- Department of Women's and Children's Health, University of Padua, Padua, Italy
Wheezing, asthma, and respiratory infections (RTI) are among the most common causes of morbidity in children and their economic and social burden could be significantly reduced by specific prevention strategies. Epidemiological studies suggest that lower levels of some nutrients are associated with higher prevalence of these conditions, but the possible protective effect of early supplementation with these nutrients has not yet been established. Aim of our review is to synthetize the available scientific evidence on the role of supplementation with pre- and probiotics, vitamin D, fish and poly-unsaturated fatty acids (PUFA), vitamin A, C, and E, given during the first year of life, in the prevention of wheezing, asthma and RTI. We searched studies published on this topic in the PubMed database between January 2000 and September 2021. As for pre- and probiotics, most of the studies showed that an early supplementation had no protective effect toward the development of asthma and wheezing, while conflicting results were reported on their role in the reduction of RTI. As for vitamin D, the available data suggest that early and regular (on a daily or weekly base) supplementation of vitamin D during infancy could have a role in the prevention of RTI, while most studies showed no effect in the prevention of wheezing or asthma. Finally, early introduction of fish in the diet in most studies has proved protective toward wheezing and asthma development.
Introduction
In recent decades the worldwide prevalence of allergic and respiratory diseases has noticeably increased, and these diseases have become a real burden for the healthcare system and society (1). Current evidence suggests that these conditions have a multifactorial etiology resulting from the interaction between genetic susceptibility, host-related factors and environmental exposure (1, 2).
Among environmental factors, diet has a high impact on individual respiratory health by regulating the immune system and nutrition during the first years of life and it is critical for enhancing health outcomes (3). Several studies have investigated the potential benefit of supplementing nutrients in early infancy for reducing the risk of developing allergic and respiratory disease during childhood (3, 4). Among the nutritional supplements available for oral administration in children, prebiotics and probiotics, vitamin D, fish and Poly-Unsatured Fatty Acids (PUFA), vitamin A, C, and E are the better studied.
Aim of our narrative review is to synthetize the available scientific evidence on the role of supplementation of these nutrients during the first year of life, in the prevention of wheezing, asthma, and respiratory tract infections (RTI) during childhood, in order to understand their potential benefit and thus supporting or discouraging their use.
Methods
We searched published studies in the PubMed database by combining the following terms: “probiotic,” “prebiotics,” “vitamin D,” “fish,” “PUFA,” “vitamin A,” “vitamin C,” “vitamin E” as nutritional supplements, and “asthma,” “wheeze,” “respiratory infections” as outcomes.
The search strategy included filters for language (English), age of study subjects (infants and children), and year of publication (we included papers published between January 2000 and September 2021).
Finally, we only included studies that considered nutrients exposure through diet or specific nutrient supplementations in the first year of life. Studies that evaluated only nutrient's serum levels or nutrient's supplementations given only to mothers during pregnancy and/or lactation were excluded.
Prebiotics and Probiotics
Probiotics are live microorganisms which confer a health benefit when administered in adequate amounts (5). Prebiotics are dietary substances (mostly polysaccharides and oligosaccharides poorly digested by human enzymes) that favor the growth of selected beneficial bacteria living in the gut (5). Synbiotics are loosely defined as mixtures of pre- and probiotics that beneficially affect the host (6).
The idea that pre- and probiotics could play a role in reducing the risk of allergic disease come from the hygiene hypothesis which states that early childhood exposure to some microorganisms protects against allergic diseases by contributing to the development of the immune system. In particular, a lack of exposure is thought to impair the development of immune tolerance. Moreover, because of a potential competitive role against resident flora and because of their immunomodulating effect, early supplementation with pre- and probiotics has been studied as possible tool to prevent RTI in children (7).
Several studies, including randomized clinical trials (RCTs), have investigated the potential benefit of various strains and forms of pre- and probiotics in decreasing the risk of asthma, wheezing, and recurrent RTI, but no clear recommendations are nowadays available about their use in children.
Prebiotics and Probiotics in Prevention of Asthma and Wheezing
Fifteen trials (8–22) (Table 1) investigated the role of supplementation with pre- and probiotics in the prevention of asthma and wheeze. In these studies, different strains of probiotics were used, the most common being Lactobacillus rhamnosus and Bifidobacterium breve. The supplementation was usually started in the mothers during the last gestational weeks (35-36 gw) and it was continued in offspring. Most of these trials were conducted in children at risk for allergic diseases (i.e., first-degree relatives with asthma or allergic diseases).

Table 1. Main features and results of studies investigating active supplementation of pre- and probiotics.
Kallio et al. (8) published the largest cohort study on the effect of a mixture of probiotics given from the 36 gw to 1,223 women carrying a child at high risk for allergy and then to the offspring up to the age of 6 months. At the 13-year follow-up, the prevalence of doctor diagnosed allergic diseases, including asthma, was not statistically different in children compared to controls.
Likewise, the vast majority of the available trials (Table 1) showed no protective effect of early pre- and probiotics supplementation with respect to the development of asthma and wheezing.
Prebiotics and Probiotics in the Prevention of RTI
Eight trials (Table 1) investigated the role of early supplementation of pre- and probiotics in the prevention of RTI. In these studies, the most commonly used probiotics were Lactobacillus rhamnosus and Bifidobacterium Lactis. Supplementation was usually started in the first months of life and it was continued for a variable period, ranging from 12 to 46 months.
The largest sample size was analyzed by DI Pierro et al. (23), who recruited 203 children (aged 6-36 months) demonstrating that the supplementation with a probiotic mixture (containing Bifidobacterium animalis subspecies lactis BB-12 and Enterococcus faecium L3) was associated with a 84% reduction of RTI episode rate and a 50% reduction of their duration.
Nonetheless, when taken altogether, the available trials provide conflicting results: in five trials (23–27) administration of pre- and/or probiotics was associated with a reduction in the prevalence of RTI, while in three trials (28–30) no significant difference was reported in children treated with pre- and/or probiotics.
Vitamin D
Vitamin D is a fat-soluble molecule mostly derived from conversion of 7-dehydrocholesterol when the skin is exposed to ultraviolet irradiation and partly derived from the diet (31).
Several studies have demonstrated that vitamin D has important biologic activities on the innate and adaptive immune systems and it can play a role in the onset and progression of immune-related diseases (31).
Here, we synthetize the available scientific literature on the role of Vitamin D supplementation in infants, to clarify whether it has a role in the prevention of wheezing, asthma, and RTI during childhood.
Vitamin D in Prevention of Wheezing and Asthma
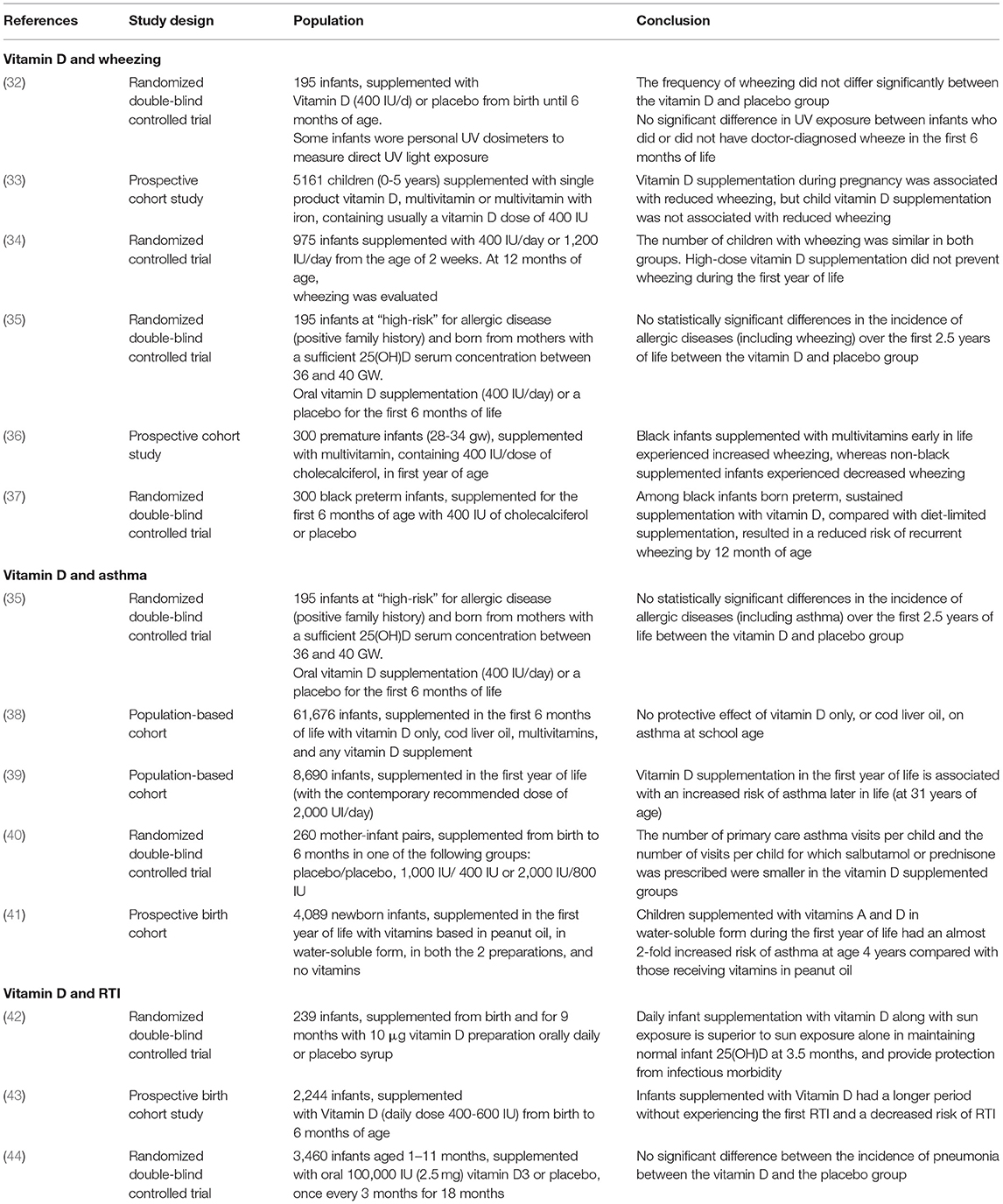
The efficacy of vitamin D supplementation in the first year of life in the prevention of wheezing and asthma is still debated. According to our research strategy, we found six studies (Table 2) that analyzed the outcome wheezing and 5 the outcome asthma.
With regard to wheezing, a recent double-blind, placebo-controlled trial (32) on 195 infants demonstrated no statistically significant difference in wheezing frequency at 12 months of life in children supplemented with vitamin D in the first 6 months of life. Likewise, Anderson et al. (33) showed in a cohort study of 5,161 children (0–5 years) that vitamin D supplementation was not associated with reduced prevalence of wheezing. Moreover, a Finnish RCT (34) on 975 vitamin D-sufficient infants demonstrated that nor standard vitamin D supplementation (400 IU) neither higher vitamin D supplementation (1,200 IU), during the first year of life, decreased allergic diseases and wheezing evaluated at 12 months of age.
Among infants at “high-risk” for allergic diseases (one first-degree relative with asthma, eczema, or allergic rhinitis), Reuter et al. (35) in a double-blind RCT showed no statistically significant differences in incidence of any doctor-diagnosed allergic disease outcomes or allergen sensitization rates between the vitamin D-supplemented and placebo groups at either 1 or 2.5 years of age.
Among preterm black infants, Hibbs et al. published two contrasting study: the first one (36) showed that supplementation with multivitamins (containing 400 IU/dose of cholecalciferol) in the first year of life was associated with a prevalence of wheezing increased in preterm black infants and reduced in non-black infants; the second one (37) showed that in preterm black infants sustained supplementation with vitamin D (400 IU/d) compared with diet-limited supplementation (200 IU/d) was associated with a reduced risk of recurrent wheeze by 12 months' adjusted age.
With regard to asthma, a recent double-blind randomized controlled trial (35) and two previous cohort studies (38, 39) showed no efficacy of early vitamin D supplementation in the prevention of asthma. On the other hand, two other studies (40, 41) report a protective role of vitamin D given in the first year of life on reducing the risk of developing asthma during childhood. Recently, both a systematic review (45) and a document of the World Allergy Organization (46) found no support for the hypothesis that vitamin D supplementation in healthy term infants reduces the risk of developing asthma in childhood.
In conclusion, although some studies report a possible protective effect, most studies did not find a significant role of vitamin D supplementation during the first year of life in the prevention of wheezing and asthma.
Vitamin D in Prevention of RTI
In vitro studies showed that vitamin D has a role in the prevention of both bacterial and also viral RTI, since it induces the production of antimicrobial peptides (47), and it reduces the inflammatory response to viral infections (48).
Considering studies conducted in vivo, a Cochrane (49) reported no benefit from vitamin D supplementation in children under 5 years of age in preventing pneumonia and tuberculosis, while a more recent individual data meta-analysis (including 11,321 participants, aged 0-95 years) (50) showed that vitamin D supplementation can prevent acute RTI with the greatest benefit in deficient subjects and in those supplemented daily or weekly.
According to our research strategy, we identified four studies (Table 2), which, indeed, differs for population, intervention, and outcomes. A double-blind, placebo-controlled trial conducted in India (42) showed that daily infant supplementation with vitamin D for 9 months after birth is superior to sun exposure alone in maintaining normal infant 25(OH)D, and provide protection from infectious morbidity. In keeping, Hong et al. (43) in a prospective birth cohort study (2,244 infants) demonstrated an inverse association between the frequency of vitamin D supplementation (400-600 IU/day) during the first 6 months of life and the risk of RTI, lower RTI, and RTI-related hospitalization. Also, Manaseki-Holland et al. (44) demonstrated that oral supplementation of vitamin D3 given to infants every 3 months for 18 months does not reduce the incidence of pneumonia.
Taken together, these studies suggest that early and regular (on a daily or weekly base) supplementation of vitamin D during infancy could have a role in the prevention of RTI.
PUFA
Polyunsaturated fatty acids (PUFAs) are fatty acids characterized by more than one double bond along the hydrocarbon chain, which have a high nutritional value and some of them, such as omega-3 and omega-6, are essential. One of the main nutritional sources of polyunsaturated fatty acids is fish (51).
Fifteen articles are nowadays available on the role of active supplementation or early introduction of fish and PUFA in the first year of life in the prevention of wheezing, asthma and RTI. We summarize the articles in Table 3 and, given that the majority of them show the same conclusions, we added quantitative information about the degree of protection.

Table 3. Main features and results of studies investigating active supplementation of PUFA and early introduction of fish in diet.
Active Supplementation of PUFA
A multicenter prospective study on 1,342 infants showed that a formula milk enriched with DHA (Docosahexanoic acid) and ARA (arachidonic acid) can reduce the incidence of upper RTI, bronchitis and bronchiolitis (52). Similar results were reported in a more recent multicenter study on 325 infants (53). Furthermore, Birch et al. (54) in a double-blind RCT showed that infants fed for the first 12 months of life with a DHA- and ARA-enriched formula showed not only a reduced incidence of RTI but also of wheezing and asthma, compared to placebo. In keeping with this, a randomized control trial with a 4-year follow-up (55) showed that children fed for the first year of life with a formula supplemented with DHA/ARA have a reduced incidence of asthma and wheeze.
On the other side, a Norwegian multicenter controlled study on 6,154 infants, followed up to the age of 2 years, showed that neither the incidence of allergic disease nor the incidence of wheezing was statistically different in children who took a diet enriched of PUFA and oily fish compared to the control cohort (56). In keeping with this study, a double blind RCT on 420 infants at high atopic risk, fed from birth to 6 months with a diet enriched with DHA and EPA (Eicosapentaenoic Acid) or with a control diet, reported no differences in the prevalence of asthma and wheeze at 12 months of age (57). Furthermore, the RCT Childhood Asthma Prevention Study shows that, in children with a family history of asthma, the implementation of fatty acids in the diet of the first years of life had no effect on reducing the prevalence of asthma in childhood (58).
Early Introduction of Fish in Diet
Among the studies analyzing the effect of an early introduction of fish on the development of respiratory outcomes, most of them (eight out of nine studies) suggest a protective role (Table 3).
A Dutch longitudinal study showed that introduction of fish between 6 and 12 months was associated with a lower prevalence of wheezing at the age of 2-year (59). In keeping with this, other studies demonstrated that fish consumption in the first year of life correlates with a reduced incidence of asthma and/or wheezing in preschool age (60–63) and up to the age of 12-year (64). Also, a recent study on 738 children showed that intake of all PUFAs in the first three years of life was inversely associated with asthma and/or recurrent wheezing, and this was statistically significant for DHA and linoleic acid (65).
On the other hand, one cohort prospective study (66) found no significant association between children's consumption of fish at 1 year of age and doctor-diagnosed asthma in subsequent years.
Taken together these studies suggest that introduction of fish in diet during the first year of life is protective toward wheezing and asthma, while the data on a possible beneficial effect of PUFA active supplementation on children's respiratory heath are more controversial.
Vitamins A, C, and E
Vitamin A comes from plants (as carotenoids) or from animal-derived food sources (as retinol) and it has a major role in lung development, respiratory epithelium and immune system. World Health Organization (WHO) (67) recommends vitamin A supplementation for children above 6 months of age living in areas characterized by vitamin A deficiency.
Vitamin C, a water-soluble vitamin, has antioxidant capacity scavenging oxygen free radicals and suppressing macrophage secretion of superoxide anions (68). Vitamin E, a lipid-soluble vitamin, is the principal defense against oxidant-induced membrane injury. It also has non-antioxidant effect on immune functions (68).
Here we summarize the available trials which analyzed the role of vitamin A, vitamin C and vitamin E supplementations in the first year of life for the prevention of wheezing, asthma, and RTI.
Vitamin A, C, and E in Prevention of Asthma and Wheeze
In the prevention of wheezing and asthma, few studies analyzed the impact of Vitamin A supplementation without unanimous results, while no studies have been published about vitamin C and E oral supplementations in the first year of life.
About Vitamin A, some authors described a higher prevalence of atopy and wheezing in children supplemented during the neonatal period with vitamin A, even if this effect is probably unrelated to later vitamin A status, influenced by selection bias and compromised by the high prevalence of drop-out during the 10 years of follow-up (69, 70). On the contrary, Kull et al. (41) demonstrated that supplementation, in the first year of life, of vitamin A and D in water-soluble form increased the risk of allergic disease up to the age of 4 years compared with supplementation with the same vitamins given in peanut oil.
Vitamin A, C, and E in Prevention of RTI
Three trials (71–73), although without unanimous results, analyzed the effects of vitamin A supplementation in the first year of life on RTI, while no study evaluated the effect of vitamin C and E.
Long et al. (71) reported on 188 children, aged 6-15 months, assigned to receive vitamin A or placebo. Vitamin A supplementation reduced cough with fever but there were no significant differences in the incidence or duration of other types of RTI.
On the contrary, Tielsch et al. (72) enrolled 11,619 live-born infants to receive oral vitamin A or placebo following delivery and at 6 months of age, and showed in the vitamin A group a slightly higher rates for acute respiratory illness. Furthermore, Long et al. (73) in a double-blind, randomized, placebo-controlled trial in 736 children, aged 6–15 months, showed that Vitamin A supplementation was associated with a statistically significant increase (23%) in cough with fever.
Conclusions
We reviewed the available scientific literature regarding early nutritional supplements in the prevention of wheezing, asthma, and RTI to understand if prebiotics and probiotics, vitamin D, PUFA, and vitamins A, C, and E could have a protective role.
As for pre- and probiotics, most of the studies showed no protective effect of early supplementation in the development of asthma and wheezing, while conflicting results were reported on their role in the reduction of RTI.
As for vitamin D, the available evidence suggests that early and regular (on a daily or weekly base) supplementation of vitamin D during infancy could have a role in the prevention of RTI, while most studies showed no protective effect toward wheezing or asthma development.
Finally, early introduction of fish in the diet seems protective toward wheezing and asthma development, while data on the effect of PUFA active supplementation on children's respiratory heath are more controversial.
Author Contributions
VF, SC, and SZanc: conceptualization, methodology, and writing-review and editing. AT, SZane, RC, and FP: writing-original draft preparation. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the Scuola di Specialitá in Pediatria, Universitá degli Studi di Padova, Padova, Italy.
References
1. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
2. Bartlett NW, McLean GR, Chang Y-S, Johnston SL. Genetics and epidemiology: asthma and infection. Curr Opin Allergy Clin Immunol. (2009) 9:395–400. doi: 10.1097/ACI.0b013e32833066fa
3. Verduci E, Martelli A, Miniello VL, Landi M, Mariani B, Brambilla M, et al. Nutrition in the first 1000 days and respiratory health: a descriptive review of the last five years' literature. Allergol Immunopathol. (2017) 45:405–13. doi: 10.1016/j.aller.2017.01.003
4. Alwarith J, Kahleova H, Crosby L, Brooks A, Brandon L, Levin SM, et al. The role of nutrition in asthma prevention and treatment. Nutr Rev. (2020) 78:928–38. doi: 10.1093/nutrit/nuaa005
5. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
6. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. (2020) 17:687–701. doi: 10.1038/s41575-020-0344-2
7. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol. (2013) 6:39–51. doi: 10.1177/1756283X12459294
8. Kallio S, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotic intervention prevented allergic disease in a Caesarean-delivered subgroup at 13-year follow-up. Clin Exp Allergy. (2019) 49:506–15. doi: 10.1111/cea.13321
9. Cabana MD, McKean M, Caughey AB, Fong L, Lynch S, Wong A, et al. Early probiotic supplementation for eczema and asthma prevention: a randomized controlled trial. Pediatrics. (2017) 140:e20163000. doi: 10.1542/peds.2016-3000
10. Loo EXL, Llanora GV, Lu Q, Aw MM, Lee BW, Shek LP. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. Int Arch Allergy Immunol. (2014) 163:25–8. doi: 10.1159/000356338
11. Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. (2007) 119:184–91. doi: 10.1016/j.jaci.2006.08.036
12. Prescott SL, Wiltschut J, Taylor A, Westcott L, Jung W, Currie H, et al. Early markers of allergic disease in a primary prevention study using probiotics: 2.5-year follow-up phase. Allergy. (2008) 63:1481–90. doi: 10.1111/j.1398-9995.2008.01778.x
13. Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol. (2012) 130:1209–11.e5. doi: 10.1016/j.jaci.2012.07.018
14. van der Aa LB, van Aalderen WMC, Heymans HSA, Henk Sillevis Smitt J, Nauta AJ, Knippels LMJ, et al. Synbiotics prevent asthma-like symptoms in infants with atopic dermatitis. Allergy. (2011) 66:170–7. doi: 10.1111/j.1398-9995.2010.02416.x
15. West CE, Hammarström M-L, Hernell O. Probiotics in primary prevention of allergic disease–follow-up at 8-9 years of age. Allergy. (2013) 68:1015–20. doi: 10.1111/all.12191
16. Peldan P, Kukkonen AK, Savilahti E, Kuitunen M. Perinatal probiotics decreased eczema up to 10 years of age, but at 5-10 years, allergic rhino-conjunctivitis was increased. Clin Exp Allergy. (2017) 47:975–9. doi: 10.1111/cea.12924
17. Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. (2007) 119:1174–80. doi: 10.1016/j.jaci.2007.01.007
18. Kuitunen M, Kukkonen K, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, et al. Probiotics prevent IgE-associated allergy until age 5 years in cesarean-delivered children but not in the total cohort. J Allergy Clin Immunol. (2009) 123:335–41. doi: 10.1016/j.jaci.2008.11.019
19. Kukkonen AK, Kuitunen M, Savilahti E, Pelkonen A, Malmberg P, Mäkelä M. Airway inflammation in probiotic-treated children at 5 years. Pediatr Allergy Immunol. (2011) 22:249–51. doi: 10.1111/j.1399-3038.2010.01079.x
20. Wickens K, Black P, Stanley TV, Mitchell E, Barthow C, Fitzharris P, et al. A protective effect of Lactobacillus rhamnosus HN001 against eczema in the first 2 years of life persists to age 4 years. Clin Exp Allergy. (2012) 42:1071–9. doi: 10.1111/j.1365-2222.2012.03975.x
21. Rose MA, Stieglitz F, Köksal A, Schubert R, Schulze J, Zielen S. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin Exp Allergy. (2010) 40:1398–405. doi: 10.1111/j.1365-2222.2010.03560.x
22. Gorissen DMW, Rutten NBMM, Oostermeijer CMJ, Niers LEM, Hoekstra MO, Rijkers GT, et al. Preventive effects of selected probiotic strains on the development of asthma and allergic rhinitis in childhood. The Panda study. Clin Exp Allergy. (2014) 44:1431–3. doi: 10.1111/cea.12413
23. DI Pierro F, Lo Russo P, Danza ML, Basile I, Soardo S, Capocasale G, et al. Use of a probiotic mixture containing Bifidobacterium animalis subsp. lactis BB-12 and Enterococcus faecium L3 as prophylaxis to reduce the incidence of acute gastroenteritis and upper respiratory tract infections in children. Minerva Pediatr. (2021) 73:222–9. doi: 10.23736/S2724-5276.20.05925-3
24. Maldonado J, Cañabate F, Sempere L, Vela F, Sánchez AR, Narbona E, et al. Human milk probiotic Lactobacillus fermentum CECT5716 reduces the incidence of gastrointestinal and upper respiratory tract infections in infants. J Pediatr Gastroenterol Nutr. (2012) 54:55–61. doi: 10.1097/MPG.0b013e3182333f18
25. Rautava S, Salminen S, Isolauri E. Specific probiotics in reducing the risk of acute infections in infancy–a randomised, double-blind, placebo-controlled study. Br J Nutr. (2009) 101:1722–6. doi: 10.1017/S0007114508116282
26. Taipale T, Pienihäkkinen K, Isolauri E, Larsen C, Brockmann E, Alanen P, et al. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in infancy. Br J Nutr. (2011) 105:409–16. doi: 10.1017/S0007114510003685
27. Taipale TJ, Pienihäkkinen K, Isolauri E, Jokela JT, Söderling EM. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr Res. (2016) 79:65–9. doi: 10.1038/pr.2015.174
28. Cohen R, Martin E, de La Rocque F, Thollot F, Pecquet S, Werner A, et al. Probiotics and prebiotics in preventing episodes of acute otitis media in high-risk children: a randomized, double-blind, placebo-controlled study. Pediatr Infect Dis J. (2013) 32:810–4. doi: 10.1097/INF.0b013e31828df4f3
29. Tano K, Grahn Håkansson E, Holm SE, Hellström S. A nasal spray with alpha-haemolytic streptococci as long term prophylaxis against recurrent otitis media. Int J Pediatr Otorhinolaryngol. (2002) 62:17–23. doi: 10.1016/S0165-5876(01)00581-X
30. Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics. (2005) 115:5–9. doi: 10.1542/peds.2004-1815
31. Charoenngam N, Holick MF. Immunologic effects of vitamin D on human health and disease. Nutrients. (2020) 12:E2097. doi: 10.3390/nu12072097
32. Rueter K, Jones AP, Siafarikas A, Lim E-M, Bear N, Noakes PS, et al. Direct infant UV light exposure is associated with eczema and immune development. J Allergy Clin Immunol. (2019) 143:1012–20.e2. doi: 10.1016/j.jaci.2018.08.037
33. Anderson LN, Chen Y, Omand JA, Birken CS, Parkin PC, To T, et al. Vitamin D exposure during pregnancy, but not early childhood, is associated with risk of childhood wheezing. J Dev Orig Health Dis. (2015) 6:308–16. doi: 10.1017/S2040174415001063
34. Rosendahl J, Pelkonen AS, Helve O, Hauta-Alus H, Holmlund-Suila E, Valkama S, et al. High-dose vitamin D supplementation does not prevent allergic sensitization of infants. J Pediatr. (2019) 209:139–45.e1. doi: 10.1016/j.jpeds.2019.02.021
35. Rueter K, Jones AP, Siafarikas A, Lim E-M, Prescott SL, Palmer DJ. In high-risk infants with sufficient vitamin D status at birth, infant vitamin D supplementation had no effect on allergy outcomes: a randomized controlled trial. Nutrients. (2020) 12:E1747. doi: 10.3390/nu12061747
36. Hibbs AM, Babineau DC, Wang X, Redline S. Race differences in the association between multivitamin exposure and wheezing in preterm infants. J Perinatol. (2015) 35:192–7. doi: 10.1038/jp.2014.176
37. Hibbs AM, Ross K, Kerns LA, Wagner C, Fuloria M, Groh-Wargo S, et al. Effect of vitamin D supplementation on recurrent wheezing in black infants who were born preterm: the D-wheeze randomized clinical trial. JAMA. (2018) 319:2086–94. doi: 10.1001/jama.2018.5729
38. Parr CL, Magnus MC, Karlstad Ø, Holvik K, Lund-Blix NA, Haugen M, et al. Vitamin A and D intake in pregnancy, infant supplementation, and asthma development: the Norwegian Mother and Child Cohort. Am J Clin Nutr. (2018) 107:789–98. doi: 10.1093/ajcn/nqy016
39. Hyppönen E, Sovio U, Wjst M, Patel S, Pekkanen J, Hartikainen A-L, et al. Infant vitamin d supplementation and allergic conditions in adulthood: northern Finland birth cohort 1966. Ann N Y Acad Sci. (2004) 1037:84–95. doi: 10.1196/annals.1337.013
40. Grant CC, Crane J, Mitchell EA, Sinclair J, Stewart A, Milne T, et al. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: a randomized controlled trial. Allergy. (2016) 71:1325–34. doi: 10.1111/all.12909
41. Kull I, Bergström A, Melén E, Lilja G, van Hage M, Pershagen G, et al. Early-life supplementation of vitamins A and D, in water-soluble form or in peanut oil, and allergic diseases during childhood. J Allergy Clin Immunol. (2006) 118:1299–304. doi: 10.1016/j.jaci.2006.08.022
42. Chandy DD, Kare J, Singh SN, Agarwal A, Das V, Singh U, et al. Effect of vitamin D supplementation, directly or via breast milk for term infants, on serum 25 hydroxyvitamin D and related biochemistry, and propensity to infection: a randomised placebo-controlled trial. Br J Nutr. (2016) 116:52–8. doi: 10.1017/S0007114516001756
43. Hong M, Xiong T, Huang J, Wu Y, Lin L, Zhang Z, et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern Child Nutr. (2020) 16:e12987. doi: 10.1111/mcn.12987
44. Manaseki-Holland S, Maroof Z, Bruce J, Mughal MZ, Masher MI, Bhutta ZA, et al. Effect on the incidence of pneumonia of vitamin D supplementation by quarterly bolus dose to infants in Kabul: a randomised controlled superiority trial. Lancet. (2012) 379:1419–27. doi: 10.1016/S0140-6736(11)61650-4
45. Yepes-Nuñez JJ, Brozek JL, Fiocchi A, Pawankar R, Cuello-García C, Zhang Y, et al. Vitamin D supplementation in primary allergy prevention: systematic review of randomized and non-randomized studies. Allergy. (2018) 73:37–49. doi: 10.1111/all.13241
46. Yepes-Nuñez JJ, Fiocchi A, Pawankar R, Cuello-Garcia CA, Zhang Y, Morgano GP, et al. World Allergy Organization-McMaster University Guidelines for Allergic Disease Prevention (GLAD-P): Vitamin D. World Allergy Organ J. (2016) 9:17. doi: 10.1186/s40413-016-0108-1
47. Olliver M, Spelmink L, Hiew J, Meyer-Hoffert U, Henriques-Normark B, Bergman P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J Infect Dis. (2013) 208:1474–81. doi: 10.1093/infdis/jit355
48. Hansdottir S, Monick MM, Lovan N, Powers L, Gerke A, Hunninghake GW. Vitamin D decreases respiratory syncytial virus induction of NF-kappaB-linked chemokines and cytokines in airway epithelium while maintaining the antiviral state. J Immunol. (2010) 184:965–74. doi: 10.4049/jimmunol.0902840
49. Yakoob MY, Salam RA, Khan FR, Bhutta ZA. Vitamin D supplementation for preventing infections in children under five years of age. Cochrane Database Syst Rev. (2016) 11:CD008824. doi: 10.1002/14651858.CD008824.pub2
50. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol Assess. (2019) 23:1–44. doi: 10.3310/hta23020
51. Saini RK, Keum Y-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance - a review. Life Sci. (2018) 203:255–67. doi: 10.1016/j.lfs.2018.04.049
52. Pastor N, Soler B, Mitmesser SH, Ferguson P, Lifschitz C. Infants fed docosahexaenoic acid- and arachidonic acid-supplemented formula have decreased incidence of bronchiolitis/bronchitis the first year of life. Clin Pediatr. (2006) 45:850–5. doi: 10.1177/1073858406289801
53. Lapillonne A, Pastor N, Zhuang W, Scalabrin DMF. Infants fed formula with added long chain polyunsaturated fatty acids have reduced incidence of respiratory illnesses and diarrhea during the first year of life. BMC Pediatr. (2014) 14:168. doi: 10.1186/1471-2431-14-168
54. Birch EE, Khoury JC, Berseth CL, Castañeda YS, Couch JM, Bean J, et al. The impact of early nutrition on incidence of allergic manifestations and common respiratory illnesses in children. J Pediatr. (2010) 156:902–6.e1. doi: 10.1016/j.jpeds.2010.01.002
55. Foiles AM, Kerling EH, Wick JA, Scalabrin DMF, Colombo J, Carlson SE. Formula with long-chain polyunsaturated fatty acids reduces incidence of allergy in early childhood. Pediatr Allergy Immunol. (2016) 27:156–61. doi: 10.1111/pai.12515
56. Dotterud CK, Storrø O, Simpson MR, Johnsen R, Øien T. The impact of pre- and postnatal exposures on allergy related diseases in childhood: a controlled multicentre intervention study in primary health care. BMC Public Health. (2013) 13:123. doi: 10.1186/1471-2458-13-123
57. D'Vaz N, Meldrum SJ, Dunstan JA, Martino D, McCarthy S, Metcalfe J, et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. (2012) 130:674–82. doi: 10.1542/peds.2011-3104
58. Toelle BG, Ng KKW, Crisafulli D, Belousova EG, Almqvist C, Webb K, et al. Eight-year outcomes of the childhood asthma prevention study. J Allergy Clin Immunol. (2010) 126:388–9, 389.e1-3. doi: 10.1016/j.jaci.2010.04.031
59. Kiefte-de Jong JC, de Vries JH, Franco OH, Jaddoe VWV, Hofman A, Raat H, et al. Fish consumption in infancy and asthma-like symptoms at preschool age. Pediatrics. (2012) 130:1060–8. doi: 10.1542/peds.2012-0875
60. Øien T, Schjelvaag A, Storrø O, Johnsen R, Simpson MR. Fish consumption at one year of age reduces the risk of eczema, asthma and wheeze at six years of age. Nutrients. (2019) 11:E1969. doi: 10.3390/nu11091969
61. Goksör E, Alm B, Thengilsdottir H, Pettersson R, Åberg N, Wennergren G. Preschool wheeze - impact of early fish introduction and neonatal antibiotics. Acta Paediatr. (2011) 100:1561–6. doi: 10.1111/j.1651-2227.2011.02411.x
62. Kull I, Bergström A, Lilja G, Pershagen G, Wickman M. Fish consumption during the first year of life and development of allergic diseases during childhood. Allergy. (2006) 61:1009–15. doi: 10.1111/j.1398-9995.2006.01115.x
63. Nafstad P, Nystad W, Magnus P, Jaakkola JJK. Asthma and allergic rhinitis at 4 years of age in relation to fish consumption in infancy. J Asthma. (2003) 40:343–8. doi: 10.1081/JAS-120018633
64. Magnusson J, Kull I, Rosenlund H, Håkansson N, Wolk A, Melén E, et al. Fish consumption in infancy and development of allergic disease up to age 12 y. Am J Clin Nutr. (2013) 97:1324–30. doi: 10.3945/ajcn.112.045377
65. Lee-Sarwar K, Kelly RS, Lasky-Su J, Kachroo P, Zeiger RS, O'Connor GT, et al. Dietary and plasma polyunsaturated fatty acids are inversely associated with asthma and atopy in early childhood. J Allergy Clin Immunol Pract. (2019) 7:529–38.e8. doi: 10.1016/j.jaip.2018.07.039
66. Oien T, Storrø O, Johnsen R. Do early intake of fish and fish oil protect against eczema and doctor-diagnosed asthma at 2 years of age? A cohort study. J Epidemiol Community Health. (2010) 64:124–9. doi: 10.1136/jech.2008.084921
67. Guideline: Vitamin A Supplementation in Infants and Children 6–59 Months of Age [Internet]. Geneva: World Health Organization (2011).
68. Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. (2005) 115:1109–17; quiz (1118). doi: 10.1016/j.jaci.2004.12.1139
69. Aage S, Kiraly N, Da Costa K, Byberg S, Bjerregaard-Andersen M, Fisker AB, et al. Neonatal vitamin A supplementation associated with increased atopy in girls. Allergy. (2015) 70:985–94. doi: 10.1111/all.12641
70. Kiraly N, Benn CS, Biering-Sørensen S, Rodrigues A, Jensen KJ, Ravn H, et al. Vitamin A supplementation and BCG vaccination at birth may affect atopy in childhood: long-term follow-up of a randomized controlled trial. Allergy. (2013) 68:1168–76. doi: 10.1111/all.12216
71. Long KZ, Rosado JL, DuPont HL, Hertzmark E, Santos JI. Supplementation with vitamin A reduces watery diarrhoea and respiratory infections in Mexican children. Br J Nutr. (2007) 97:337–43. doi: 10.1017/S0007114507257757
72. Tielsch JM, Rahmathullah L, Thulasiraj RD, Katz J, Coles C, Sheeladevi S, et al. Newborn vitamin A dosing reduces the case fatality but not incidence of common childhood morbidities in South India. J Nutr. (2007) 137:2470–4. doi: 10.1093/jn/137.11.2470
73. Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double-blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrheal disease and respiratory tract infections in children in Mexico City, Mexico. Am J Clin Nutr. (2006) 83:693–700. doi: 10.1093/ajcn.83.3.693
Keywords: wheezing, pediatric asthma, respiratory tract infection (RTI), prebiotics and probiotics, vitamin D, fish, PUFA, children
Citation: Trivillin A, Zanella S, Castaldo RJ, Prati F, Zanconato S, Carraro S and Ferraro VA (2022) Early Oral Nutritional Supplements in the Prevention of Wheezing, Asthma, and Respiratory Infections. Front. Pediatr. 10:866868. doi: 10.3389/fped.2022.866868
Received: 31 January 2022; Accepted: 22 February 2022;
Published: 25 March 2022.
Edited by:
Philip Keith Pattemore, University of Otago, New ZealandReviewed by:
Zorica Momcilo Zivkovic, University Hospital Center Dr. Dragiša Mišović, SerbiaCopyright © 2022 Trivillin, Zanella, Castaldo, Prati, Zanconato, Carraro and Ferraro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Trivillin, YW5uYS50cml2aWxsaW5AZ21haWwuY29t
 Anna Trivillin
Anna Trivillin Sara Zanella
Sara Zanella