
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 25 April 2022
Sec. Pediatric Infectious Diseases
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.865099
 David Chun-Ern Ng1
David Chun-Ern Ng1 Kah Kee Tan2
Kah Kee Tan2 Grace Sieng Sing TING1
Grace Sieng Sing TING1 Chin Ling1
Chin Ling1 Nur Fadzreena Binti Fadzilah1
Nur Fadzreena Binti Fadzilah1 Shir Fong TAN1
Shir Fong TAN1 Thayasheri Subramaniam1
Thayasheri Subramaniam1 Nur Emylia Binti Zailanalhuddin1
Nur Emylia Binti Zailanalhuddin1 Hui Yi LIM1
Hui Yi LIM1 Suhaila Binti Baharuddin3
Suhaila Binti Baharuddin3 Yee Lean LEE1
Yee Lean LEE1 Airena Mohamad Nor1
Airena Mohamad Nor1 Erwin Jiayuan Khoo4*
Erwin Jiayuan Khoo4*Objectives: We described the etiology of severe pneumonia in children during the height of the COVID-19 pandemic in Malaysia and compared the clinical features of severe SARS-CoV-2 to other respiratory viruses.
Methods: This retrospective study included all children aged 12 years and below hospitalized with severe pneumonia in Negeri Sembilan, Malaysia, between 1 April 2021 and 31 October 2021. We extracted demographic and clinical data and used logistic regression to examine risk factors associated with severe SARS-CoV-2 or other viral pneumonia.
Results: A total of 111 children were included. The median age was 15 months. Human rhinovirus/enterovirus, SARS-CoV-2 and respiratory syncytial virus were the most common etiology of severe pneumonia. Codetection of >1 viral pathogen was present in 14 (12.6%) patients. Children with severe COVID-19 presented early in the course of illness and had lower rates of pediatric intensive care admission. The presence of sick contact with an adult was a predictor for SARS-CoV-2, whereas adventitious breath sounds were predictive of other respiratory viruses.
Conclusions: The etiology of severe pneumonia in children evolved with the epidemic curve of COVID-19 and school closures. Children with severe pneumonia due to SARS-CoV-2 experienced a milder clinical course when compared to other respiratory viruses.
The coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an unprecedented global crisis. Many countries implemented strict control strategies to contain the spread of the pandemic before widespread vaccination coverage was achieved, including lockdowns and school closures and the adoption of non-pharmaceutical interventions (wearing face masks, social distancing measures and travel restrictions). These interventions, which were originally aimed at reducing the impact of the COVID-19 pandemic also affected the transmission dynamics of other viral respiratory infections (1).
Acute respiratory infections are the major cause of morbidity and mortality in young children, especially in developing countries (2). It is of interest to know the etiology of severe pneumonia in children during the COVID-19 pandemic considering the alterations in transmission patterns of respiratory viruses during this period, and its impact on public health control and clinical case management. Therefore, we studied the clinical and epidemiologic aspects of severe viral respiratory infections during the outbreak of COVID-19. We present the findings on circulating viruses causing severe pneumonia in children and the clinical course of COVID-19 in comparison with other respiratory viruses at the height of the COVID-19 pandemic in Malaysia.
This was a hospital-based, retrospective observational study of children aged ≤ 12 years hospitalized for severe pneumonia between 1 April 2021 and 31 October 2021. The study was performed at Tuanku Ja'afar Seremban Hospital. The hospital is the only tertiary referral center in the state of Negeri Sembilan, Malaysia, serving approximately 1,100,000 people, including 215,000 children aged ≤ 12 years. Children vaccinated under the national immunization program would have received diphtheria, tetanus and pertussis with Haemophilus influenza b (Hib) and inactivated poliovirus (IPV) vaccine (DTaP/Hib/IPV) at 2, 3, and 5 months of age. Malaysia has reported high immunization coverage over more than 98% among children (3). Pneumococcal 10-valent conjugate vaccine was introduced into the national immunization program in December 2020, whereas influenza vaccination was not mandatory in the population. This study was performed before COVID-19 vaccinations were available to children below 12 years old in the country.
We included all children aged 12 years and below who were admitted to the pediatric respiratory ward or pediatric intensive care unit (ICU) between 1 April 2021 and 31 October 2021 with a diagnosis of severe viral pneumonia or severe SARS-CoV-2 pneumonia. Patients who were tested negative for a viral etiology and patients who had an incomplete virological workup were excluded.
Data were extracted from the patient's electronic medical records into a manual case record form for patients who met the eligibility criteria. Information retrieved included demographic characteristics, comorbidities, immunization status, sick contact, presenting symptoms and their duration, lung auscultation findings, laboratory parameters, treatment received and outcomes.
Severe pneumonia was modified from the World Health Organization's definition (4), as the presence of cough or difficulty breathing with signs of severe respiratory distress, requiring high dependency care in the pediatric respiratory ward or admission to the pediatric ICU. Severe SARS-CoV-2 pneumonia was defined as a patient with confirmed SARS-CoV-2 infection with clinical features of pneumonia requiring supplemental oxygen therapy, according to the local guidelines (5). Patients were admitted to the pediatric ICU if they required inotropes, non-invasive ventilation, mechanical ventilation support, or continuous vital sign monitoring based on the clinician's discretion. All symptoms and signs were collected at the time of presentation. Fever was defined as temperature ≥37.5 C. Duration of illness was calculated from the onset of the first reported symptom. An adult sick contact was defined as a sick contact with an index case above 12 years old, whereas child sick contact was defined as sick contact with an index case below 12 years old. Sick contact was defined as a symptomatic household or social contact (spending face-to-face contact within 1 meter for >15 min) in the preceding 2 weeks prior to the onset of the patient's symptoms. When multiple family members were symptomatic, the first family member who became symptomatic was regarded as the index case. Adventitious breath sounds were defined as the presence of either crackles, wheeze or rhonchi on lung auscultation.
A nasopharyngeal aspirate or endotracheal aspirate (if ventilated) was obtained from each patient within the first 24 h of admission. Pathogen detection techniques were differentiated based on whether the patients were admitted to the pediatric ICU or the pediatric respiratory ward. Our healthcare system restricted multiplex real-time polymerase chain reaction (RT-PCR) assay testing to the patients in the pediatric ICU to conserve test supplies. The patients admitted to the pediatric ICU were tested with the QIAstat-Dx® Respiratory SARS-CoV-2 Panel (QIAGEN, Germany). The QIAstat-Dx® Respiratory SARS-CoV-2 panel is a multiplex RT-PCR panel that simultaneously detects 22 viral and bacterial respiratory pathogens, including SARS-CoV-2 (E and Orf1 genes), human adenovirus (hAdv), human bocavirus (hBoV), human coronaviruses (229E, HKU 1, NL63, OC43), human metapneumovirus (hMPV), human rhinovirus/enterovirus (hRV/EV), influenza A (H1N1/2009, H1, H3), influenza B virus, parainfluenza viruses (1, 2, 3 and 4), respiratory syncytial virus A/B (RSV-A/B), Bordetella pertussis, Legionella pneumophilia and Mycoplasma pneumoniae. Patients admitted to the pediatric respiratory ward were tested for SARS-CoV-2 using real-time PCR (Allplex SARS-CoV-2 Assay, Seegene Inc, Republic of South Korea) and other viruses such as RSV, influenza A, influenza B and hAdv using chromatographic immunoassay antigen testing (CerTest Biotech S.L, Zaragoza, Spain).
Categorical variables were presented as frequency and percentage (%), and continuous variables were presented as median and interquartile range (IQR). Non-parametric two-tailed Mann–Whitney U-test was used for continuous variables and the Chi-squared test or Fisher's exact test was used for categorical variables, as appropriate. Variables with a p-value < 0.05 in the univariate analysis were entered into the multivariate logistic regression analysis to identify independent risk factors associated with severe SARS-CoV-2 or other viral pneumonia. All probabilities were two-tailed, and p < 0.05 was considered to indicate statistical significance. Data analysis was performed using SPSS Version 26.0 (IBM Corp., Armonk, NY, USA).
The study was registered with the National Medical Research Register (NMRR-21-1158-60295) and approved by the Medical Research and Ethics Committee, Ministry of Health Malaysia (KKM/NIHSEC/P21-1195).
A total of 129 patients were diagnosed with severe pneumonia in our institution during the study period. 18 patients were excluded because some had incomplete data while others had negative microbiological investigation results (Figure 1). Eventually, 111 patients were included in the analysis, including 66 males (59.5%) and 45 females (40.5%). The baseline characteristics of the study population are summarized in Table 1. The median age was 15 months (IQR 6-30), with more than two-thirds of patients aged below 24 months. At least one comorbidity was identified in 27 patients (24.3%), with respiratory conditions the most common comorbidity. The number of patients who received at least one dose of Hib vaccination and pneumococcal vaccination was 96 (86.5%) and 42 (37.8%), respectively. Only three patients (2.7%) received influenza vaccination in the preceding 12 months, whereas no patients received Palivizumab in the preceding month before hospitalization. Overall, the median duration of hospitalization was 5 days (IQR 4-8), and all patients were discharged alive.
97 patients (87.4%) had a single viral pathogen detected. Detection of >1 viral pathogen was present in 14 patients (12.6%). The most common single pathogen detected was HRV/EV (n = 40, 36.0%), followed by SARS-CoV-2 (n = 27, 24.3%) and RSV (n = 26, 23.4%). Figure 2 shows the trends of the three major viruses causing severe pneumonia during the study period in relation to the incidence of pediatric SARS-CoV-2 in the state. The etiology of severe pneumonia evolved with the activity of COVID-19 and school closures. SARS-CoV-2 accounted for most of the severe pneumonia cases at the height of the COVID-19 pandemic. However, as COVID-19 dissipates, HRV/EV and RSV resurgence were seen.
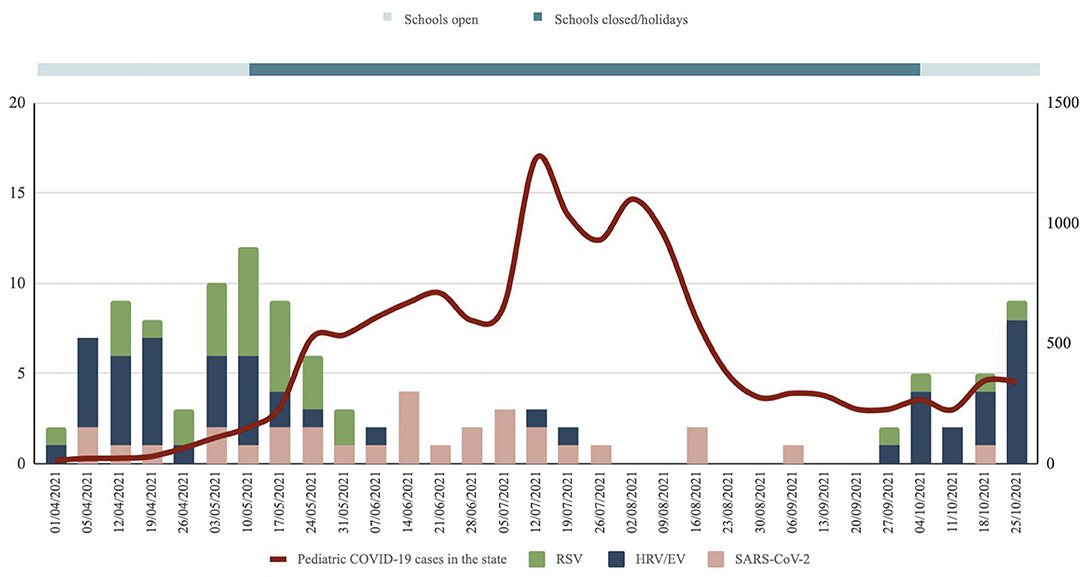
Figure 2. Trends of the three major viruses causing severe pneumonia during the study period in relation to the incidence of pediatric SARS-Cov-2 in the state. Data for pediatric SARS-Cov-2 cases in the state obtained from the state health department.
The most common dual pathogen combinations were HRV/EV and RSV in 4 patients (3.6%) and HRV/EV and Adenovirus in 3 patients (2.7%). Out of the 14 patients with codetection of multiple viruses, HRV/EV was most commonly present with RSV (4 samples), Adenovirus (3 samples), SARS-CoV-2 (2 samples), and Parainfluenza 3 and HBoV (1 sample each).
The median age of patients with severe pneumonia due to SARS-CoV-2 was 17.5 months (IQR 2.0 – 46.9 months) vs. 15.2 months (IQR 6.5 – 26.2 months; p = 0.863) for those due to other respiratory viruses. There was no significant difference between gender and underlying comorbidities of both groups. The proportion of patients exposed to a symptomatic adult index was significantly higher in the SARS-CoV-2 group (77.8 vs. 6.3%, p < 0.001). In contrast, the proportion of patients who had sick contact with a child was higher in the other respiratory virus group (40 vs. 11.1%, p = 0.006) (Table 2).
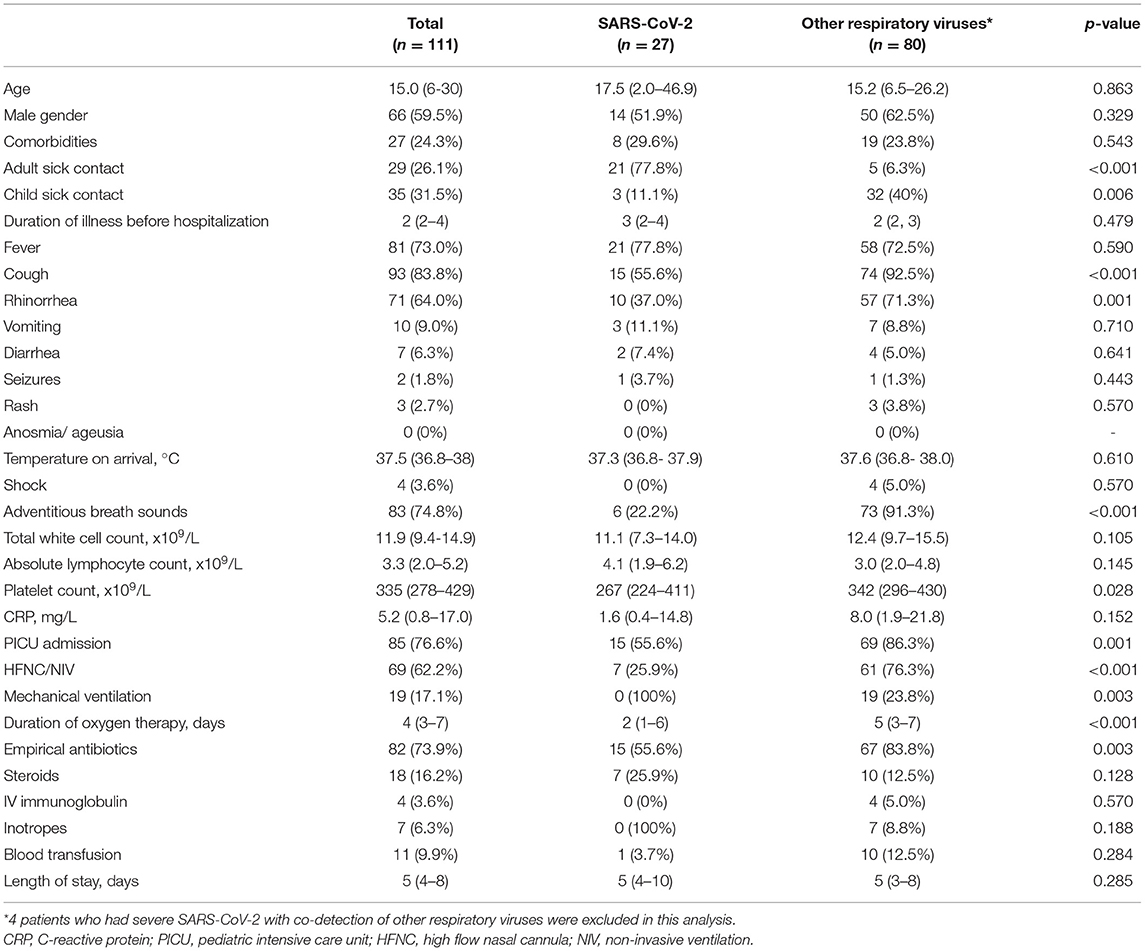
Table 2. Demographics, clinical characteristics and outcomes of patients presenting with severe SARS-CoV-2 compared to other respiratory viruses.
On admission, cough (83.8.%, 93/111), fever (73%, 81/111), and rhinorrhea (64%, 71/111) were the three most common presenting symptoms for patients presenting with severe pneumonia. 55.6% of patients with severe SARS-CoV-2 had cough, significantly less than patients with other respiratory viruses (92.5%, p < 0.001). The proportion of patients with rhinorrhea (37.0%) and adventitious breath sounds (22.2%) was significantly lower in patients with severe SARS-CoV-2 than those with other respiratory viruses (71.3%, p = 0.001; 91.3%, p < 0.001, respectively). No significant differences were found in the proportion of patients who presented with fever, rash, vomiting or diarrhea (p > 0.05). The median duration of illness before hospitalization, the temperature on arrival to hospital, total white count, absolute lymphocyte count, platelet count and C-reactive protein levels did not differ significantly between both groups.
The proportions of patients with severe COVID-19 requiring pediatric ICU admission were lower than those with other respiratory viruses (55.6 vs. 86.3%, p = 0.001). Similarly, the proportion of patients who received non-invasive ventilation was lower in the SARS-CoV-2 group (25.9 vs. 76.3%, p < 0.001). None of the patients with severe COVID-19 required invasive mechanical ventilation, whereas 23.8% of patients with other respiratory viruses received mechanical ventilation (p = 0.003). The median duration of oxygen therapy was significantly lower in the SARS-CoV-2 group (2 days vs. 5 days, p < 0.001). 55.6% of patients with severe COVID-19 received antibiotics, significantly lower than the proportion of patients with other respiratory viruses (83.8%, p = 0.003). There was no difference in the duration of hospitalization between both groups, and no mortality was reported in both groups.
Patients with severe COVID-19 were more likely to have sick contact with an adult than those with severe pneumonia due to other respiratory viruses (aOR 26.86, 95% CI 5.79–124.76; p < 0.001). On the other hand, patients with severe COVID-19 had a lower disposition to exhibit adventitious breath sounds than those with other respiratory viruses (aOR 0.05, 95% CI 0.01–0.26, p < 0.001) (Table 3).
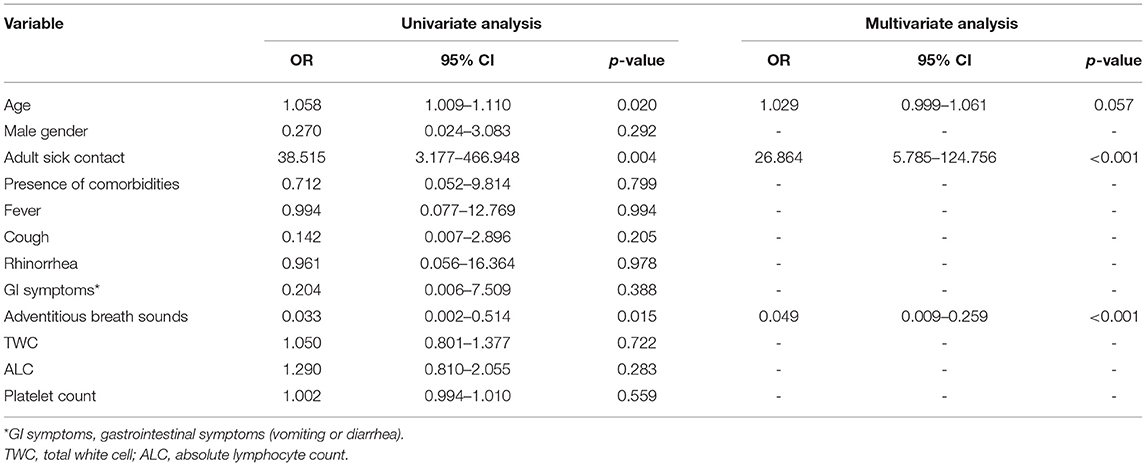
Table 3. Multivariate analysis of independent risk factors for differentiating severe COVID-19 from other viral Pneumonia.
Our study revealed three major viruses circulating during the COVID-19 pandemic in 2021 which caused severe pneumonia in children, namely HRV/EV, SARS-CoV-2 and RSV. 106 out of the 111 respiratory samples (95.5%) sent detected either one of these three viruses, some in combination with other respiratory viruses. HRV/EV was the most common etiology for severe pneumonia in our population during the study period, accounting for 36.0% of the samples with a single viral pathogen. The etiological role of HRV/EV has been questioned due to high detection rates even in asymptomatic subjects (6, 7). Nevertheless, we deduced HRV/EV as the causative agent for these patients, given the absence of other detectable viral or bacterial pathogens. These results were consistent with findings elsewhere documenting high HRV/EV transmission during the COVID-19 pandemic (8–11).
There were several plausible explanations for the dominance of rhinovirus amongst other respiratory viruses during the COVID-19 pandemic. Many rhinovirus infections are asymptomatic and therefore easily spread as asymptomatic carriers unknowingly transmit the virus (12). In our study, more than half of the patients with severe HRV/EV did not report a sick contact (Supplementary Table), suggesting that there could have been transmission by asymptomatic carriers at home. Additionally, face masks appeared less efficient at filtering rhinoviruses out of exhaled breaths than they are at reducing influenza droplets and seasonal coronavirus aerosols (13). This could contribute to rhinovirus transmission despite universal masking, particularly in adults who develop asymptomatic infection and subsequently transmit it to their children. Furthermore, the non-enveloped rhinoviruses tend to be more environmentally stable and relatively resistant to ethanol-containing disinfectants (14). This could be one of the reasons for the resurgence of rhinovirus cases as the schools reopened due to the greater propensity for contact transmission in addition to droplet transmission.
The etiology of severe pneumonia during the COVID-19 pandemic evolved over the study period (Figure 2). The closure of schools reduced the circulation of common childhood respiratory viruses such as rhinovirus and RSV. During that period, cases of severe pneumonia were caused by SARS-CoV-2. With children confined to their homes, they were most likely infected by adults in the household who had interactions with the community. We observed a shift in the etiology of severe pneumonia from SARS-CoV-2 to HRV/EV as COVID-19 activity came down and schools reopened. The rebound of HRV/EV with the reopening of schools and daycares was well-documented during the COVID-19 pandemic (15, 16). This is understandable because children are the main reservoirs and transmission drivers of rhinovirus (17). Most children experienced an “immunity debt” or a lack of immune stimulation due to reduced exposures with prolonged school closures. This led to a surge of cases as school attendance resumed, compounded by the children's difficulty maintaining social distancing in school.
Notably, only one patient had Influenza (codetection of Influenza B with SARS-CoV-2) despite only 2.7% of the study population who received Influenza vaccination in the preceding year. Our results support previous findings that Influenza activity was low during the COVID-19 pandemic (18, 19). Although empirical antibiotics were started for 73.9% of cases with severe pneumonia, none of the patients' blood cultures were positive. There were no invasive pneumococcal coinfections despite the low pneumococcal vaccination rates among the study population. The low incidence of Streptococcus pneumoniae during the COVID-19 pandemic likely contributed to this (20).
We observed that severe COVID-19 in children exhibits different characteristics compared to the classical adult-type disease described in literature. Severe COVID-19 in adults is characterized by a hyperinflammatory response that typically occurs in the second week of illness with abnormal biomarkers such as lymphopenia and raised C-reactive protein (CRP) levels (21, 22). In contrast, our patients with severe COVID-19 presented early in the course of illness (median 3 days after symptom onset), had no lymphopenia (median absolute lymphocyte count, ALC 4.1 x 109/L) and normal CRP levels (median CRP 1.6 mg/L). These findings were consistent with studies that had observed children were hospitalized at a median of 2 days from onset of symptoms (23) and were less likely to exhibit a profound inflammatory response even in severe disease (24). The relatively mild clinical course of COVID-19 in children is somewhat unusual, considering that other viral respiratory infections such as RSV or Influenza are generally more severe in children. The differences in disease progression between children and adults are not fully understood but are likely a combination of factors that place adults at higher risk for severe disease and those that protect children (25). Patients hospitalized for multisystem inflammatory syndrome (MIS-C) were not part of the inclusion criteria, although some required admission to the pediatric ICU as we decided to focus on acute respiratory infections in children. Furthermore, many of our patients with MIS-C did not have significant respiratory system involvement.
Although fever, cough and rhinorrhea were the most common symptoms of children with severe COVID-19, we observed that the proportion of patients with cough and rhinorrhea was significantly lower than that of other respiratory viruses. The presence of cough was only reported in around one-third of children admitted to the ICU in a previous study (26). In our study, COVID-19 results in less severe disease when compared to other respiratory viruses, evidenced by the lower proportion of patients who required ICU admission, high flow nasal cannula/non-invasive ventilation, mechanical ventilation and empirical antibiotics. Additionally, the duration of oxygen therapy was significantly shorter among patients with SARS-CoV-2 compared to other respiratory viruses. The presence of an adult sick contact was a predictor for SARS-CoV-2, whereas adventitious breath sounds in a child with severe pneumonia were predictive of other respiratory viruses as the etiology.
This study has several important clinical implications. Firstly, children with severe COVID-19 presented early in the course of illness without abnormal biomarkers such as lymphopenia and raised CRP. This suggests that the pathophysiology of severe COVID-19 in children is different from adults, where a transition to a hyperinflammatory state could occur during later stages. Although the usage of corticosteroids has been shown beneficial in hospitalized adults requiring respiratory support (27), our study showed that routine corticosteroids for pediatric COVID-19 are not required even in those who require respiratory support. In our study, only 25.9% of children with severe SARS-CoV-2 received corticosteroids during hospitalization. Secondly, 4/31 (12.9%) of patients with severe SARS-CoV-2 had codetection of other respiratory pathogens, namely RSV (two patients), Influenza B (1 patient) and Parainfluenza 3 (one patient). Since SARS-CoV-2 is generally a milder disease when compared to other respiratory pathogens, attempts to search for coinfections with other respiratory pathogens should be made when a child presents with severe pneumonia due to SARS-CoV-2. The presence of coinfections of SARS-CoV-2 with other respiratory pathogens was as high as 33–51% in previous studies (28, 29). Thirdly, we observed that in a child who presents with severe pneumonia, the presence of adventitious breath sounds with a child sick contact suggests a non-SARS-CoV-2 etiology. This is understandable as HRV/EV was the predominant circulating virus during the study period, and children are important reservoirs of these viruses. On the other hand, adults were the primary source of household transmission of SARS-CoV-2 as shown in a local study (30), and sick contact with an adult was a predictor for SARS-CoV-2.
Our findings are subject to several limitations. The respiratory virus detection method was not standardized for all patients. Multiplex PCR respiratory viral panel (RVP) testing was only available for patients admitted to the pediatric ICU due to budget limitations. The usage of chromatographic immunoassays with lower sensitivity and a limited panel of pathogens would affect our codetection rates. Our viral codetection rates could have been potentially higher if multiplex PCR RVP assays had been used for all cases. Nevertheless, our codetection rate of 12.6% (14/111) was higher than other studies using multiplex PCR panel assays (8), suggesting that most of our patients likely had a single pathogen infection even if the respiratory viral panel was used universally for all. Patients with a negative or incomplete virological workup who were omitted from the final analysis could have had severe pneumonia of another etiology. While this omission allowed us to compare the clinical features of patients with a confirmed viral etiology, it could have introduced ascertainment bias when comparing the clinical features of patients with severe SARS-CoV-2 and those with severe pneumonia of other etiology. Further details of the patients who were omitted were available in the supplementary table. Additionally, being a retrospective study, we did not have complete data for other laboratory parameters such as procalcitonin, serum transaminases and arterial blood gas analysis as these investigations were not routinely carried out for all cases. We did not collect data on chest radiography results as radiological reporting for the chest radiographs was not routinely performed at the height of the COVID-19 pandemic. The documented symptoms and laboratory results were the baseline upon hospital admission, and this study did not capture longitudinal data regarding the development of new symptoms or changes in laboratory parameters. Lastly, genomic sequencing for SARS-CoV-2 variants and molecular studies to distinguish the various species of HRV/EV were not performed. Nevertheless, the study period coincides with the peak of the COVID-19 epidemic in Malaysia, as the Delta variant displaced Beta and became the dominant strain in the country (31). Despite those limitations, this study provides a sufficiently broad overview of the etiology and clinical features of severe pneumonia in children during the height of the COVID-19 pandemic in Malaysia.
In conclusion, our study showed three major viruses causing severe pneumonia in children during the COVID-19 pandemic, namely HRV/EV, SARS-CoV-2 and RSV. COVID-19 pneumonia in children appeared less severe than pneumonia caused by other respiratory viruses circulating during the period. The presence of coinfections with other respiratory viruses should be considered in a child who presents with severe pneumonia due to SARS-CoV-2. Children with severe COVID-19 presented early in the course of illness without any biochemical evidence of hyperinflammation. Lastly, the epidemiological characteristics of respiratory viral pathogens, especially HRV/EV should be evaluated more intensely with the reopening of schools and increased social interaction among children.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical Research and Ethics Committee, Ministry of Health Malaysia (KKM/NIHSEC/P21-1195). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
DN, KT, and SB made substantial contributions to the conception and design of the work. GT, CL, NF, TS, ST, NZ, and HL made substantial contribution to data collection and data analysis. DN, KT, GT, CL, NF, TS, ST, NZ, HL, SB, YL, AM, and EK made substantial contributions to the interpretation of data for the manuscript. DN and EK drafted the initial manuscript. DN, KT, YL, AM, and EK supported literature review. All authors reviewed and revised the manuscript for important intellectual content, giving approval of the version to be published, and gave their agreement to be accountable for all aspects of the work.
Open access publication fees are funded by the International Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge all healthcare workers involved in the treatment of pediatric cases of COVID-19 in the state. We thank the Director-General of Health Malaysia for permission to publish this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.865099/full#supplementary-material
1. Olsen SJ, Winn AK, Budd AP, Prill MM, Steel J, Midgley CM, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic - United States, 2020-2021. MMWR Morb Mortal Wkly Rep. (2021) 70:1013–9. doi: 10.15585/mmwr.mm7029a1
2. Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. (2002) 2:25–32. doi: 10.1016/S1473-3099(01)00170-0
3. Department of Statistics Malaysia. (2019). Children Statistics. Available online at: https://www.dosm.gov.my/v1/index.php?r=column/cthemeByCat&cat=333&bul_id=c3I4eitkb1RZTlMvUjNLZVRBMExVQT09&menu_id=U3VPMldoYUxzVzFaYmNkWXZteGduZz09 (accessed January 18, 2022).
4. World Health Organisation. (2013). Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. Available online at: https://apps.who.int/iris/handle/10665/81170 (accessed January 18, 2022).
5. Ministry of Health Malaysia. (2020). COVID-19 Management Guidelines in Malaysia No 5/2020. Available online at: https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm/ANNEX_2e_CLINICAL_MANAGEMENT_OF_CONFIRMED_COVID-19_CASE_IN_ADULT_AND_PAEDIATRIC_13082021.pdf. (accessed January 5, 2022).
6. Advani S, Sengupta A, Forman M, Valsamakis A, Milstone AM. Detecting respiratory viruses in asymptomatic children. Pediatr Infect Dis J. (2012) 31:1221–6. doi: 10.1097/INF.0b013e318265a804
7. Rhedin S, Lindstrand A, Rotzen-Ostlund M, Tolfvenstam T, Ohrmalm L, Rinder MR, et al. Clinical utility of PCR for common viruses in acute respiratory illness. Pediatrics. (2014) 133:e538–545. doi: 10.1542/peds.2013-3042
8. Diesner-Treiber SC, Voitl P, Voitl JJM, Langer K, Kuzio U, Riepl A, et al. Respiratory infections in children during a Covid-19 pandemic winter. Front Pediatr. (2021) 9:740785. doi: 10.3389/fped.2021.740785
9. Kuitunen I, Artama M, Haapanen M, Renko M. Rhinovirus spread in children during the COVID-19 pandemic despite social restrictions-A nationwide register study in Finland. J Med Virol. (2021) 93:6063–7. doi: 10.1002/jmv.27180
10. Takashita E, Kawakami C, Momoki T, Saikusa M, Shimizu K, Ozawa H, et al. Increased risk of rhinovirus infection in children during the coronavirus disease-19 pandemic. Influenza Other Respir Viruses. (2021) 15:488–94. doi: 10.1111/irv.12854
11. Wan WY, Thoon KC, Loo LH, Chan KS, Oon LLE, Ramasamy A, et al. Trends in respiratory virus infections during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. (2021) 4:e2115973. doi: 10.1001/jamanetworkopen.2021.15973
12. Shaman J, Morita H, Birger R, Boyle M, Comito D, Lane B, et al. Asymptomatic summertime shedding of respiratory viruses. J Infect Dis. (2018) 217:1074–7. doi: 10.1093/infdis/jix685
13. Leung NHL, Chu DKW, Shiu EYC, Chan KH, McDevitt JJ, Hau BJP, et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. (2020) 26:676–80. doi: 10.1038/s41591-020-0843-2
14. Savolainen-Kopra C, Korpela T, Simonen-Tikka ML, Amiryousefi A, Ziegler T, Roivainen M, et al. Single treatment with ethanol hand rub is ineffective against human rhinovirus–hand washing with soap and water removes the virus efficiently. J Med Virol. (2012) 84:543–7. doi: 10.1002/jmv.23222
15. Fong MW, Leung NHL, Cowling BJ, Wu P. Upper respiratory infections in schools and childcare centers reopening after COVID-19 dismissals, Hong Kong. Emerg Infect Dis. (2021) 27:1525–7. doi: 10.3201/eid2705.210277
16. Oh DY, Buda S, Biere B, Reiche J, Schlosser F, Duwe S, et al. Trends in respiratory virus circulation following COVID-19-targeted non-pharmaceutical interventions in Germany, January - September 2020: analysis of national surveillance data. Lancet Reg Health Eur. (2021) 6:100112. doi: 10.1016/j.lanepe.2021.100112
17. Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypia T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. (2008) 197:382–9. doi: 10.1086/525542
18. Adlhoch C, Mook P, Lamb F, Ferland L, Melidou A, Amato-Gauci AJ, et al. Very little influenza in the WHO European Region during the 2020/21 season, weeks 40 2020 to 8 2021. Euro Surveill. (2021) 26:2100221. doi: 10.2807/1560-7917.ES.2021.26.11.2100221
19. Sullivan SG, Carlson S, Cheng AC, Chilver MB, Dwyer DE, Irwin M, et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro Surveill. (2020) 25:2001847. doi: 10.2807/1560-7917.ES.2020.25.47.2001847
20. Brueggemann AB., Jansen van Rensburg MJ, Shaw D, McCarthy ND, Jolley KA, Maiden MCJ, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health. (2021) 3:e360–70. doi: 10.1016/S2589-7500(21)00077-7
21. Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, et al. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. (2020) 25:30. doi: 10.1186/s40001-020-00432-3
22. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39:405–7. doi: 10.1016/j.healun.2020.03.012
23. Zachariah P, Johnson CL, Halabi KC, Ahn D, Sen AI, Fischer A, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. (2020) 174:e202430. doi: 10.1001/jamapediatrics.2020.2430
24. Qian G, Zhang Y, Xu Y, Hu W, Hall IP, Yue J, et al. Reduced inflammatory responses to SARS-CoV-2 infection in children presenting to hospital with COVID-19 in China. EClin Med. (2021) 34:100831. doi: 10.1016/j.eclinm.2021.100831
25. Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. (2021) 106:429–39. doi: 10.1136/archdischild-2020-320338
26. Bhalala US, Gist KM, Tripathi S, Boman K, Kumar VK, Retford L, et al. Characterization and outcomes of hospitalized children with coronavirus disease 2019: a report from a multicenter, viral infection and respiratory illness universal study (coronavirus disease 2019) registry. Crit Care Med. (2022) 50:e40–51. doi: 10.1097/CCM.0000000000005232
27. RECOVERY Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. (2021) 384:693–704. doi: 10.1056/NEJMoa2021436
28. Li Y, Wang H, Wang F, Lu X, Du H, Xu J, et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected children: A retrospective study. Medicine. (2021) 100:e24315. doi: 10.1097/MD.0000000000024315
29. Wu Q, Xing Y, Shi L, Li W, Gao Y, Pan S, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. (2020) 146:e20200961. doi: 10.1542/peds.2020-0961
30. Ng DC, Tan KK, Chin L, Cheng XL, Vijayakulasingam T, Liew DWX, et al. Risk factors associated with household transmission of SARS-CoV-2 in Negeri Sembilan, Malaysia. J Paediatr Child Health. (2021). In press. doi: 10.1111/jpc.15821. [Epub ahead of print].
31. Hodcroft EB. (2022). CoVariants: Overview of Variants in Countries. Available online at: https://covariants.org/per-country (accessed January 18, 2022).
Keywords: children, COVID-19, SARS-CoV-2, severe pneumonia, viral pneumonia
Citation: Ng DC-E, Tan KK, TING GSS, Ling C, Fadzilah NFB, TAN SF, Subramaniam T, Zailanalhuddin NEB, LIM HY, Baharuddin SB, LEE YL, Mohamad Nor A and Khoo EJ (2022) Comparison of Severe Viral Pneumonia Caused by SARS-CoV-2 and Other Respiratory Viruses Among Malaysian Children During the COVID-19 Pandemic. Front. Pediatr. 10:865099. doi: 10.3389/fped.2022.865099
Received: 29 January 2022; Accepted: 28 March 2022;
Published: 25 April 2022.
Edited by:
Muralidharan Jayashree, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Susan Coffin, University of Pennsylvania, United StatesCopyright © 2022 Ng, Tan, TING, Ling, Fadzilah, TAN, Subramaniam, Zailanalhuddin, LIM, Baharuddin, LEE, Mohamad Nor and Khoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erwin Jiayuan Khoo, amlheXVhbl9raG9vQGltdS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.