- 1Department of Neurology, Wake Forest University School of Medicine, Atrium Health Wake Forest Baptist, Winston-Salem, NC, United States
- 2Department of Neurology, University of Rochester Medical Center, Rochester, NY, United States
Background: There has been a rise in explosive onset of tic-like behaviors during the COVID-19 pandemic. Historically, this is an uncommon phenomenology of functional movement disorders across all ages. Both the psychological burden of the pandemic and social media usage have been implicated in the rise of these tic-like behaviors.
Methods: This paper provides a narrative review of the literature on chronic tic disorders, functional tics, and mass functional illness with particular focus on the key distinguishing features, role of social media, and the role of COVID-19.
Results: The COVID-19 pandemic has profoundly affected the mental health of many individuals, including children, adolescents, and their caregivers. Implementation of lockdowns, lifestyle disruptions, school closures, and social distancing have driven a surge in social media and digital technology use. The combination of predisposing factors, the psychological burden of the COVID-19 pandemic, and social media are implicated in the rise and spread of tic-like behaviors; which may represent a modern-day form of mass functional illness. While many of the features overlap with functional tics, there are emerging distinctive features that are important to recognize. A more encompassing term, Functional Tic-Like Behaviors, is used to better reflect multiple contributing factors.
Conclusion: Knowledge of these differences is essential to mitigate downstream health effects and poor outcomes.
Introduction
In December 2019, a novel coronavirus (COVID-19) was discovered in Wuhan, China. By March 11, 2020, the World Health Organization (WHO) declared a global pandemic of severe acute respiratory syndrome due to coronavirus-2 (SARS-CoV-2)1. This led to implementation of lockdowns, lifestyle disruptions, school closures, and social distancing. To date, there have been 246 million confirmed cases and 4.9 million deaths from COVID-19 worldwide2.
There is mounting evidence of potential neurological and psychological sequela of COVID-19. Peripheral and central neurological complications of COVID-19 infections have reported (1–5) as have rising rates of stress, anxiety, depression, and behavioral problems (6).
During this time, there has been an increase in functional tics (FT) and functional tic-like behaviors (FTLB). Abrupt onset, atypical progression of symptoms, poorly localized premonitory sensation, high-degree of suggestibility, lack of suppressibility, and complete distractibility help distinguish FT from chronic tic disorders (CTD), including Tourette Syndrome (TS). Previously an uncommon phenomenology of functional movement disorders (FMD) (7–11), the rise in FTLB presents an opportunity to understand the overlapping and distinguishing features of this disorder from CTD and FT. Both social media and the psychological burden of the COVID-19 pandemic have been implicated in this increase.
As research in this area is rapidly evolving, the purpose of this article is to provide a narrative summary of our current understanding of FT, FTLB, and mass functional illness, with particular focus on the distinguishing features, social media, and the role of COVID-19. Early recognition of FT and FTLB is essential for improved outcomes (12, 13).
Methods
A narrative review was chosen as the synthesis method due to rapidly evolving research in this area. The literature search was conducted using PubMed, Embase, and Web of Science between 2006 through 2021. A modified Patient Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) figure is included (Figure 1). A combination of search terms produced 6,925 results. Initial search terms included “tic” or “tourette” and “COVID,” “social media,” “TikTok,” “functional,” “psychogenic,” or “like-behavior.” Both “functional” and “psychogenic movement disorders” in context of COVID and pediatrics were also searched. Additionally, the various names for mass functional illness were searched. Inclusion criteria included English literature with publication dates between 2006 and 2021. No specific type of study or age range were excluded from the search. Use of the term functional yielded many non-relevant results related to functional imaging, disability, and anatomy. After removal of duplicates, non-relevant, and non-English literature, as well as backwards snowballing, 118 articles were included for final review. Given the topic of social media, additional publicly available content was included in the review as noted in the footnotes.
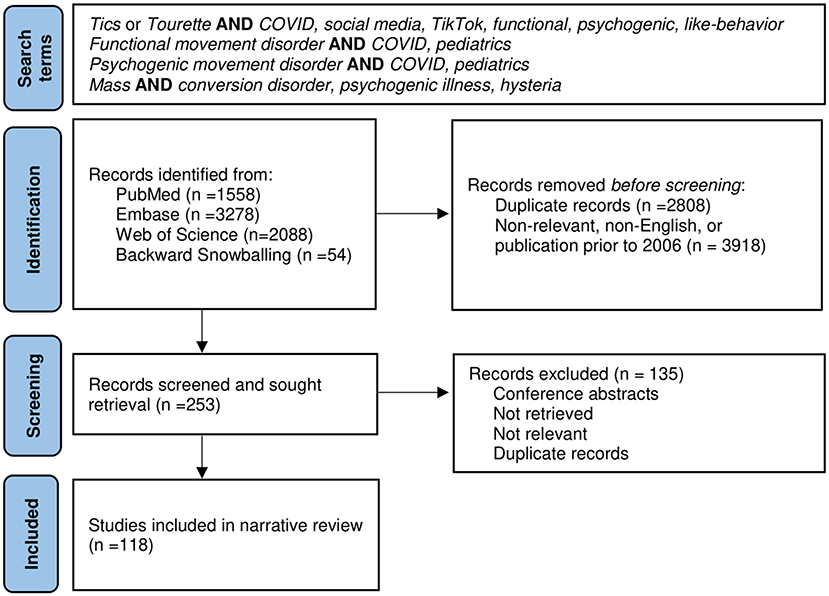
Figure 1. Modified Patient Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Figure.
Chronic Tic Disorders
Tics are sudden, rapid, non-rhythmic movements or vocalizations, which occur in 1 in 5 school-aged children (14). Chronic Tic Disorders (CTD), including Tourette Syndrome (TS), are developmental neurobiological disorders characterized by multiple motor and/or vocal tics for at least 1 year. Co-occurring conditions such as attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), and anxiety occur in 90% of individuals with CTD (15–19).
Tics begin gradually in early childhood, fluctuate, and change over time (16, 18, 20). There is a male predominance of tics, 4:1 male to female ratio, which diminishes in adulthood (18, 20, 21). For the vast majority of individuals with CTD, tics peak in the peri-pubertal period and improve through adolescence (20).
Tics often begin as simple motor tics and progress over time in a rostro-caudal distribution (22). Tics are preceded by a premonitory urge, often localized to the affected body region (23). Premonitory urges are an itch, tension, need or unpleasant sensation that builds with voluntary suppression and is relieved by completing the tic (24, 25). Recognition of premonitory urges tends to occur between 8 and 10 years old, although is reported by the majority of individuals with CTD (24, 26, 27).
Whether the phenotype of TS is different in affected females is poorly understood. There are conflicting reports on the sex-differences of comorbidity prevalence (16, 18, 21, 28–32). Some studies have reported TS-affected females have later onset of tics (16, 33, 34), later peak of tic severity (29, 34), motor tic severity (32, 33), and lower likelihood of tic remission; however, others have shown conflicting results or no significant sex-differences in these factors (18, 28, 32, 33, 35).
Complex tics, including coprophenomena, echophenomena and self-injurious behaviors (SIB) are often misunderstood by the public (36). Risk of coprophenomena such as obscene gestures (copropraxia) or words (coprolalia) increases with age and co-occurring conditions. Coprolalia is three times more common than copropraxia, with a lifetime prevalence of 8–18.5 and 5.7% respectively and often occurs within 5 years of tic onset (18, 37–39).
Echophenomena refers to the repetition of other's actions (echopraxia) or sounds/words (echolalia). In the original publications from Georges Gilles de la Tourette, the persistence of echophenomena beyond normal expected childhood development was essential for the diagnosis of TS (40). Given the heterogeneity of CTD, echophenomena are now considered a distinctive feature rather than a requirement for diagnosis (41), with an estimated lifetime prevalence 43–56% (42, 43).
SIB can occur in 4–53% of individuals with CTD (18, 44, 45). Estimates vary depending on the definition of SIB, which can range from skin picking or scratching, biting, head banging, or self-hitting, to more severe symptoms such as self-cutting, body deformation, or self-mutilation (46). Rarely, individuals affected with TS can have life-threating symptoms (44, 47).
Functional Movement Disorders
Functional movement disorders (FMD) are a common presentation in neurology clinics (48). Many different terms have described these disorders including conversion disorder, psychogenic, nonorganic, medically unexplained, and hysteria (49, 50). Although psychogenic and functional are often use interchangeably, functional is the preferred terminology. Functional is freer of stigma and more reflective of the current understanding of the pathophysiology on FMD, which suggest a neurobiological basis of these disorders (12, 49, 51, 52). A combination of predisposing, precipitating, and perpetuating factors play a role in development of FMD. Psychosocial stressors, low socioeconomic status, psychiatric comorbidities, female gender, and adverse experiences may increase risk of FMD. It is hypothesized that both epigenetic and genetic factors may contribute to FMD, but current evidence is limited (53). Additionally, brain maladaptation and plasticity may serve as perpetuating factors for FMD (12, 49, 54–56). The Diagnostic and Statistical Manual for Mental Disorders–Fifth Edition (DSM-5) adopted new emphasis on diagnosing based on positive features and removed the necessity of a precipitating stressor as with many patients none are found (57).
Epidemiology of Functional Tics
The true prevalence of FMD is hard to discern given diagnostic uncertainty, inconsistent terminology, and variability of utilized billing codes (48, 58). Diagnosis relies on inconsistencies and incongruences with known movement disorders. Across all ages, tremor, dystonia and gait disorders are the most common phenomenology of FMD, and FT were rarely reported (13, 59–61).
Estimates of pediatric FT prevalence vary from 0 to 17% (61–64). The rarity of FT may be attributed to the challenge in distinguishing FT from CTD. Many of the positive features used to diagnose FMDs such as distractibility, suggestibility, and fluctuating course are common amongst CTD. Clinical expertise and prior case studies suggest there are some key distinguishing features (10, 12, 65–68). While most individuals with pre-existing FMD reported no change in symptoms with the COVID-19 pandemic, there has been a dramatic increase in new FT (7–11, 69, 70).
Clinical Phenomenology of Functional Tics
FT have key clinical characteristics that distinguish them from CTD. FT present in adolescents often without a prior history of tics. There is a 3:1 to 9:1 female predominance in FTs. This is in contrast to CTD, which are heavily male dominant (13, 20, 59, 61, 71). Common features include abrupt onset followed by a static or progressive course, high-degree of suggestibility, and complete distractibility. FT lack suppressibility, build up with voluntary suppression, or relief upon completion of the tics (65, 66). Although the presence or absence of a premonitory urge is less definitive, when present in FT it is less often localized to the area of the tic-like behavior (11, 72, 73).
Unlike CTD, FT include complex and large amplitude movements at onset (11). Complex tics such as pali-, echo-, and copro-phenomena are less common in FT and are often more complex, variable, longer in duration, or context-dependent (72, 74, 75). The progression of FT tends to disregard the expected rostro-caudal distribution seen in CTD (66, 76). Frequently other functional neurological or somatic symptoms are present (65, 66).
Some studies note a lack of family history of tics in individuals with FT; however, an important caveat is that there may be a heredity component to FMD (51, 65, 77, 78). There is also potential for false-negative family history, as some may not recognize they had tics previously. Alternatively, a false-positive family history can occur given the prevalence of CTD. Additionally, while a precipitating event can occur, it is important to note stressors and adverse experiences are risk factors rather than requirements for diagnosis (13, 50, 64, 65). Treatment resistance to typical tic medications may also occur (65, 66).
Mass Functional Illness
Mass Functional Illness (MFI), also known as mass psychogenic illness, mass hysteria, mass conversion disorder, or mass sociogenic illness, is “the rapid spread of illness signs and symptoms affecting members of a cohesive group” (79). MFI has been described for many centuries and occurs in varied cultures, ethnic groups, and religious settings (79, 80).
Historically, there are two categories of MFI: anxiety or motor phenomena. Anxiety MFI is characterized by transient, benign symptoms typically resolving within 24 h when there is a sudden, extreme stress or perceived threat in a cohesive group (81). Symptoms can include dizziness, headache, fatigue or hyperventilation. Motor MFI typically presents with gradual onset of motor symptoms including hyper- or hypo-kinetic movements, gait abnormalities and speech difficulties. Symptoms evolve over weeks to months and gradually remit.
Over the past two decades there has been increased motor presentations. There are many examples throughout recent history of MFI including outbreaks of non-epileptic spells, weakness, twitching, and gait abnormalities often in adolescent females (82–86)3.
Perhaps one of the most notable relevant examples was the outbreak of sudden onset of tic-like behaviors in August 2011 through January 2012 at Le Roy High School in Western New York State (68). The 19 affected individuals (18 females, 1 male), who did not belong to the same social group initially, formed a new social group based on their common disorder. Similarly, there were two outbreaks of hiccups and vocal tic-like behaviors of over a dozen students in two nearby Massachusetts high schools in November 2012 and January 2013 (87).
Like FMD, females have been reported to have a higher propensity to MFI (88, 89). A recent meta-analysis of gender differences showed 2.4:1 female predominance of MFI in children and adolescent (90). These outbreaks become the target of substantial media attention as well as thorough investigations into exposures, recent vaccinations, or environmental triggers (90–93).
Role of Social Media
Presence of movement disorders on social media is not novel. Review of videos on YouTube in 2011 by movement disorder specialists revealed 66% of movement-related videos were FMD (94). Interestingly enough, 18% (5,450/30,095) of movement-related videos reviewed were categorized as tic-related content.
Historically, MFI has been limited to a cohesive group; however, in the modern-day era, social media breaks the geographic barriers that typically confine such symptoms. Bartholomew was one of the first to propose the role of social media in MFI (81, 87). YouTube and Facebook were implicated in the spread of tic-like behaviors in Le Roy, as affected individuals were uploading videos of their symptoms onto these social media sites4,5,6,7.
A similar phenomena occurred in Germany in June 2019 when German Neurologists saw a vast rise in FT strikingly similar to a popular YouTube Channel “Gewitter im Kopf [Thunderstorm in the Brain]”, staring a young man Jan Zimmerman (95, 96). The channel gained rapid popularity and has more than 2.2 million subscribers and 312 million views8. Zimmerman has a similarly large presence across multiple platforms. Individuals presented with near identical complex movements, vocalizations, and unique words or phrases often seen in Zimmerman's videos. Given the specific role of social media, a more specific term was suggested - mass social media-induced illness (MSMI) (95).
The benefits and risks of social media remain controversial. Some argue that social media and digital technology help maintain social connection despite social distancing and lockdowns (97). Social media can also serve as a platform for individuals to share their experiences, advocate, and educate about medical conditions including tics. However, drawing attention to tics and/or exposure to other's tic-like behaviors may serve as precipitating or perpetuating risk factors for both FT and CTD. While social media provides access to communities that may not be readily available locally, this may also serve as a medium for continued spread of FT. Additionally, overuse of social media is associated with anxiety, depression, and psychological distress all of which may serve as risk factors for FT (98, 99).
TikTok Tics
Tic-related videos are gaining popularity across social media and the rapid spread of tic-like behaviors is a global phenomenon. On TikTok alone, hashtags of #tourette (4.9 billion views) and #tic (3.1 billion) have grown substantially during the COVID-19 pandemic9, hence what some are calling “TikTok Tics”.
TikTok is a popular social media platform where users can create, watch and share short videos. TikTok has experienced a surge in monthly active users between January 2018 and August 2020. Globally TikTok's active monthly users has grown from 54 million users in January 2018 to over 1 billion users as of September 202110,11. For comparison of active monthly users across other social media platforms Facebook has 2.9 billion, YouTube 2.3 billion, WhatsApp 2 billion, Instagram 1.4 billion, Snapchat 538 million, and Twitter 436 million12.
It is important to note that tic-related videos have grown substantially across multiple social media platforms and are not exclusive to TikTok. For example, TikTok influencer Evie Meg, better known as @thistrippyhippie, has 14 million followers for her tic-like behavior but also 791k followers on Instagram and 25 million views on YouTube13,14,15. Her videos often feature complex movements, coprophenomena, unique triggers and context-dependent tics. This influencer discloses her diagnosis of FND and features other videos of functional dystonia and psychogenic non-epileptic spells (PNES).
Two studies assessed the phenomenology of tic-like behavior on TikTok based on expert review. Both studies found a high degree of coprophenomena, context-dependence, aggression toward others, and self-injurious behavior (7, 100). Tic-like behaviors were highly variable and nonstereotyped. While tic-like behavior often involved the face and neck, there was a higher percentage of movements involving arms or body. Tic severity was overall severe with a high degree of tic attacks reported. There was a female predominance with 64.3% female, 17.6% male, and 14.3% nonbinary based on self-report of gender identity in the user's profile (7). Mean age reported was 18.8 years old although limitations included lack of age disclosure as well as unclear timing of video to onset of symptoms (7).
While these descriptive analyses are important to exploring the relationship of social media and tic-like behaviors, these conclusions were based on observations of social media videos rather than in-depth in-person evaluations. Additionally, negative portrayals of CTD are more popular on social media (101) and may influence the phenomenology reviewed by these two studies.
There has been some question of secondary gain in use of social media. Many of these social media influencers have merchandise for purchase (7). Jan Zimmerman sells merchandise, a book and recently released a Google app with his most popular vocal tics including “tics of the month.” Evie Meg released her new book “My Non-Identical Twin: What I'd like you to know about living with Tourette's” (7, 102, 103). It is important to note that factitious disorders and malingering are distinctly different from FMD and are beyond the scope of this article.
Functional Tic-Like Behaviors
While many of the features overlap with FT, there are emerging distinctive features (Figure 2, Supplementary Table 1). A more encompassing term is used, Functional Tic-Like Behaviors (FTLB) to better reflect the combined role of social media and the pandemic.
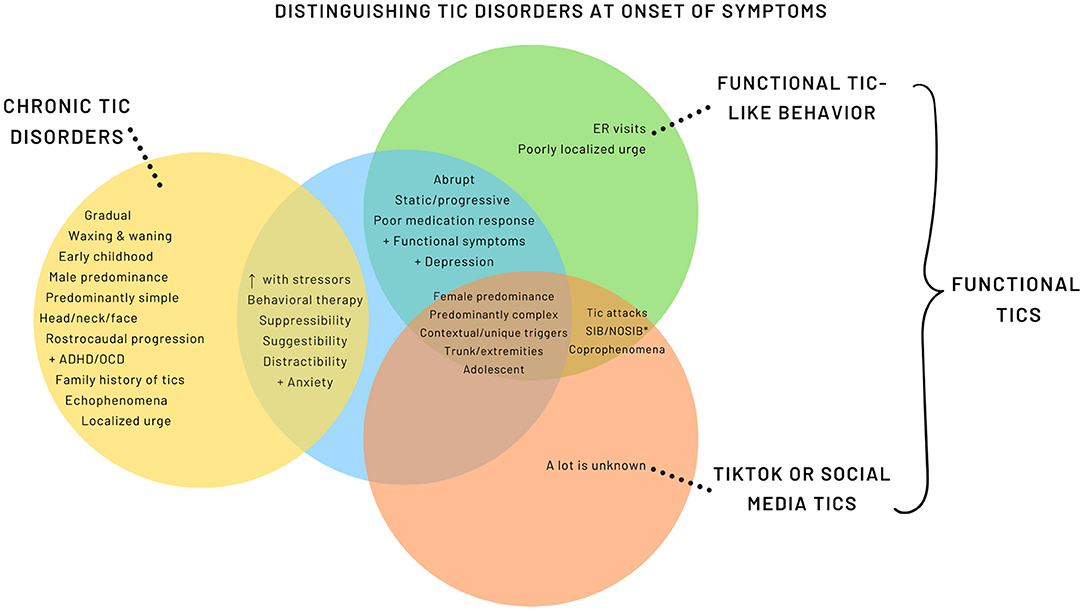
Figure 2. Distinguishing Tic Disorders at Onset of Symptoms. *SIB, Self-injurious behavior; NOSIB, Non-obscene socially inappropriate behavior.
FLTB have a female predominance (11) with the exception of one report from Germany (96), which had a male predominance. It is possible that this is related to the German social media influencer previously discussed. Median age of FTLB onset ranges from 14.2 to 15.3 years old with initial presentations being abrupt onset, non-fluctuating, and predominately complex tic-like behavior (11, 96, 104). Studies note a higher prevalence of tic-like behavior involving the trunk and extremities relative to the expected rostro-caudal progression seen in CTD (11, 96, 104). Pringsheim et al. reported a higher proportion of anxiety and depression diagnoses in FTLB compared to primary tic disorders (11), whereas others have found no significant difference (96).
FTLB are associated with high prevalence of coprophenomena, odd words or phrases, self-injurious (SIB) and non-obscene socially inappropriate behavior (NOSIB) (7, 71). Additionally, unique or contextual triggers such as particular words, flashing lights, or loud noises can trigger the tics or tic attacks (96, 104). Common SIB include hitting, punching or slapping one's self. Conversely, NOSIB can present as hitting others, throwing or hitting objects (11, 104). Tic attacks and presence of other functional or somatic symptoms were commonly reported (104). There are more limited data and variability in the degree of suppressibility, family history of tics, and presence of premonitory urge in patients with FTLB (11, 96). Careful questioning may endorse exposure to tic-related videos on social media; however, it should be considered one of many risk factors and is not always found (11, 96).
Although the predominance of adolescent females is reported, other sociodemographic features associated with FTLB remain unknown. Further exploration of risk factors and social determinants of health would be useful for prevention and intervention planning.
Role of the COVID-19 Pandemic
There is mounting evidence on the neurological sequela of COVID-19 infections including new development of movement disorders. Although one may consider post-infectious or infectious phenomena of COVID-19, a recent review of de novo movement disorders related to COVID-19 infections did not report any cases of new tics or tic-like behavior (105).
However, the COVID-19 pandemic has profoundly affected the mental health of many individuals, including children and adolescents. Nearly 168 million children globally missed an entire year of school due to COVID-19 according to the United Nations Education, Scientific and Cultural Organization (UNESCO). In April 2020, 1.5 billion learners were affected by school closures in 195 countries and as of November 2021, 55 million learners were still impacted by school closures with lower socio-economic statuses disproportionally affected (106)16,17.
Prior studies demonstrated both short- and long-term psychological effects of pandemics/epidemics including increased post-traumatic stress symptoms, anxiety, depression, helplessness, and risky behaviors (107–113). The overall rates of depression and anxiety are higher during COVID-19 than prior pandemics (114), with increased risk in females, adolescents, and remote learners (106, 107, 114–121). Periods of intense stress, such as the pandemic, can be associated with increased functional symptoms (122–124).
Parental stress, mental health, and wellbeing are also impacted during the pandemic, which is associated with poorer child wellbeing (125–128). Disruptions from the pandemic altered diets, sleep schedules, and social relationships. Additionally, parents reported interrupted access to medical care and to their support networks. Parents and/or caregivers suffered from isolation, employment changes, food insecurity, housing instability, and financial constraints all while balancing remote-learning and their child's wellbeing (125). Lower socioeconomic status, younger parents, and families of healthcare workers have been reported to be at increased risk of poorer wellbeing (129, 130). Additionally, there are increased reports of childhood adverse experiences during the pandemic, such as witnessed domestic violence, emotional abuse, and physical abuse (131, 132).
There has been an overall increase in new FMD presenting to neurology clinics during the COVID-19 pandemic (10). Despite this increase, individuals with preexisting FMD did not show significant variability or worsening of their symptoms during the pandemic (133). However, up to two-thirds of parents or individuals reported worsening of CTD symptoms (134, 135). Children with neurodevelopmental disorders, such as CTD, report higher behavioral and psychological impacts of the pandemic compared to peers (136). The same is true for children with preexisting mental health diagnoses (125). Acute psychosocial stressors, routine disruption, and increased mental health burden likely play a role in symptom exacerbation or development; however, these relationships need to be further explored (137).
Discussion
Knowledge of these disorders are vital in mitigating downstream health effects and poor outcomes. A common concern in FMD is fear of misdiagnosis; however, in the modern medical setting the frequency of misdiagnosis is consistently low (138, 139). Recognition of the positive features to support a diagnosis of FMD is essential. While behavioral therapy is the first line treatment for both FMD and CTD, it is critical to establish the diagnosis early and engage familial support (12, 140). Longer duration of symptoms before diagnosis and pre-existing personality disorders lead to poorer outcomes (141, 142). A multidisciplinary approach is essential in effective treatment and psychological support is crucial. The overall mental health burden of the pandemic poses challenges for accessibility to knowledgeable therapists and mental health resources.
Clinicians should also be mindful that FTLBs may co-occur in individuals with CTD or other neurological conditions (143). A sudden or explosive emergence of atypical tic-like behaviors should raise concern of functional overlay (144, 145). Failure to recognize this can lead to unnecessary medication trials, sense of pseudo-refractoriness, potential invasive surgical procedures or delay in diagnosis (61, 146, 147).
The role of social media in these tic-like behaviors has gained significant media attention, which likely contributes to parental fear and uncertainty. Explosive onset of FTLB can be both bothersome and intrusive to the daily function of the individual and their family. This may result in missed days at school, parental missed days of work, missed social events, and/or financial constraints that impacts parental stress and wellbeing. Many patients with explosive onset of FTLB are utilizing emergency room services (9). FMD admissions have higher work-up rates but shorter length of stays. In 2017, the estimated US economic impact of ER and inpatient care of FNDs was more than $1.2 billion annually, comparable to other high-utilization neurological conditions such as refractory epilepsy or demyelinating disorders (58). Recognition of FTLB may reduce unnecessary admissions, diagnostic testing, medication trials, time to treatment, and economic impacts.
The impact this global phenomena has had on the CTD community must also be considered. A look through the comments on these influencers' videos suggests a step backwards in awareness, attitudes, and stigmatization of not only CTD but also FMD community. In CTD, female gender, tic severity and complex tics increase stigmatization risk, which is associated with lower quality of life, depression, and lower self-esteem (36, 148–151). The commonality of these features with FT and FTLB may contribute to ongoing public misconception of individuals with CTD and FMD. Future research should aim to understand the intricacies of stigmatization in these disorders.
Lastly, the rarity of FT previously may have limited our understanding of this disorder. With the rise of FTLB, there is an opportunity to evaluate overlapping and distinguishing features of FT, FTLB, and CTD to establish evidence-based guidelines for evaluation and treatment. Previous studies have suggested some common predisposing factors between CTD and FT such as family history, adverse experiences, and psychosocial stressors (11, 72). Lastly, the etiology of FLTB is likely multifactorial. Future research is necessary to better define the relationship between social media, the pandemic, and these entities as well as further understand shared predisposing, precipitating, and perpetuating factors.
Author Contributions
JMM: conceptualized, drafted the initial manuscript, reviewed, and revised the manuscript. JWM: conceptualized, critically reviewed the manuscript for important intellectual content, and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Sean Keui-Hsin Wang and Scott Otallah for their editing of the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.863919/full#supplementary-material
Abbreviations
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome due to coronavirus-2; FMD, functional movement disorder; FT, functional tics; FND, functional neurological disorder; CTD, chronic tic disorders; TS, Tourette Syndrome; ADHD, attention deficit-hyperactivity disorder; OCD, obsessive-compulsive disorder; MFI, mass functional illness; PNES, psychogenic non-epileptic spells; MSMI, mass social media-induced illness; UNESCO, United Nations Education, Scientific and Cultural Organization.
Footnotes
1. ^World Health Organization. (2020). Director-General media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-−11-march-2020 (Accessed 11/4/2021).
2. ^World Health Organization. (2020). Coronavirus (COVID-19) Dashboard. https://covid19.who.int/ (Accessed 11/2/2021).
3. ^The New York Times. (2007). Mysterious illness strikes teenage girls in Mexico. https://www.nytimes.com/2007/04/16/world/americas/16iht-mexico.3.5306132.html (Accessed 10/26/2021).
4. ^The New York Times Magazine. (2012). What Happened to the Girls in Le Roy. https://www.nytimes.com/2012/03/11/magazine/teenage-girls-twitching-le-roy.html (Accessed 10/26/2021).
5. ^The Daily Mail. (2012). Facebook to blame for the panic surrounding mysterious Tourette's-like illness spreading in rural New York town. https://www.dailymail.co.uk/news/article-2096813/Could-infection-mysterious-Tourettes-like-syndrome-affecting-teenagers.html (Accessed 10/26/2021).
6. ^TODAY. (2012). Facebook, YouTube could be spreading 'mystery illness,' doctor says. https://www.today.com/health/facebook-youtube-could-be-spreading-mystery-illness-doctor-says-1C9381793 (Accessed 10/26/2021).
7. ^Huffpost. (2014). When Social Media Makes Something Go Viral In Real Life. https://www.huffpost.com/entry/dont-look-now-social-medi_b_5534200 (Accessed 10/26/2021).
8. ^YouTube. (2005). Gewitterimkopf. https://www.youtube.com/c/gewitterimkopf/about (Accessed 10/28/2021).
9. ^TikTok. (2016). Tag: Tourette's. https://www.tiktok.com/tag/tourettes?lang=en (Accessed 10/28/2021).
10. ^CNBC. (2021). TikTok says 1 billion people use the app each month. https://www.cnbc.com/2021/09/27/tiktok-reaches-1-billion-monthly-users.html (Accessed 10/27/2021).
11. ^TikTok. (2021) TikTok Newsroom: Thanks a billion!. https://newsroom.tiktok.com/en-us/1-billion-people-on-tiktok (Accessed 10/27/2021).
12. ^Datareportal. (2021) Global Social Media Stats. https://datareportal.com/social-media-users (Accessed 10/27/2021).
13. ^Social Blade. (2018). User Summary: This Trippy Hippie - YouTube. https://socialblade.com/youtube/channel/UCJHvN0zYgO2ZLjePERRLjKQ (Accessed 10/28/2021).
14. ^Social Blade. (2018). User Summary thistrippyhippie – TikTok. https://socialblade.com/tiktok/user/thistrippyhippie (Accessed 10/28/2021).
15. ^Social Blade. (2018) User Summary eviemeg – Instagram. https://socialblade.com/instagram/user/eviemeg (Accessed 10/28/2021).
16. ^UNESCO. (2021). Education: From disruption to recovery. https://en.unesco.org/covid19/educationresponse#schoolclosures (Accessed 11/01/2021).
17. ^UNESCO. (2020). 1.3 billion learners are still affected by school or university closures, as educational institutions start reopening around the world, says UNESCO. https://en.unesco.org/news/13-billion-learners-are-still-affected-school-university-closures-educational-institutions (Accessed 11/1/2021).
References
1. Abdel-Mannan O, Eyre M, Lobel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and Radiographic Findings Associated With COVID-19 infection in children. JAMA Neurol. (2020) 77:1440–5. doi: 10.1001/jamaneurol.2020.2687
2. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US children and adolescents. N Engl J Med. (2020) 383:334–46. doi: 10.1056/NEJMoa2021680
3. Lin JE, Asfour A, Sewell TB, Hooe B, Pryce P, Earley C, et al. Neurological issues in children with COVID-19. Neurosci Lett. (2021) 743:135567. doi: 10.1016/j.neulet.2020.135567
4. LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. (2021) 78:536–47. doi: 10.1001/jamaneurol.2021.0504
5. O'Loughlin L, Alvarez Toledo N, Budrie L, Waechter R, Rayner J. A systematic review of severe neurological manifestations in pediatric patients with coexisting SARS-CoV-2 infection. Neurol Int. (2021) 13:410–27. doi: 10.3390/neurolint13030041
6. Panda PK, Gupta J, Chowdhury SR, Kumar R, Meena AK, Madaan P, et al. Psychological and behavioral impact of lockdown and quarantine measures for COVID-19 pandemic on children, adolescents and caregivers: a systematic review and meta-analysis. J Trop Pediatr. (2021) 67:fmaa122. doi: 10.1093/tropej/fmaa122
8. Forsyth RJ. Tics, TikTok and COVID-19. Arch Dis Child. (2021) 106:417. doi: 10.1136/archdischild-2021-321885
9. Heyman I, Liang H, Hedderly T. COVID-19 related increase in childhood tics and tic-like attacks. Arch Dis Child. (2021) 106:420–21. doi: 10.1136/archdischild-2021-321748
10. Hull M. Increased Incidence of Functional (Psychogenic) movement disorders in children and adults admist the COVID-19 Pandemic: a cross-sectional study. Neurol Clin Pract. (2021). doi: 10.1212/CPJ.0000000000001082
11. Pringsheim T, Ganos C, McGuire JF, Hedderly T, Woods D, Gilbert DL, et al. Rapid onset functional tic-like behaviors in young females during the COVID-19 pandemic. Mov Disord. (2021) 36:2707–13. doi: 10.1002/mds.28778
12. Espay AJ, Aybek S, Carson A, Edwards MJ, Goldstein LH, Hallett M, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol. (2018) 75:1132–41. doi: 10.1001/jamaneurol.2018.1264
13. Schwingenschuh P, Pont-Sunyer C, Surtees R, Edwards MJ, Bhatia KP. Psychogenic movement disorders in children: a report of 15 cases and a review of the literature. Mov Disord. (2008) 23:1882–8. doi: 10.1002/mds.22280
14. Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioral spectrum of tic disorders: a community-based study. Neurology. (2002) 59:414–20. doi: 10.1212/WNL.59.3.414
15. Comings DE, Comings BG. A controlled study of Tourette syndrome. I Attention-deficit disorder, learning disorders, and school problems. Am J Hum Genet. (1987) 41:701–41.
16. Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. (2015) 72:325–33. doi: 10.1001/jamapsychiatry.2014.2650
17. Martino D, Ganos C, Pringsheim TM. Tourette syndrome and chronic tic disorders: the clinical spectrum beyond tics. Int Rev Neurobiol. (2017) 134:1461–90. doi: 10.1016/bs.irn.2017.05.006
18. Freeman RD, Fast DG, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol. (2000) 42:436–47. doi: 10.1017/S0012162200000839
19. Cavanna AE, Servo S, Monaco F, Robertson MM. The behavioral spectrum of Gilles de la Tourette syndrome. J Neuropsychiatry Clin Neurosci. (2009) 21:13–23. doi: 10.1176/jnp.2009.21.1.13
20. Leckman JF, Zhang HP, Vitale A, Lahnin F, Lynch K, Bondi C, et al. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. (1998) 102:14–9. doi: 10.1542/peds.102.1.14
21. Garris J, Quigg M. The female Tourette patient: Sex differences in Tourette Disorder. Neurosci Biobehav Rev. (2021) 129:261–8. doi: 10.1016/j.neubiorev.2021.08.001
22. Albin RL. Tourette syndrome: a disorder of the social decision-making network. Brain. (2018) 141:332–47. doi: 10.1093/brain/awx204
23. Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. (2014) 13:100–12. doi: 10.1016/S1474-4422(13)70213-8
24. Leckman JF, Walker DE, Cohen DJ. Premonitory urges in Tourette's syndrome. Am J Psychiatry. (1993) 150:98–102. doi: 10.1176/ajp.150.1.98
25. Prado HS, Rosario MC, Lee J, Hounie AG, Shavitt RG, Miguel EC. Sensory phenomena in obsessive-compulsive disorder and tic disorders: a review of the literature. CNS Spectr. (2008) 13:425–32. doi: 10.1017/S1092852900016606
26. McGuire JF, McBride N, Piacentini J, Johnco C, Lewin AB, Murphy TK, et al. The premonitory urge revisited: An individualized premonitory urge for tics scale. J Psychiatr Res. (2016) 83:176–83. doi: 10.1016/j.jpsychires.2016.09.007
27. Kwak C, Dat Vuong K, Jankovic J. Premonitory sensory phenomenon in Tourette's syndrome. Mov Disord. (2003) 18:1530–3. doi: 10.1002/mds.10618
28. Garcia-Delgar B, Servera M, Coffey BJ, Lázaro L, Openneer T, Benaroya-Milshtein N, et al. Tic disorders in children and adolescents: does the clinical presentation differ in males and females? A report by the EMTICS group. Eur Child Adolesc Psychiatry. (2021). doi: 10.1007/s00787-021-01751-4
29. Burd L, Kerbeshian J, Barth A, Klug MG, Avery K, Benz B. Long-term follow-up of an epidemiologically defined cohort of patients with Tourette syndrome. J Child Neurol. (2001) 16:431–7. doi: 10.1177/088307380101600609
30. Bloch MH, Peterson BS, Scahill L, Otka J, Katsovich L, Zhang H, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. (2006) 160:65–9. doi: 10.1001/archpedi.160.1.65
31. Bloch MH, Leckman JF. Clinical course of Tourette syndrome. J Psychosom Res. (2009) 67:497–501. doi: 10.1016/j.jpsychores.2009.09.002
32. Girgis J, Martino D, Pringsheim T. Influence of sex on tic severity and psychiatric comorbidity profile in patients with pediatric tic disorder. Dev Med Child Neurol. (2022) 64:488–94. doi: 10.1111/dmcn.15088
33. Eapen V, Fox-Hiley P, Banerjee S, Robertson M. Clinical features and associated psychopathology in a Tourette syndrome cohort. Acta Neurol Scand. (2004) 109:255–60. doi: 10.1046/j.1600-0404.2003.00228.x
34. Shprecher DR, Rubenstein LA, Gannon K, Frank SA, Kurlan R. Temporal course of the tourette syndrome clinical triad. Tremor Other Hyperkinet Mov (N Y). (2014) 4:243. doi: 10.5334/tohm.195
35. Groth C, Debes NM, Skov L. Phenotype development in adolescents with Tourette syndrome: a large clinical longitudinal study. J Child Neurol. (2017) 32:1047–57. doi: 10.1177/0883073817729917
36. Malli MA, Forrester-Jones R, Murphy G. Stigma in youth with Tourette's syndrome: a systematic review and synthesis. Eur Child Adolesc Psychiatry. (2016) 25:127–39. doi: 10.1007/s00787-015-0761-x
37. Goldenberg JN, Brown SB, Weiner WJ. Coprolalia in younger patients with Gilles de la Tourette syndrome. Mov Disord. (1994) 9:622–5. doi: 10.1002/mds.870090607
38. Freeman RD, Zinner SH, Muller-Vahl KR, Fast DK, Burd LJ, Kano Y, et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol. (2009) 51:218–27. doi: 10.1111/j.1469-8749.2008.03135.x
39. Singer C. Coprolalia and other coprophenomena. Neurol Clin. (1997) 15:299–308. doi: 10.1016/S0733-8619(05)70314-5
40. Lajonchere C, Nortz M, Finger S. Gilles de la Tourette and the discovery of tourette syndrome: includes a translation of his 1884 article. Arch Neurol. (1996) 53:567–74. doi: 10.1001/archneur.1996.00550060111024
41. Ganos C, Ogrzal T, Schnitzler A, Münchau A. The pathophysiology of echopraxia/echolalia: Relevance to Gilles De La Tourette syndrome. Movement Disord. (2012) 27:1222–9. doi: 10.1002/mds.25103
42. Muller N, Putz A, Kathmann N, Lehle R, Gunther W, Straube A. Characteristics of obsessive-compulsive symptoms in Tourette's syndrome, obsessive-compulsive disorder, and Parkinson's disease. Psychiatry Res. (1997) 70:105–14. doi: 10.1016/S0165-1781(97)02658-9
43. Cavanna AE, Critchley HD, Orth M, Stern JS, Young MB, Robertson MM. Dissecting the Gilles de la Tourette spectrum: a factor analytic study on 639 patients. J Neurol Neurosurg Psychiatry. (2011) 82:1320–3. doi: 10.1136/jnnp.2010.225029
44. Mathews CA, Waller J, Glidden D, Lowe TL, Herrera LD, Budman CL, et al. Self injurious behaviour in Tourette syndrome: correlates with impulsivity and impulse control. J Neurol Neurosurg Psychiatry. (2004) 75:1149–55. doi: 10.1136/jnnp.2003.020693
45. Robertson MM, Trimble MR, Lees AJ. Self-injurious behaviour and the Gilles de la Tourette syndrome: a clinical study and review of the literature. Psychol Med. (1989) 19:611–25. doi: 10.1017/S0033291700024211
46. Szejko N, Jakubczyk A, Janik P. Prevalence and clinical correlates of self-harm behaviors in gilles de la Tourette Syndrome. Front Psychiatry. (2019) 10:638. doi: 10.3389/fpsyt.2019.00638
47. Cheung MY, Shahed J, Jankovic J. Malignant Tourette syndrome. Mov Disord. (2007) 22:1743–50. doi: 10.1002/mds.21599
48. Stone J, Carson A, Duncan R, Roberts R, Warlow C, Hibberd C, et al. Who is referred to neurology clinics?–the diagnoses made in 3781 new patients. Clin Neurol Neurosurg. (2010) 112:747–51. doi: 10.1016/j.clineuro.2010.05.011
49. Baizabal-Carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis. (2019) 127:32–44. doi: 10.1016/j.nbd.2019.02.013
50. Edwards MJ, Stone J, Lang AE. From psychogenic movement disorder to functional movement disorder: it's time to change the name. Mov Disord. (2014) 29:849–52. doi: 10.1002/mds.25562
51. Ellenstein A, Kranick SM, Hallett M. An update on psychogenic movement disorders. Curr Neurol Neurosci Rep. (2011) 11:396–403. doi: 10.1007/s11910-011-0205-z
52. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. (2012) 11:250–60. doi: 10.1016/S1474-4422(11)70310-6
54. Chouksey A, Pandey S. Functional movement disorders in children. Front Neurol. (2020) 11:570151. doi: 10.3389/fneur.2020.570151
55. Harris SR. Psychogenic movement disorders in children and adolescents: an update. Eur J Pediatr. (2019) 178:581–5. doi: 10.1007/s00431-019-03317-8
56. Rakesh K, Kamble N, Yadav R, Bhattacharya A, Holla VV, Netravathi M, et al. Pediatric functional movement disorders: experience from a tertiary care centre. Can J Neurol Sci. (2021) 48:518–24. doi: 10.1017/cjn.2020.213
57. American Psychiatric Association. American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association. (2013). xliv, p. 947.
58. Stephen CD, Fung V, Lungu CI, Espay AJ. Assessment of emergency department and inpatient use and costs in adult and pediatric functional neurological disorders. JAMA Neurol. (2021) 78:88–101. doi: 10.1001/jamaneurol.2020.3753
59. Fernandez-Alvarez E. Movement disorders of functional origin (psychogenic) in children. Rev Neurol. (2005) 40 (Suppl 1):S75–7.
60. Baizabal-Carvallo JF, Jankovic J. Gender differences in functional movement disorders. Mov Disord Clin Pract. (2020) 7:182–7. doi: 10.1002/mdc3.12864
61. Ferrara J, Jankovic J. Psychogenic movement disorders in children. Mov Disord. (2008) 23:1875–81. doi: 10.1002/mds.22220
62. Ahmed MA, Martinez A, Yee A, Cahill D, Besag FM. Psychogenic and organic movement disorders in children. Dev Med Child Neurol. (2008) 50:300–4. doi: 10.1111/j.1469-8749.2008.02043.x
63. Cubo E, Hinson VK, Goetz CG, Garcia Ruiz P, Garcia de Yebenes J, Marti MJ, et al. Transcultural comparison of psychogenic movement disorders. Mov Disord. (2005) 20:1343–5. doi: 10.1002/mds.20561
64. Pandey S, Koul A. Psychogenic movement disorders in adults and children: a clinical and video profile of 58 Indian patients. Mov Disord Clin Pract. (2017) 4:763–7. doi: 10.1002/mdc3.12516
65. Baizabal-Carvallo JF, Jankovic J. The clinical features of psychogenic movement disorders resembling tics. J Neurol Neurosurg Psychiatry. (2014) 85:573–5. doi: 10.1136/jnnp-2013-305594
66. Demartini B, Ricciardi L, Parees I, Ganos C, Bhatia KP, Edwards MJ, et al. positive diagnosis of functional (psychogenic) tics. Eur J Neurol. (2015) 22:527–e36. doi: 10.1111/ene.12609
67. Perisa A, Telarovic S. On Differences between Gilles De La Tourette Syndrome and psychogenic/functional tics: a narrative review. Psychiat Danub. (2019) 31:10–4.
68. Mink JW. Conversion disorder and mass psychogenic illness in child neurology. Ann N Y Acad Sci. (2013) 1304:40–4. doi: 10.1111/nyas.12298
69. Schneider SA, Hennig A, Martino D. Relationship between COVID-19 and movement disorders: a narrative review. Eur J Neurol. (2021) 29:1243–1253. doi: 10.1111/ene.15217
70. Delgado C, Parees I, Jimenez-Huete A, Kurtis MM. Impact of the coronavirus disease 2019 pandemic on functional movement disorders: lessons from a specialized clinic. Mov Disord. (2020) 35:1723–4. doi: 10.1002/mds.28278
71. Buts S, Duncan M, Owen T, Martino D, Pringsheim T, Byrne S, et al. Paediatric tic-like presentations during the COVID-19 pandemic. Arch Dis Child. (2021). doi: 10.1136/archdischild-2021-323002
72. Ganos C, Martino D, Espay AJ, Lang AE, Bhatia KP, Edwards MJ. Tics and functional tic-like movements: can we tell them apart? Neurology. (2019) 93:750–8. doi: 10.1212/WNL.0000000000008372
73. Baizabal-Carvallo JF, Fekete R. Recognizing uncommon presentations of psychogenic (functional) movement disorders. Tremor Other Hyperkinet Mov (N Y). (2015) 5:279. doi: 10.5334/tohm.266
74. Ganos C, Erro R, Cavanna AE, Bhatia KP. Functional tics and echophenomena. Parkinsonism Relat Disord. (2014) 20:1440–1. doi: 10.1016/j.parkreldis.2014.10.001
75. Ganos C, Edwards MJ, Muller-Vahl K. “I swear it is Tourette's!”: on functional coprolalia and other tic-like vocalizations. Psychiatry Res. (2016) 246:821–6. doi: 10.1016/j.psychres.2016.10.021
76. Ganos C, Münchau A, Bhatia KP. The semiology of tics, Tourette's, and Their Associations. Mov Disord Clin Pract. (2014) 1:145–53. doi: 10.1002/mdc3.12043
77. Stamelou M, Cossu G, Edwards MJ, Murgia D, Parees I, Melis M, et al. Familial psychogenic movement disorders. Mov Disord. (2013) 28:1295–8. doi: 10.1002/mds.25463
78. Black K, Black E, Greene D, Schlaggar B. Provisional tic disorder: what to tell parents when their child first starts ticcing [version 1; peer review: 3 approved]. F1000Res. (2016) 5:696. doi: 10.12688/f1000research.8428.1
79. Bartholomew RE, Wessely S. Protean nature of mass sociogenic illness: from possessed nuns to chemical and biological terrorism fears. Br J Psychiatry. (2002) 180:300–6. doi: 10.1192/bjp.180.4.300
80. Ajemu KF, Weldearegay TW, Bezabih NM, Meles Y, Mehari G, Desta AA, et al. Mass psychogenic illness in haraza elementary school, Erop District, Tigray, Northern Ethiopia: investigation to the nature of an episode. Psychiatry J. (2020) 2020:2693830. doi: 10.1155/2020/2693830
81. Bartholomew RE, Wessely S, Rubin GJ. Mass psychogenic illness and the social network: is it changing the pattern of outbreaks? J R Soc Med. (2012) 105:509–12. doi: 10.1258/jrsm.2012.120053
82. Roach ES, Langley RL. Episodic neurological dysfunction due to mass hysteria. Arch Neurol. (2004) 61:1269–72. doi: 10.1001/archneur.61.8.1269
83. Sapkota RP, Brunet A, Kirmayer LJ. Characteristics of adolescents affected by mass psychogenic illness outbreaks in schools in Nepal: a case-control study. Front Psychiatry. (2020) 11:493094. doi: 10.3389/fpsyt.2020.493094
84. Roach ES. Mass hysteria and the media: Folie à troupeau? Pediatr Neurol. (2013) 49:6–7. doi: 10.1016/j.pediatrneurol.2013.04.013
85. Govender I. Mass hysteria among South African primary school learners in Kwa-Dukuza, Kwazulu-Natal. South Afr Fam Pract. (2010) 52:318–21. doi: 10.1080/20786204.2010.10873998
86. Buttery JP, Madin S, Crawford NW, Elia S, La Vincente S, Hanieh S, et al. Mass psychogenic response to human papillomavirus vaccination. Med J Australia. (2008) 189:261–2. doi: 10.5694/j.1326-5377.2008.tb02018.x
88. Lorber W, Mazzoni G, Kirsch I. Illness by suggestion: expectancy, modeling, and gender in the production of psychosomatic symptoms. Ann Behav Med. (2007) 33:112–6. doi: 10.1207/s15324796abm3301_13
89. Cheng Q, Xie L, Hu Y, Hu J, Gao W, Lv Y, et al. Gender differences in the prevalence and impact factors of hysterical tendencies in adolescents from three eastern Chinese provinces. Environ Health Prev Med. (2018) 23:5. doi: 10.1186/s12199-018-0695-2
90. Zhao G, Cheng Q, Dong X, Xie L. Mass hysteria attack rates in children and adolescents: a meta-analysis. J Int Med Res. (2021) 49. doi: 10.1177/03000605211039812
91. Huang WT, Hsu CC, Lee PI, Chuang JH. Mass psychogenic illness in nationwide in-school vaccination for pandemic influenza A(H1N1) 2009, Taiwan, November 2009-January 2010. Euro Surveill. (2010) 15:19575. doi: 10.2807/ese.15.21.19575-en
92. Loharikar A, Suragh TA, MacDonald NE, Balakrishnan MR, Benes O, Lamprianou S, et al. Anxiety-related adverse events following immunization (AEFI): A systematic review of published clusters of illness. Vaccine. (2018) 36:299–305. doi: 10.1016/j.vaccine.2017.11.017
93. Singh OP, Mandal N, Biswas A, Mondal S, Sen S, Mukhopadhyay S. An investigation into a mass psychogenic illness at Burdwan, West Bengal. Indian J Public Health. (2009) 53:55–7.
94. Stamelou M, Edwards MJ, Espay AJ, Fung VS, Hallett M, Lang AE, et al. Movement disorders on YouTube–caveat spectator. N Engl J Med. (2011) 365:1160–1. doi: 10.1056/NEJMc1107673
95. Müller-Vahl KR, Pisarenko A, Jakubovski E, Fremer C. Stop that! It's not Tourette's but a new type of mass sociogenic illness. Brain. (2021) 45:476–80. doi: 10.1093/brain/awab316
96. Paulus T, Baumer T, Verrel J, Weissbach A, Roessner V, Beste C, et al. Pandemic Tic-like Behaviors Following Social Media Consumption. Mov Disord. (2021) 36:2932–35. doi: 10.1002/mds.28800
97. Pancani L, Marinucci M, Aureli N, Riva P. Forced social isolation and mental health: a study on 1,006 Italians under COVID-19 lockdown. Front Psychol. (2021) 12:663799. doi: 10.3389/fpsyg.2021.663799
98. Keles B, McCrae N, Grealish A. A systematic review: the influence of social media on depression, anxiety and psychological distress in adolescents. Int J Adolesc Youth. (2020) 25:79–93. doi: 10.1080/02673843.2019.1590851
99. Garfin DR, Silver RC, Holman EA. The novel coronavirus (COVID-2019) outbreak: Amplification of public health consequences by media exposure. Health Psychol. (2020) 39:355–7. doi: 10.1037/hea0000875
100. Zea Vera A, Bruce A, Garris J, Tochen L, Bhatia P, Lehman RK, et al. The Phenomenology of Tics and Tic-Like Behavior in TikTok. Pediatr Neurol. (2022) 130:14–20. doi: 10.1016/j.pediatrneurol.2022.02.003
101. Fat MJL, Sell E, Barrowman N, Doja A. Public perception of Tourette Syndrome on YouTube. Ann Neurol. (2011) 70:S153-S. doi: 10.1177/0883073811432294
102. Gewitter Im Shop: Holymesh (2021). Available online at: https://gewitterimshop.de/ (accessed April 11, 2021).
103. Gewitter Im Kopf Google Beyto GmbH Entertainment (2021). Available online at: https://play.google.com/store/apps/details?id=com.gik_android&hl=en_US&gl=US (accessed April 11, 2021).
104. Hull M, Parnes M. Tics and TikTok: Functional tics spread through social media. Mov Disord Clin Pract. (2021) 8:1248–52. doi: 10.1002/mdc3.13267
105. Ghosh R, Biswas U, Roy D, Pandit A, Lahiri D, Ray BK, et al. De novo movement disorders and COVID-19: exploring the interface. Mov Disord Clin Pract. (2021) 8:669–80. doi: 10.1002/mdc3.13224
106. Hawrilenko M, Kroshus E, Tandon P, Christakis D. The association between school closures and child mental health during COVID-19. JAMA Netw Open. (2021) 4:e2124092. doi: 10.1001/jamanetworkopen.2021.24092
107. Saurabh K, Ranjan S. Compliance and psychological impact of quarantine in children and adolescents due to COVID-19 pandemic. Indian J Pediatr. (2020) 87:532–6. doi: 10.1007/s12098-020-03347-3
108. Buzzi C, Tucci M, Ciprandi R, Brambilla I, Caimmi S, Ciprandi G, et al. The psycho-social effects of COVID-19 on Italian adolescents' attitudes and behaviors. Ital J Pediatr. (2020) 46:69. doi: 10.1186/s13052-020-00833-4
109. Blakey SM, Reuman L, Jacoby RJ, Abramowitz JS. Tracing “Fearbola”: psychological predictors of anxious responding to the threat of Ebola. Cognit Ther Res. (2015) 39:816–25. doi: 10.1007/s10608-015-9701-9
110. Imran N, Aamer I, Sharif MI, Bodla ZH, Naveed S. Psychological burden of quarantine in children and adolescents: A rapid systematic review and proposed solutions. Pak J Med Sci. (2020) 36:1106–16. doi: 10.12669/pjms.36.5.3088
111. Shah T, Shah Z, Yasmeen N, Ma ZR. Psychological impact of the COVID-19 pandemic on Chinese population: an online survey. World J Clin Cases. (2021) 9:9500–8. doi: 10.12998/wjcc.v9.i31.9500
112. Nisticò V, Goeta D, Gambini O, Demartini B. The psychological impact of COVID-19 among a sample of Italian patients with functional neurological disorders: a preliminary study. Parkinsonism Relat Disord. (2020) 78:79–81. doi: 10.1016/j.parkreldis.2020.07.019
113. Dubey S, Biswas P, Ghosh R, Chatterjee S, Dubey MJ, Chatterjee S, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr. (2020) 14:779–88. doi: 10.1016/j.dsx.2020.05.035
114. Meherali S, Punjani N, Louie-Poon S, Rahim KA, Das JK, Salam RA, et al. Mental health of children and adolescents amidst COVID-19 and past pandemics: a rapid systematic review. Int J Environ Res Pubic Health. (2021) 18:3432. doi: 10.3390/ijerph18073432
115. Duan L, Shao X, Wang Y, Huang Y, Miao J, Yang X, et al. An investigation of mental health status of children and adolescents in china during the outbreak of COVID-19. J Affect Disord. (2020) 275:112–8. doi: 10.1016/j.jad.2020.06.029
116. Liu X, Luo WT, Li Y, Li CN, Hong ZS, Chen HL, et al. Psychological status and behavior changes of the public during the COVID-19 epidemic in China. Infect Dis Poverty. (2020) 9:58. doi: 10.1186/s40249-020-00678-3
117. Tian FY, Li HX, Tian SC, Yang J, Shao J, Tian CN. Psychological symptoms of ordinary Chinese citizens based on SCL-90 during the level I emergency response to COVID-19. Psychiat Res. (2020) 288. doi: 10.1016/j.psychres.2020.112992
118. Wang CY, Pan RY, Wan XY, Tan YL, Xu LK, Ho CS, et al. Immediate Psychological Responses and Associated Factors during the Initial Stage of the 2019 Coronavirus Disease (COVID-19) Epidemic among the General Population in China. Int J Environ Res Public Health. (2020) 17:1729. doi: 10.3390/ijerph17051729
119. Zhou SJ, Zhang LG, Wang LL, Guo ZC, Wang JQ, Chen JC, et al. Prevalence and socio-demographic correlates of psychological health problems in Chinese adolescents during the outbreak of COVID-19. Eur Child Adolesc Psychiatry. (2020) 29:749–58. doi: 10.1007/s00787-020-01541-4
120. Nearchou F, Flinn C, Niland R, Subramaniam SS, Hennessy E. Exploring the impact of COVID-19 on mental health outcomes in children and adolescents: a systematic review. Int J Environ Res Public Health. (2020) 17:8479. doi: 10.3390/ijerph17228479
121. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. (2020) 89:531–42. doi: 10.1016/j.bbi.2020.05.048
122. Guerriero RM, Pier DB, de Gusmao CM, Bernson-Leung ME, Maski KP, Urion DK, et al. Increased pediatric functional neurological symptom disorders after the Boston marathon bombings: a case series. Pediatr Neurol. (2014) 51:619–23. doi: 10.1016/j.pediatrneurol.2014.07.011
123. Liu SY, Liu Y, Liu Y. Somatic symptoms and concern regarding COVID-19 among Chinese college and primary school students: a cross-sectional survey. Psychiat Res. (2020) 289:113070. doi: 10.1016/j.psychres.2020.113070
124. Hassett AL, Sigal LH. Unforeseen consequences of terrorism: medically unexplained symptoms in a time of fear. Arch Intern Med. (2002) 162:1809–13. doi: 10.1001/archinte.162.16.1809
125. Rizzo R, Karlov L, Maugeri N, di Silvestre S, Eapen V. Impact of the COVID-19 Pandemic on family wellbeing in the context of neurodevelopmental disorders. Neuropsychiatr Dis Treat. (2021) 17:3007–14. doi: 10.2147/NDT.S327092
126. Kim SJ, Lee S, Han H, Jung J, Yang SJ, Shin Y. Parental mental health and children's behaviors and media usage during COVID-19-related school closures. J Korean Med Sci. (2021) 36:e184. doi: 10.3346/jkms.2021.36.e184
127. Lee SJ, Ward KP, Chang OD, Downing KM. Parenting activities and the transition to home-based education during the COVID-19 pandemic. Child Youth Serv Rev. (2021) 122:105585. doi: 10.1016/j.childyouth.2020.105585
128. Singletary B, Schmeer KK, Purtell KM, Sayers RC, Justice LM, Lin TJ, et al. Understanding family life during the COVID-19 shutdown. Fam Relat. (2022) 71:475–93. doi: 10.1111/fare.12655
129. Bates CR, Nicholson LM, Rea EM, Hagy HA, Bohnert AM. Life interrupted: family routines buffer stress during the COVID-19 pandemic. J Child Fam Stud. (2021) 30:2641–51. doi: 10.1007/s10826-021-02063-6
130. V CF, Iarocci G. Child and family outcomes following pandemics: a systematic review and recommendations on COVID-19 policies. J Pediatr Psychol. (2020) 45:1124–43. doi: 10.1093/jpepsy/jsaa092
131. Calvano C, Engelke L, Di Bella J, Kindermann J, Renneberg B, Winter SM. Families in the COVID-19 pandemic: parental stress, parent mental health and the occurrence of adverse childhood experiences-results of a representative survey in Germany. Eur Child Adolesc Psychiatry. (2021) 35: 1–13. doi: 10.1007/s00787-021-01739-0
132. Lawson M, Piel MH, Simon M. Child maltreatment during the COVID-19 pandemic: consequences of parental job loss on psychological and physical abuse towards children. Child Abuse Negl. (2020) 110(Pt 2):104709. doi: 10.1016/j.chiabu.2020.104709
133. Sandri A, Di Vico IA, Riello M, Marotta A, Tinazzi M. The impact of recurrent Covid-19 waves on patients with Functional Movement Disorders: a follow-up study. Clin Park Relat Disord. (2022) 6:100139. doi: 10.1016/j.prdoa.2022.100139
134. Conte G, Baglioni V, Valente F, Chiarotti F, Cardona F. Adverse mental health impact of the COVID-19 lockdown in individuals with Tourette syndrome in Italy: an online survey. Front Psychiatry. (2020) 11:583744. doi: 10.3389/fpsyt.2020.583744
135. Mataix-Cols D, Ringberg H, Fernandez de la Cruz L. Perceived Worsening of Tics in Adult Patients with Tourette Syndrome after the COVID-19 Outbreak. Mov Disord Clin Pract. (2020) 7:725–6. doi: 10.1002/mdc3.13004
136. Termine C, Dui LG, Borzaga L, Galli V, Lipari R, Vergani M, et al. Investigating the effects of COVID-19 lockdown on Italian children and adolescents with and without neurodevelopmental disorders: a cross-sectional study. Curr Psychol. (2021). doi: 10.1007/s12144-021-02321-2
137. Buonsenso D, De Rose C, Mariotti P. Children experienced new or worsening tic issues when they were separated from their parents during the Italian COVID-19 lockdown. Acta Paediatr. (2021) 110:394–6. doi: 10.1111/apa.15684
138. Stone J, Smyth R, Carson A, Lewis S, Prescott R, Warlow C, et al. Systematic review of misdiagnosis of conversion symptoms and “hysteria”. BMJ. (2005) 331:989. doi: 10.1136/bmj.38628.466898.55
139. Stone J, Carson A, Duncan R, Coleman R, Roberts R, Warlow C, et al. Symptoms 'unexplained by organic disease' in 1144 new neurology out-patients: how often does the diagnosis change at follow-up? Brain. (2009) 132(Pt 10):2878–88. doi: 10.1093/brain/awp220
140. Faust J, Soman T. Psychogenic movement disorders in children: characteristics and predictors of outcome. Movement Disord. (2011) 26:S231. doi: 10.1177/0883073811422753
141. Gelauff J, Stone J. Prognosis of functional neurologic disorders. Handb Clin Neurol. (2016) 139:523–41. doi: 10.1016/B978-0-12-801772-2.00043-6
142. Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry. (2014) 85:220–6. doi: 10.1136/jnnp-2013-305321
143. Lidstone SC, Araujo R, Stone J, Bloem BR. Ten myths about functional neurological disorder. Eur J Neurol. (2020) 27:e62–e4. doi: 10.1111/ene.14310
144. Robinson S, Hedderly T. Novel psychological formulation and treatment of “Tic Attacks” in Tourette Syndrome. Front Pediatr. (2016) 4:46. doi: 10.3389/fped.2016.00046
145. Sachdev P, Chee KY, Wilson A. Tics status. Aust Nz J Psychiat. (1996) 30:392–6. doi: 10.3109/00048679609065004
146. Kious BM, Jimenez-Shahed J, Shprecher DR. Treatment-refractory Tourette Syndrome. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 70:227–36. doi: 10.1016/j.pnpbp.2016.02.003
147. Szejko N, Lombroso A, Bloch MH, Landeros-Weisenberger A, Leckman JF. Refractory Gilles de la Tourette Syndrome-Many Pieces That Define the Puzzle. Front Neurol. (2020) 11:589511. doi: 10.3389/fneur.2020.589511
148. Fernandes PT, Snape DA, Beran RG, Jacoby A. Epilepsy stigma: what do we know and where next? Epilepsy Behav. (2011) 22:55–62. doi: 10.1016/j.yebeh.2011.02.014
149. Zinner SH, Conelea CA, Glew GM, Woods DW, Budman CL. Peer victimization in youth with Tourette syndrome and other chronic tic disorders. Child Psychiatry Hum Dev. (2012) 43:124–36. doi: 10.1007/s10578-011-0249-y
150. Debes N, Hjalgrim H, Skov L. The presence of attention-deficit hyperactivity disorder (ADHD) and obsessive-compulsive disorder worsen psychosocial and educational problems in Tourette syndrome. J Child Neurol. (2010) 25:171–81. doi: 10.1177/0883073809336215
Keywords: tourette, tic, functional tic, functional movement disorders, mass psychogenic illness, TikTok, COVID-19, tic-like behavior
Citation: Martindale JM and Mink JW (2022) The Rise of Functional Tic-Like Behaviors: What Do the COVID-19 Pandemic and Social Media Have to Do With It? A Narrative Review. Front. Pediatr. 10:863919. doi: 10.3389/fped.2022.863919
Received: 27 January 2022; Accepted: 24 June 2022;
Published: 11 July 2022.
Edited by:
Amanda Ludlow, University of Hertfordshire, United KingdomReviewed by:
Rita Barone, University of Catania, ItalyCopyright © 2022 Martindale and Mink. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaclyn M. Martindale, am1hcnRpbmRAd2FrZWhlYWx0aC5lZHU=
 Jaclyn M. Martindale
Jaclyn M. Martindale Jonathan W. Mink
Jonathan W. Mink