feature of Kawasaki disease. Indian J Pediatr. (2014) 81:415. doi: 10.1007/s12098-014-1367-x
104. Choi HS, Lee SB, Kwon JH, Kim HS, Sohn S, Hong YM. Uveitis as an important ocular sign to help early diagnosis in Kawasaki disease. Korean J Pediatr. (2015) 58:374–9. doi: 10.3345/kjp.2015.58.10.374
105. Kaur S, Maheshwari A, Aneja S, Seth A, Beri S, Agarwal S, et al. Henoch-Schonlein purpura with uveitis: an unusual case and review of literature. Rheumatol Int. (2012) 32:4057–9. doi: 10.1007/s00296-011-2087-4
106. Muqit MM, Gallagher MJ, Gavin M, Roberts F, Jardine AG. Henoch-Schonlein purpura with keratitis and granulomatous anterior uveitis. Br J Ophthalmol. (2005) 89:1221–2. doi: 10.1136/bjo.2004.064519
107. Kubaisi B, Abu Samra K, Foster CS. Granulomatosis with polyangiitis (Wegener's disease): an updated review of ocular disease manifestations. Intractable Rare Dis Res. (2016) 5:61–9. doi: 10.5582/irdr.2016.01014
108. Sfiniadaki E, Tsiara I, Theodossiadis P, Chatziralli I. Ocular manifestations of granulomatosis with polyangiitis: a review of the literature. Ophthalmol Ther. (2019) 8:227–34. doi: 10.1007/s40123-019-0176-8
109. Orazbekov L, Issergepova B, Assainova M, Ruslanuly K. Granulomatosis with polyangiitis with ocular manifestations. Case Rep Ophthalmol. (2021) 12:98–104. doi: 10.1159/000510959
110. Muller K, Lin JH. Orbital granulomatosis with polyangiitis (Wegener granulomatosis): clinical and pathologic findings. Arch Pathol Lab Med. (2014) 138:1110–4. doi: 10.5858/arpa.2013-0006-RS
111. Nakase H, Uchino M, Shinzaki S, Matsuura M, Matsuoka K, Kobayashi T, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J Gastroenterol. (2021) 56:489–526. doi: 10.1007/s00535-021-01784-1
112. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. (2015) 21:1982–92. doi: 10.1097/MIB.0000000000000392
113. Biedermann L, Renz L, Fournier N, Rossel JB, Butter M, Bluemel S, et al. Uveitis manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. Therap Adv Gastroenterol. (2019) 12:1756284819865142.
114. Jansen FM, Vavricka SR, den Broeder AA, de Jong EM, Hoentjen F, van Dop WA. Clinical management of the most common extra-intestinal manifestations in patients with inflammatory bowel disease focused on the joints, skin and eyes. United Eur Gastroenterol J. (2020) 8:1031–44. doi: 10.1177/2050640620958902
115. Troncoso LL, Biancardi AL, de Moraes HV Jr., Zaltman C. Ophthalmic manifestations in patients with inflammatory bowel disease: a review. World J Gastroenterol. (2017) 23:5836–48. doi: 10.3748/wjg.v23.i32.5836
116. Maccora I, Marrani E, Mastrolia MV, Abu-Rumeileh S, Maniscalco V, Fusco E, et al. Ocular involvement in monogenic autoinflammatory disease. Autoimmun Rev. (2021) 20:102944. doi: 10.1016/j.autrev.2021.102944
117. Meng T, Wu D, Luo Y, Wu N, Zhao M, Shen M, et al. Ocular manifestations in Chinese adult patients with NLRP3-associated autoinflammatory disease. Sci Rep. (2021) 11:11904. doi: 10.1038/s41598-021-91315-y
118. Sota J, Vitale A, Fabiani C, Frediani B, Rigante D, Tosi GM, et al. The eye involvement in monogenic autoinflammatory diseases: literature review and update. Clin Exp Rheumatol. (2018) 36(Suppl. 110):44–53.
119. Welzel T, Kuemmerle-Deschner JB. Diagnosis and management of the cryopyrin-associated periodic syndromes (CAPS): what do we know today? J Clin Med. (2021) 10:128. doi: 10.3390/jcm10010128
120. Tarabishy AB, Hise AG, Traboulsi EI. Ocular manifestations of the autoinflammatory syndromes. Ophthalmic Genet. (2012) 33:179–86. doi: 10.3109/13816810.2012.695421
121. Acharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. (2013) 131:1405–12. doi: 10.1001/jamaophthalmol.2013.4237
122. Avar-Aydin PO, Cakar N, Ozcakar ZB, Yalcindag N, Yalcinkaya F. Ocular inflammatory diseases in children with familial Mediterranean fever: a true association or a coincidence? Int Ophthalmol. (2022) 42:1249–57. doi: 10.21203/rs.3.rs-409833/v1
123. Lachmann HJ, Papa R, Gerhold K, Obici L, Touitou I, Cantarini L, et al. The phenotype of TNF receptor-associated autoinflammatory syndrome (TRAPS) at presentation: a series of 158 cases from the Eurofever/EUROTRAPS international registry. Ann Rheum Dis. (2014) 73:2160–7. doi: 10.1136/annrheumdis-2013-204184
124. Gundogan FC, Akay F, Uzun S, Ozge G, Toyran S, Genc H. Choroidal thickness changes in the acute attack period in patients with familial mediterranean fever. Ophthalmologica. (2016) 235:72–7. doi: 10.1159/000442216
125. Papa R, Doglio M, Lachmann HJ, Ozen S, Frenkel J, Simon A, et al. A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry. Orphanet J Rare Dis. (2017) 12:167. doi: 10.1186/s13023-017-0720-3
126. Karaca EE, Ozek D, Omma A, Evren Kemer O. Comparison of optical coherence tomography angiography results of adult patients with Familial Mediterranean fever and healthy individuals. Ther Adv Ophthalmol. (2019) 11:2515841419892056. doi: 10.1177/2515841419892056
127. Kurt T, Aydin F, Sezer M, Tekgoz PN, Tekin ZE, Celikel E, et al. Performance of diagnostic criteria in pediatric Behcet's disease. Rheumatol Int. (2022) 42:127–32. doi: 10.1007/s00296-020-04777-0
128. Tugelbayeva A, Ivanova R, Goremykina M, Rymbayeva T, Toktabayeva B. Reactive arthritis in children (review). Georgian Med News. (2021) 311:130–5.
129. Pathanapitoon K, Dodds EM, Cunningham ET Jr., Rothova A. Clinical spectrum of HLA-B27-associated ocular inflammation. Ocul Immunol Inflamm. (2017) 25:569–76. doi: 10.1080/09273948.2016.1185527
130. Al-Mayouf SM, Al-Hemidan AI. Ocular manifestations of systemic lupus erythematosus in children. Saudi Med J. (2003) 24:964–6.
131. Gallagher K, Viswanathan A, Okhravi N. Association of systemic lupus erythematosus with uveitis. JAMA Ophthalmol. (2015) 133:1190–3. doi: 10.1001/jamaophthalmol.2015.2249
132. Shoughy SS, Tabbara KF. Ocular findings in systemic lupus erythematosus. Saudi J Ophthalmol. (2016) 30:117–21. doi: 10.1016/j.sjopt.2016.02.001
134. Smith JA, Mackensen F, Sen HN, Leigh JF, Watkins AS, Pyatetsky D, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. (2009) 116:1544–51, 51.e1. doi: 10.1016/j.ophtha.2009.05.002
135. Soheilian M, Aletaha M, Yazdani S, Dehghan MH, Peyman GA. Management of pediatric Vogt-Koyanagi- Harada (VKH)-associated panuveitis. Ocul Immunol Inflamm. (2006) 14:91–8. doi: 10.1080/09273940600557001
136. Rathinam SR, Vijayalakshmi P, Namperumalsamy P, Nozik RA, Cunningham ET Jr. Vogt-Koyanagi-Harada syndrome in children. Ocul Immunol Inflamm. (1998) 6:155–61. doi: 10.1076/ocii.6.3.155.4041
137. Kaza H, Tyagi M, Agarwal K, Behera S, Pappuru RR, Mohan S, et al. Vogt Koyanagi Harada disease in paediatric age group: clinical characteristics, remission, recurrences and complications in Asian Indian Population. Semin Ophthalmol. (2022) 37:187–92. doi: 10.1080/08820538.2021.1948067
138. O'Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. (2017) 62:1–25. doi: 10.1016/j.survophthal.2016.05.002
139. Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. (2001) 131:647–52. doi: 10.1016/S0002-9394(01)00925-4
140. Kaya D, Kaya M, Ozakbas S, Idiman E. Uveitis associated with multiple sclerosis: complications and visual prognosis. Int J Ophthalmol. (2014) 7:1010–3. doi: 10.3980/j.issn.2222-3959.2014.06.18
141. Olsen TG, Frederiksen J. The association between multiple sclerosis and uveitis. Surv Ophthalmol. (2017) 62:89–95. doi: 10.1016/j.survophthal.2016.07.002
142. Ozdal PC, Berker N, Tugal-Tutkun I. Pars planitis: epidemiology, clinical characteristics, management and visual prognosis. J Ophthalmic Vis Res. (2015) 10:469–80. doi: 10.4103/2008-322X.176897
143. Mora P, Calzetti G, Ghirardini S, Rubino P, Gandolfi S, Orsoni J. Cogan's syndrome: State of the art of systemic immunosuppressive treatment in adult and pediatric patients. Autoimmun Rev. (2017) 16:385–90. doi: 10.1016/j.autrev.2017.02.009
144. Fricker M, Baumann A, Wermelinger F, Villiger PM, Helbling A. A novel therapeutic option in Cogan diseases? TNF-alpha blockers. Rheumatol Int. (2007) 27:493–5. doi: 10.1007/s00296-006-0252-y
145. Orsoni JG, Lagana B, Rubino P, Zavota L, Bacciu S, Mora P. Rituximab ameliorated severe hearing loss in Cogan's syndrome: a case report. Orphanet J Rare Dis. (2010) 5:18. doi: 10.1186/1750-1172-5-18
146. Khan IJ, Allroggen H, Pagliarini S. Susac's syndrome: the value of fundus fluorescein angiography. BMJ Case Rep. (2014) 2014:546. doi: 10.1136/bcr-2014-206546
147. Boukouvala S, Jacob S, Lane M, Denniston AK, Burdon MA. Detection of branch retinal artery occlusions in Susac's syndrome. BMC Res Notes. (2014) 7:56. doi: 10.1186/1756-0500-7-56
148. Heng LZ, Bailey C, Lee R, Dick A, Ross A. A review and update on the ophthalmic implications of Susac syndrome. Surv Ophthalmol. (2019) 64:477–85. doi: 10.1016/j.survophthal.2019.01.007
149. Grygiel-Gorniak B, Puszczewicz M, Czaplicka E. Susac syndrome–clinical insight and strategies of therapy. Eur Rev Med Pharmacol Sci. (2015) 19:1729–35.
150. Sood AB, Angeles-Han ST. An update on treatment of pediatric chronic non-infectious uveitis. Curr Treatm Opt Rheumatol. (2017) 3:1–16. doi: 10.1007/s40674-017-0057-z
151. Quartier P. Juvenile idiopathic arthritis-associated chronic uveitis: recent therapeutic approaches. J Clin Med. (2021) 10:934. doi: 10.3390/jcm10132934
152. Gregory AC II, Kempen JH, Daniel E, Kacmaz RO, Foster CS, Jabs DA, et al. Risk factors for loss of visual acuity among patients with uveitis associated with juvenile idiopathic arthritis: the Systemic Immunosuppressive Therapy for Eye Diseases Study. Ophthalmology. (2013) 120:186–92. doi: 10.1016/j.ophtha.2012.07.052
153. Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. (2020) 127:S21–S6. doi: 10.1016/j.ophtha.2020.01.036
154. Shrestha S, Thapa M, Shah DN. Pattern of intraocular pressure fluctuation in uveitic eyes treated with corticosteroids. Ocul Immunol Inflamm. (2014) 22:110–5. doi: 10.3109/09273948.2013.824106
155. Holroyd CR, Seth R, Bukhari M, Malaviya A, Holmes C, Curtis E, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatology. (2019) 58:e3–e42. doi: 10.1093/rheumatology/key208
156. Onel KB, Horton DB, Lovell DJ, Shenoi S, Cuello CA, Angeles-Han ST, et al. 2021 American College of Rheumatology Guideline for the Treatment of Juvenile Idiopathic Arthritis: recommendations for nonpharmacologic therapies, medication monitoring, immunizations, and imaging. Arthritis Rheumatol. (2022) 74:570–85. doi: 10.1002/art.42036
157. Ashkenazy N, Saboo US, Robertson ZM, Cao J. The effect of patient compliance on remission rates in pediatric noninfectious uveitis. J AAPOS. (2019) 23:334.e1–e6. doi: 10.1016/j.jaapos.2019.08.280
158. Fisher JP, Bate J, Hambleton S. Preventing varicella in children with malignancies: what is the evidence? Curr Opin Infect Dis. (2011) 24:203–11. doi: 10.1097/QCO.0b013e328345d666
159. Lachiewicz AM, Srinivas ML. Varicella-zoster virus post-exposure management and prophylaxis: a review. Prev Med Rep. (2019) 16:101016. doi: 10.1016/j.pmedr.2019.101016
160. Costa G, Orbach D, Saulpic J, Sarda-Thibault H, Hanslik T, Brethon B, et al. Varicella post-exposure management for pediatric oncology patients. Bull Cancer. (2022) 109:287–95. doi: 10.1016/j.bulcan.2021.11.016
161. Shamriz O, Ben-Ami R, Averbuch D, Reif S. Low complication rate in immunocompromised children with varicella-zoster virus infections in a single centre. Acta Paediatr. (2020) 109:1409–16. doi: 10.1111/apa.15118
162. Keller M, Pittet LF, Zimmermann P. Immunogenicity and safety of routine vaccines in children and adolescents with rheumatic diseases on immunosuppressive treatment - a systematic review. Eur J Pediatr. (2022) 181:1329–62. doi: 10.1007/s00431-021-04283-w
163. Heijstek MW, van Gageldonk PG, Berbers GA, Wulffraat NM. Differences in persistence of measles, mumps, rubella, diphtheria and tetanus antibodies between children with rheumatic disease and healthy controls: a retrospective cross-sectional study. Ann Rheum Dis. (2012) 71:948–54. doi: 10.1136/annrheumdis-2011-200637
164. Uziel Y, Moshe V, Onozo B, Kulcsar A, Trobert-Sipos D, Akikusa JD, et al. Live attenuated MMR/V booster vaccines in children with rheumatic diseases on immunosuppressive therapy are safe: Multicenter, retrospective data collection. Vaccine. (2020) 38:2198–201. doi: 10.1016/j.vaccine.2020.01.037
165. Heijstek MW, Pileggi GC, Zonneveld-Huijssoon E, Armbrust W, Hoppenreijs EP, Uiterwaal CS, et al. Safety of measles, mumps and rubella vaccination in juvenile idiopathic arthritis. Ann Rheum Dis. (2007) 66:1384–7. doi: 10.1136/ard.2006.063586
166. Heijstek MW, Kamphuis S, Armbrust W, Swart J, Gorter S, de Vries LD, et al. Effects of the live attenuated measles-mumps-rubella booster vaccination on disease activity in patients with juvenile idiopathic arthritis: a randomized trial. JAMA. (2013) 309:2449–56. doi: 10.1001/jama.2013.6768
167. Nathan N, Marcelo P, Houdouin V, Epaud R, de Blic J, Valeyre D, et al. Lung sarcoidosis in children: update on disease expression and management. Thorax. (2015) 70:537–42. doi: 10.1136/thoraxjnl-2015-206825
168. Gedalia A, Khan TA, Shetty AK, Dimitriades VR, Espinoza LR. Childhood sarcoidosis: Louisiana experience. Clin Rheumatol. (2016) 35:1879–84. doi: 10.1007/s10067-015-2870-9
169. Milman N, Hoffmann AL. Childhood sarcoidosis: long-term follow-up. Eur Respir J. (2008) 31:592–8. doi: 10.1183/09031936.00011507
170. Rose CD, Pans S, Casteels I, Anton J, Bader-Meunier B, Brissaud P, et al. Blau syndrome: cross-sectional data from a multicentre study of clinical, radiological and functional outcomes. Rheumatology. (2015) 54:1008–16. doi: 10.1093/rheumatology/keu437
171. Sag E, Bilginer Y, Ozen S. Autoinflammatory diseases with periodic fevers. Curr Rheumatol Rep. (2017) 19:41. doi: 10.1007/s11926-017-0670-8
172. Alsharief AN, Laxer RM, Wang Q, Stimec J, Man C, Babyn P, et al. Monogenic autoinflammatory diseases in children: single center experience with clinical, genetic, and imaging review. Insights Imaging. (2020) 11:87. doi: 10.1186/s13244-020-00889-0
173. Kendall JL, Springer JM. The many faces of a monogenic autoinflammatory disease: adenosine deaminase 2 deficiency. Curr Rheumatol Rep. (2020) 22:64. doi: 10.1007/s11926-020-00944-1
174. Clive DM, Vanguri VK. The syndrome of tubulointerstitial nephritis with uveitis (TINU). Am J Kidney Dis. (2018) 72:118–28. doi: 10.1053/j.ajkd.2017.11.013
175. Regusci A, Lava SAG, Milani GP, Bianchetti MG, Simonetti GD, Vanoni F. Tubulointerstitial nephritis and uveitis syndrome: a systematic review. Nephrol Dial Transplant. (2022) 37:876–86. doi: 10.1093/ndt/gfab030
176. Srinivasalu H, Sikora KA, Colbert RA. Recent updates in juvenile spondyloarthritis. Rheum Dis Clin North Am. (2021) 47:565–83. doi: 10.1016/j.rdc.2021.07.001
177. Ouedraogo F, Navara R, Thapa R, Patel KG. Reactive arthritis post-SARS-CoV-2. Cureus. (2021) 13:e18139. doi: 10.7759/cureus.18139
178. Guillo L, Abreu M, Panaccione R, Sandborn WJ, Azevedo VF, Gensler L, et al. Endpoints for extraintestinal manifestations in inflammatory bowel disease trials: the EXTRA consensus from the International Organization for the Study of Inflammatory Bowel Diseases. Lancet Gastroenterol Hepatol. (2022) 7:254–61. doi: 10.1016/S2468-1253(21)00297-1
179. Hintzen RQ, Dale RC, Neuteboom RF, Mar S, Banwell B. Pediatric acquired CNS demyelinating syndromes: features associated with multiple sclerosis. Neurology. (2016) 87(Suppl. 2):S67–73. doi: 10.1212/WNL.0000000000002881
180. de Mol CL, Wong YYM, van Pelt ED, Ketelslegers IA, Bakker DP, Boon M, et al. Incidence and outcome of acquired demyelinating syndromes in Dutch children: update of a nationwide and prospective study. J Neurol. (2018) 265:1310–9. doi: 10.1007/s00415-018-8835-6
181. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation. (2017) 135:e927–e99. doi: 10.1161/CIR.0000000000000484
182. Dietz SM, van Stijn D, Burgner D, Levin M, Kuipers IM, Hutten BA, et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. (2017) 176:995–1009. doi: 10.1007/s00431-017-2937-5
183. Weiss PF. Pediatric vasculitis. Pediatr Clin North Am. (2012) 59:407–23. doi: 10.1016/j.pcl.2012.03.013
184. Sivaraj RR, Durrani OM, Denniston AK, Murray PI, Gordon C. Ocular manifestations of systemic lupus erythematosus. Rheumatology. (2007) 46:1757–62. doi: 10.1093/rheumatology/kem173
185. Massias JS, Smith EMD, Al-Abadi E, Armon K, Bailey K, Ciurtin C, et al. Clinical and laboratory characteristics in juvenile-onset systemic lupus erythematosus across age groups. Lupus. (2020) 29:474–81. doi: 10.1177/0961203320909156
186. Tayer-Shifman OE, Ilan O, Tovi H, Tal Y. Cogan's syndrome–clinical guidelines and novel therapeutic approaches. Clin Rev Allergy Immunol. (2014) 47:65–72. doi: 10.1007/s12016-013-8406-7
187. Olfat M, Al-Mayouf SM. Cogan's syndrome in childhood. Rheumatol Int. (2001) 20:246–9. doi: 10.1007/s002960100123
188. Pereira S, Vieira B, Maio T, Moreira J, Sampaio F. Susac's syndrome: an updated review. Neuroophthalmology. (2020) 44:355–60. doi: 10.1080/01658107.2020.1748062
189. Dorr J, Krautwald S, Wildemann B, Jarius S, Ringelstein M, Duning T, et al. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. (2013) 9:307–16. doi: 10.1038/nrneurol.2013.82
190. Susac JO, Egan RA, Rennebohm RM, Lubow M. Susac's syndrome: 1975-2005 microangiopathy/autoimmune endotheliopathy. J Neurol Sci. (2007) 257:270–2. doi: 10.1016/j.jns.2007.01.036
191. Vishnevskia-Dai V, Chapman J, Sheinfeld R, Sharon T, Huna-Baron R, Manor RS, et al. Susac syndrome: clinical characteristics, clinical classification, and long-term prognosis. Medicine. (2016) 95:e5223. doi: 10.1097/MD.0000000000005223
192. Choi RY, Rivera-Grana E, Rosenbaum JT. Reclassifying idiopathic uveitis: lessons from a tertiary uveitis center. Am J Ophthalmol. (2019) 198:193–9. doi: 10.1016/j.ajo.2018.10.018
193. Richard Colmant G, Kodjikian L, De Parisot De Bernecourt A, Guillaud M, Gerfaud-Valentin M, Denis P, et al. Uveitis of unknown etiology: clinical and outcome features. A retrospective analysis of 355 patients. Ocul Immunol Inflamm. (2019) 27:1251–8. doi: 10.1080/09273948.2018.1522356
194. Barisani-Asenbauer T, Maca SM, Mejdoubi L, Emminger W, Machold K, Auer H. Uveitis- a rare disease often associated with systemic diseases and infections- a systematic review of 2619 patients. Orphanet J Rare Dis. (2012) 7:57. doi: 10.1186/1750-1172-7-57
195. Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML, et al. (2019). American College of Rheumatology/Arthritis Foundation Guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. (2019) 71:703–16. doi: 10.1002/acr.23871
196. Angeles-Han ST, Pelajo CF, Vogler LB, Rouster-Stevens K, Kennedy C, Ponder L, et al. Risk markers of juvenile idiopathic arthritis-associated uveitis in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry. J Rheumatol. (2013) 40:2088–96. doi: 10.3899/jrheum.130302
197. Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. (2018) 77:1107–17. doi: 10.1136/annrheumdis-2018-213131
198. Kim L, Li A, Angeles-Han S, Yeh S, Shantha J. Update on the management of uveitis in children: an overview for the clinician. Expert Rev Ophthalmol. (2019) 14:211–8. doi: 10.1080/17469899.2019.1663731
199. Simonini G, Cantarini L, Bresci C, Lorusso M, Galeazzi M, Cimaz R. Current therapeutic approaches to autoimmune chronic uveitis in children. Autoimmun Rev. (2010) 9:674–83. doi: 10.1016/j.autrev.2010.05.017
200. Henderson LA, Zurakowski D, Angeles-Han ST, Lasky A, Rabinovich CE, Lo MS, et al. Medication use in juvenile uveitis patients enrolled in the Childhood Arthritis and Rheumatology Research Alliance Registry. Pediatr Rheumatol Online J. (2016) 14:9. doi: 10.1186/s12969-016-0069-5
201. Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology. (2010) 117:1436–41. doi: 10.1016/j.ophtha.2009.12.003
202. Slabaugh MA, Herlihy E, Ongchin S, van Gelder RN. Efficacy and potential complications of difluprednate use for pediatric uveitis. Am J Ophthalmol. (2012) 153:932–8. doi: 10.1016/j.ajo.2011.10.008
203. Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, Liesegang TL, Levy-Clarke GA, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. (2014) 121:2275–86. doi: 10.1016/j.ophtha.2014.05.021
204. Hunter RS, Lobo AM. Dexamethasone intravitreal implant for the treatment of noninfectious uveitis. Clin Ophthalmol. (2011) 5:1613–21. doi: 10.2147/OPTH.S17419
205. Stroh IG, Moradi A, Burkholder BM, Hornbeak DM, Leung TG, Thorne JE. Occurrence of and risk factors for ocular hypertension and secondary glaucoma in juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm. (2017) 25:503–12. doi: 10.3109/09273948.2016.1142573
206. Massa H, Georgoudis P, Panos GD. Dexamethasone intravitreal implant (OZURDEX((R))) for macular edema secondary to noninfectious uveitis: a review of the literature. Ther Deliv. (2019) 10:343–51. doi: 10.4155/tde-2019-0024
207. Gaballa SA, Kompella UB, Elgarhy O, Alqahtani AM, Pierscionek B, Alany RG, et al. Corticosteroids in ophthalmology: drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv Transl Res. (2021) 11:866–93. doi: 10.1007/s13346-020-00843-z
208. Axelsson I, Naumburg E, Prietsch SO, Zhang L. Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database Syst Rev. (2019) 6:CD010126. doi: 10.1002/14651858.CD010126.pub2
209. Fuhlbrigge AL, Kelly HW. Inhaled corticosteroids in children: effects on bone mineral density and growth. Lancet Respir Med. (2014) 2:487–96. doi: 10.1016/S2213-2600(14)70024-4
210. Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term systemic corticosteroid exposure: a systematic literature review. Clin Ther. (2017) 39:2216–29. doi: 10.1016/j.clinthera.2017.09.011
211. Bechtold S, Simon D. Growth abnormalities in children and adolescents with juvenile idiopathic arthritis. Rheumatol Int. (2014) 34:1483–8. doi: 10.1007/s00296-014-3022-2
212. McErlane F, Carrasco R, Kearsley-Fleet L, Baildam EM, Wedderburn LR, Foster HE, et al. Growth patterns in early juvenile idiopathic arthritis: Results from the Childhood Arthritis Prospective Study (CAPS). Semin Arthritis Rheum. (2018) 48:53–60. doi: 10.1016/j.semarthrit.2017.11.002
213. Malik S, Ahmed SF, Wilson ML, Shah N, Loganathan S, Naik S, et al. The effects of anti-TNF-alpha treatment with adalimumab on growth in children with Crohn's disease (CD). J Crohns Colitis. (2012) 6:337–44. doi: 10.1016/j.crohns.2011.09.004
214. Faubion WA, Dubinsky M, Ruemmele FM, Escher J, Rosh J, Hyams JS, et al. Long-term efficacy and safety of adalimumab in pediatric patients with Crohn's Disease. Inflamm Bowel Dis. (2017) 23:453–60. doi: 10.1097/MIB.0000000000001021
215. Kalinina Ayuso V, van de Winkel EL, Rothova A, de Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol. (2011) 151:217–22. doi: 10.1016/j.ajo.2010.08.021
216. Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. (2014) 121:785–96.e3. doi: 10.1016/j.ophtha.2013.09.048
217. Li Y, Mao X, Tang X, Mao H. Efficacy and safety of anti-TNFalpha therapy for uveitis associated with juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatol Ther. (2021) 8:711–27. doi: 10.1007/s40744-021-00296-x
218. Kruh JN, Yang P, Suelves AM, Foster CS. Infliximab for the treatment of refractory noninfectious Uveitis: a study of 88 patients with long-term follow-up. Ophthalmology. (2014) 121:358–64. doi: 10.1016/j.ophtha.2013.07.019
219. Vallet H, Seve P, Biard L, Baptiste Fraison J, Bielefeld P, Perard L, et al. Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the french uveitis network. Arthritis Rheumatol. (2016) 68:1522–30. doi: 10.1002/art.39667
220. Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. (2017) 376:1637–46. doi: 10.1056/NEJMoa1614160
221. Quartier P, Baptiste A, Despert V, Allain-Launay E, Kone-Paut I, Belot A, et al. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. (2018) 77:1003–11. doi: 10.1136/annrheumdis-2017-212089
222. Fabiani C, Vitale A, Rigante D, Emmi G, Lopalco G, Sota J, et al. Efficacy of anti-tumour necrosis factor-alpha monoclonal antibodies in patients with non-infectious anterior uveitis. Clin Exp Rheumatol. (2019) 37:301–5.
223. Leal I, Rodrigues FB, Sousa DC, Duarte GS, Romao VC, Marques-Neves C, et al. Anti-TNF drugs for chronic uveitis in adults-A systematic review and meta-analysis of randomized controlled trials. Front Med. (2019) 6:104. doi: 10.3389/fmed.2019.00104
224. Sharma SM, Damato E, Hinchcliffe AE, Andrews CD, Myint K, Lee R, et al. Long-term efficacy and tolerability of TNFalpha inhibitors in the treatment of non-infectious ocular inflammation: an 8-year prospective surveillance study. Br J Ophthalmol. (2021) 105:1256–62. doi: 10.1136/bjophthalmol-2018-312767
225. Mercier AE, Ribeiro E, Korobelnik JF, Delyfer MN, Rougier MB. Efficacy of anti-TNF-alpha therapy for the treatment of non-infectious uveitis: a retrospective study of 21 patients. Ocul Immunol Inflamm. (2018) 26:477–84. doi: 10.1080/09273948.2016.1236968
226. Trivedi A, Katelaris C. The use of biologic agents in the management of uveitis. Intern Med J. (2019) 49:1352–63. doi: 10.1111/imj.14215
227. Schmeling H, Horneff G. Etanercept and uveitis in patients with juvenile idiopathic arthritis. Rheumatology. (2005) 44:1008–11. doi: 10.1093/rheumatology/keh658
228. Smith JA, Thompson DJ, Whitcup SM, Suhler E, Clarke G, Smith S, et al. A randomized, placebo-controlled, double-masked clinical trial of etanercept for the treatment of uveitis associated with juvenile idiopathic arthritis. Arthritis Rheum. (2005) 53:18–23. doi: 10.1002/art.20904
229. Lanz S, Seidel G, Skrabl-Baumgartner A. Golimumab in juvenile idiopathic arthritis-associated uveitis unresponsive to Adalimumab. Pediatr Rheumatol Online J. (2021) 19:132. doi: 10.1186/s12969-021-00630-1
230. Palmou-Fontana N, Calvo-Rio V, Martin-Varillas JL, Fernandez-Diaz C, Mesquida M, Adan A, et al. Golimumab in refractory uveitis associated to juvenile idiopathic arthritis: multicentre study of 7 cases and literature review. Clin Exp Rheumatol. (2018) 36:652–7.
231. Tosi GM, Sota J, Vitale A, Rigante D, Emmi G, Lopalco G, et al. Efficacy and safety of certolizumab pegol and golimumab in the treatment of non-infectious uveitis. Clin Exp Rheumatol. (2019) 37:680–3.
232. Maccora I, Fusco E, Marrani E, Ramanan AV, Simonini G. Changing evidence over time: updated meta-analysis regarding anti-TNF efficacy in childhood chronic uveitis. Rheumatology. (2021) 60:568–87. doi: 10.1093/rheumatology/keaa595
233. Armaroli G, Klein A, Ganser G, Ruehlmann MJ, Dressler F, Hospach A, et al. Long-term safety and effectiveness of etanercept in JIA: an 18-year experience from the BiKeR registry. Arthritis Res Ther. (2020) 22:258. doi: 10.1186/s13075-020-02326-5
234. Kearsley-Fleet L, Klotsche J, van Straalen JW, Costello W, D'Angelo G, Giancane G, et al. Burden of comorbid conditions in children and young people with juvenile idiopathic arthritis: a collaborative analysis of 3 JIA registries. Rheumatology. (2022) 61:2524–34. doi: 10.1093/rheumatology/keab641
235. Tappeiner C, Schenck S, Niewerth M, Heiligenhaus A, Minden K, Klotsche J. Impact of antiinflammatory treatment on the onset of uveitis in juvenile idiopathic arthritis: longitudinal analysis from a nationwide pediatric rheumatology database. Arthritis Care Res. (2016) 68:46–54. doi: 10.1002/acr.22649
236. Deitch I, Amer R, Tomkins-Netzer O, Habot-Wilner Z, Friling R, Neumann R, et al. The effect of anti-tumor necrosis factor alpha agents on the outcome in pediatric uveitis of diverse etiologies. Graefes Arch Clin Exp Ophthalmol. (2018) 256:801–8. doi: 10.1007/s00417-018-3928-6
237. Castiblanco C, Meese H, Foster CS. Treatment of pediatric uveitis with adalimumab: the MERSI experience. J AAPOS. (2016) 20:145–7. doi: 10.1016/j.jaapos.2015.12.006
238. Nagakura T, Wakiguchi H, Kubota T, Yamatou T, Yamasaki Y, Nonaka Y, et al. Tumor necrosis factor inhibitors provide longterm clinical benefits in pediatric and young adult patients with blau syndrome. J Rheumatol. (2017) 44:536–8. doi: 10.3899/jrheum.160672
239. Sota J, Gentileschi S, Perfetti MO, Frediani B, Tosi GM, Cantarini L, et al. Role of adalimumab biosimilar in the treatment of non-anterior uveitis associated with Behcet's syndrome. Ophthalmol Ther. (2021) 10:1129–35. doi: 10.1007/s40123-021-00387-6
240. Munoz-Gallego A, Barral E, Enriquez E, Tejada P, Barcelo A, de Inocencio J. Adalimumab for the treatment of refractory noninfectious paediatric uveitis. Int Ophthalmol. (2017) 37:719–25. doi: 10.1007/s10792-016-0293-5
241. Silvestri E, Bitossi A, Bettiol A, Emmi G, Urban ML, Mattioli I, et al. Adalimumab effectively controls both anterior and posterior noninfectious uveitis associated with systemic inflammatory diseases: focus on Behcet's syndrome. Inflammopharmacology. (2020) 28:711–8. doi: 10.1007/s10787-020-00697-4
242. Norcia LF, Kiappe OP, Jorge EC. Biological therapy in noninfectious pediatric uveitis: a systematic review. Clin Ophthalmol. (2021) 15:3765–76. doi: 10.2147/OPTH.S322445
243. Valenzuela RA, Flores I, Urrutia B, Fuentes F, Sabat PE, Llanos C, et al. New pharmacological strategies for the treatment of non-infectious uveitis. A minireview. Front Pharmacol. (2020) 11:655. doi: 10.3389/fphar.2020.00655
244. Leclercq M, Andrillon A, Maalouf G, Seve P, Bielefeld P, Gueudry J, et al. Anti-TNF-alpha versus tocilizumab in the treatment of refractory uveitic macular edema: a multicenter study from the French Uveitis Network. Ophthalmology. (2022) 129:520–9. doi: 10.1016/j.ophtha.2021.11.013
245. Ramanan AV, Dick AD, Guly C, McKay A, Jones AP, Hardwick B, et al. Tocilizumab in patients with anti-TNF refractory juvenile idiopathic arthritis-associated uveitis (APTITUDE): a multicentre, single-arm, phase 2 trial. Lancet Rheumatol. (2020) 2:e135–e41. doi: 10.1016/S2665-9913(20)30008-4
246. Atienza-Mateo B, Calvo-Rio V, Beltran E, Martinez-Costa L, Valls-Pascual E, Hernandez-Garfella M, et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behcet's disease: multicentre retrospective study. Rheumatology. (2018) 57:856–64. doi: 10.1093/rheumatology/kex480
247. Doeleman MJH, van Maarseveen EM, Swart JF. Immunogenicity of biologic agents in juvenile idiopathic arthritis: a systematic review and meta-analysis. Rheumatology. (2019) 58:1839–49. doi: 10.1093/rheumatology/kez030
248. Papamichael K, Vogelzang EH, Lambert J, Wolbink G, Cheifetz AS. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev Clin Immunol. (2019) 15:837–48. doi: 10.1080/1744666X.2019.1630273
249. Doeleman MJH, de Roock S, El Amrani M, van Maarseveen EM, Wulffraat NM, Swart JF. Association of adalimumab trough concentrations and treatment response in patients with juvenile idiopathic arthritis. Rheumatology. (2021) 61:377–82. doi: 10.1093/rheumatology/keab354
250. Parikh CR, Ponnampalam JK, Seligmann G, Coelewij L, Pineda-Torra I, Jury EC, et al. Impact of immunogenicity on clinical efficacy and toxicity profile of biologic agents used for treatment of inflammatory arthritis in children compared to adults. Ther Adv Musculoskelet Dis. (2021) 13:1759720X211002685. doi: 10.1177/1759720X211002685
251. Verstegen RHJ, McMillan R, Feldman BM, Ito S, Laxer RM. Towards therapeutic drug monitoring of TNF inhibitors for children with juvenile idiopathic arthritis: a scoping review. Rheumatology. (2020) 59:386–97. doi: 10.1093/rheumatology/kez285
252. Correll CK, Shrader P, Dennos A, Phillips T, Shiff NJ, Verstegen RHJ, et al. Effectiveness and safety of high-dose biologics in juvenile idiopathic arthritis in the childhood arthritis and rheumatology research alliance. Arthritis Care Res. (2021). doi: 10.1002/acr.24727
253. Leinonen ST, Aalto K, Kotaniemi KM, Kivela TT. Anti-adalimumab antibodies in juvenile idiopathic arthritis-related uveitis. Clin Exp Rheumatol. (2017) 35:1043–6.
254. Skrabl-Baumgartner A, Seidel G, Langner-Wegscheider B, Schlagenhauf A, Jahnel J. Drug monitoring in long-term treatment with adalimumab for juvenile idiopathic arthritis-associated uveitis. Arch Dis Child. (2019) 104:246–50. doi: 10.1136/archdischild-2018-315060
255. Syversen SW, Goll GL, Jorgensen KK, Sandanger O, Sexton J, Olsen IC, et al. Effect of therapeutic drug monitoring vs. standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. (2021) 325:1744–54. doi: 10.1001/jama.2021.4172
256. Wen J, Hu H, Chen M, Yang H, Zhao Y, Liu Y. Role of Janus Kinase (JAK) inhibitor in autoimmune ocular inflammation: a systematic review. J Immunol Res. (2021) 2021:2324400. doi: 10.1155/2021/2324400
257. Ramanan AV, Guly CM, Keller SY, Schlichting DE, de Bono S, Liao R, et al. Clinical effectiveness and safety of baricitinib for the treatment of juvenile idiopathic arthritis-associated uveitis or chronic anterior antinuclear antibody-positive uveitis: study protocol for an open-label, adalimumab active-controlled phase 3 clinical trial (JUVE-BRIGHT). Trials. (2021) 22:689. doi: 10.1186/s13063-021-05651-5
258. Le Voyer T, Gitiaux C, Authier FJ, Bodemer C, Melki I, Quartier P, et al. JAK inhibitors are effective in a subset of patients with juvenile dermatomyositis: a monocentric retrospective study. Rheumatology. (2021) 60:5801–8. doi: 10.1093/rheumatology/keab116
259. Sanchez GAM, Reinhardt A, Ramsey S, Wittkowski H, Hashkes PJ, Berkun Y, et al. JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest. (2018) 128:3041–52. doi: 10.1172/JCI98814
260. Ruperto N, Brunner HI, Synoverska O, Ting TV, Mendoza CA, Spindler A, et al. Tofacitinib in juvenile idiopathic arthritis: a double-blind, placebo-controlled, withdrawal phase 3 randomised trial. Lancet. (2021) 398:1984–96. doi: 10.1016/S0140-6736(21)01255-1
261. Acharya NR, Ebert CD, Kelly NK, Porco TC, Ramanan AV, Arnold BF, et al. Discontinuing adalimumab in patients with controlled juvenileidiopathic arthritis-associated uveitis (ADJUST-Adalimumab in Juvenile Idiopathic Arthritis-associated Uveitis Stopping Trial): study protocol for a randomised controlled trial. Trials. (2020) 21:887. doi: 10.1186/s13063-020-04796-z
262. Angeles-Han ST, Griffin KW, Harrison MJ, Lehman TJ, Leong T, Robb RR, et al. Development of a vision-related quality of life instrument for children ages 8-18 years for use in juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. (2011) 63:1254–61. doi: 10.1002/acr.20524
263. McDonald J, Cassedy A, Altaye M, Andringa J, Cooper AM, Drews-Botsch C, et al. Comprehensive assessment of quality of life, functioning and mental health in children with juvenile idiopathic arthritis and non-infectious uveitis. Arthritis Care Res. (2021) 10.1002/acr.24551. doi: 10.1002/acr.24551
264. Parker DM, Angeles-Han ST, Stanton AL, Holland GN. Chronic anterior uveitis in children: psychosocial challenges for patients and their families. Am J Ophthalmol. (2018) 191:xvi–xxiv. doi: 10.1016/j.ajo.2018.03.028
265. Narayan AR, Rahi JS, Solebo AL. Temporal Trends in childhood uveitis: using administrative health data to investigate the impact of health policy and clinical practice. Ocul Immunol Inflamm. (2021) 1–5. doi: 10.1080/09273948.2021.1976215
Keywords: uveitis, inflammation, pediatric uveitis, pediatrician, ocular inflammation
Citation: Shivpuri A, Turtsevich I, Solebo AL and Compeyrot-Lacassagne S (2022) Pediatric uveitis: Role of the pediatrician. Front. Pediatr. 10:874711. doi: 10.3389/fped.2022.874711
Received: 12 February 2022; Accepted: 08 July 2022;
Published: 01 August 2022.
Edited by:
Séverine Guillaume-Czitrom, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Mikhail Kostik, Saint Petersburg State Pediatric Medical University, RussiaIlaria Testi, Moorfields Eye Hospital NHS Foundation Trust, United Kingdom
Copyright © 2022 Shivpuri, Turtsevich, Solebo and Compeyrot-Lacassagne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhay Shivpuri, abhay_shivpuri@yahoo.com
†These authors have contributed equally to this work
 Abhay Shivpuri
Abhay Shivpuri