- 1Almazov National Medical Research Centre, Saint Petersburg, Russia
- 2Saint-Petersburg State Pediatric Medical University, Saint Petersburg, Russia
It is known that the SARS-CoV-2 virus may cause neurologic damage. Rapid-onset obesity, hypoventilation, hypothalamus dysfunction, and autonomic dysregulation (ROHHAD) syndrome is a disease of unknown etiology with a progressive course and unclear outcomes. The etiology of ROHHAD syndrome includes genetic, epigenetic, paraneoplastic, and immune-mediated theories, but to our knowledge, viral-associated cases of the disease have not been described yet. Here we present the case of a 4-year-old girl who developed a ROHHAD syndrome-like phenotype after a COVID-19 infection and the results of 5 months of therapy. She had COVID-19 pneumonia, followed by electrolyte disturbances (hypernatremia and hyperchloremia), hypocorticism and hypothyroidism, central hypoventilation—requiring prolonged assisted lung ventilation—bulimia, and progressive obesity with hypertriglyceridemia, dyslipidemia, hyperuricemia, and hyperinsulinemia. The repeated MRI of the brain and hypothalamic–pituitary region with contrast enhancement showed mild post-hypoxic changes. Prader–Willi/Angelman syndrome as well as PHOX2B-associated variants was ruled out. Treatment with non-steroidal anti-inflammatory drugs and monthly courses of intravenous immunoglobulin led to a dramatic improvement. Herein the first description of ROHHAD-like syndrome is timely associated with a previous COVID-19 infection with possible primarily viral or immune-mediated hypothalamic involvement.
Introduction
COVID-19 viral infection may trigger immune dysregulation affecting different targets. It is known that COVID-19 may cause severe neurologic damage, ranging from anosmia, headache, and memory disturbances to several cases of encephalitis (1–3).
Rapid-onset obesity, hypoventilation, hypothalamus dysfunction, and autonomic dysregulation (ROHHAD) syndrome is a rare and complicated condition presenting in previously healthy children. It was first described in 1965 (4, 5). However, its etiology and pathogenesis remain unknown. Usually, it has a progressive course and unclear outcomes. There are several hypotheses about the etiology of this syndrome, including genetic (6–11), epigenetic (7, 12–14), and paraneoplastic theories (4, 15), but autoimmune etiology is becoming more widely discussed lately (6–9, 11–13, 16–24). Viral-associated cases of the ROHHAD syndrome have not been described yet.
Herein we describe the case of a 4-year-old girl who presented with ROHHAD syndrome-like phenotype timely associated with a previous COVID-19 infection. To our knowledge, this is the first description of ROHHAD-like phenotype timely associated with COVID-19 infection.
Case Description
A 4-year-old girl was transferred to our hospital with a suspicion of ROHHAD syndrome. She was tall for her age since birth (53 cm, +2.1 SD) and obese since 2 years of age (BMI, 21.0 kg/m2, +3.4 SD) but otherwise doing well until 3.5 years (Figures 1A–C).
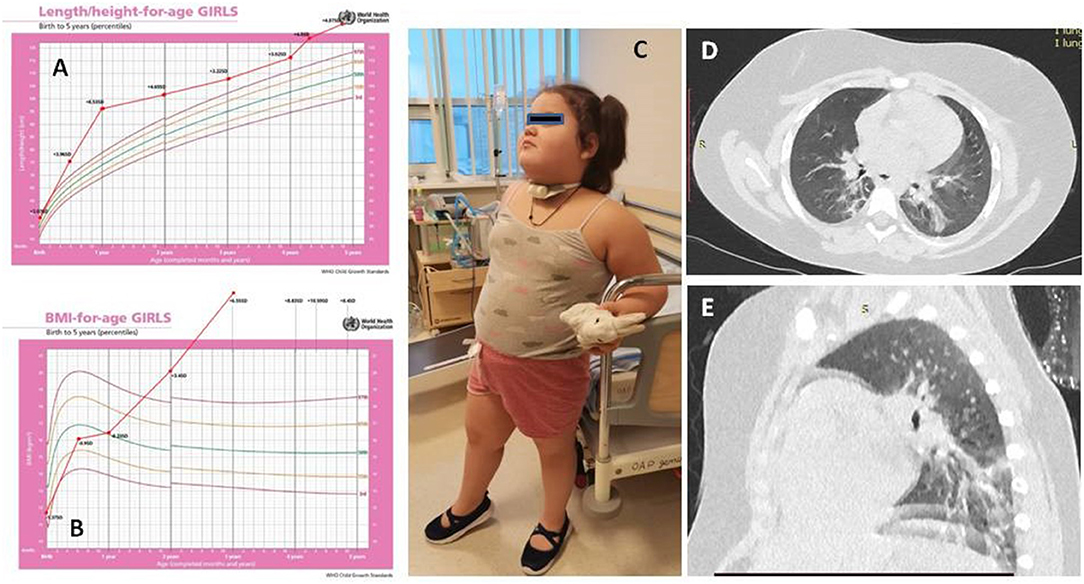
Figure 1. The dynamics of height (A) and body mass index (B) of the patient. Patient's picture (C). Chest CT (D,E).
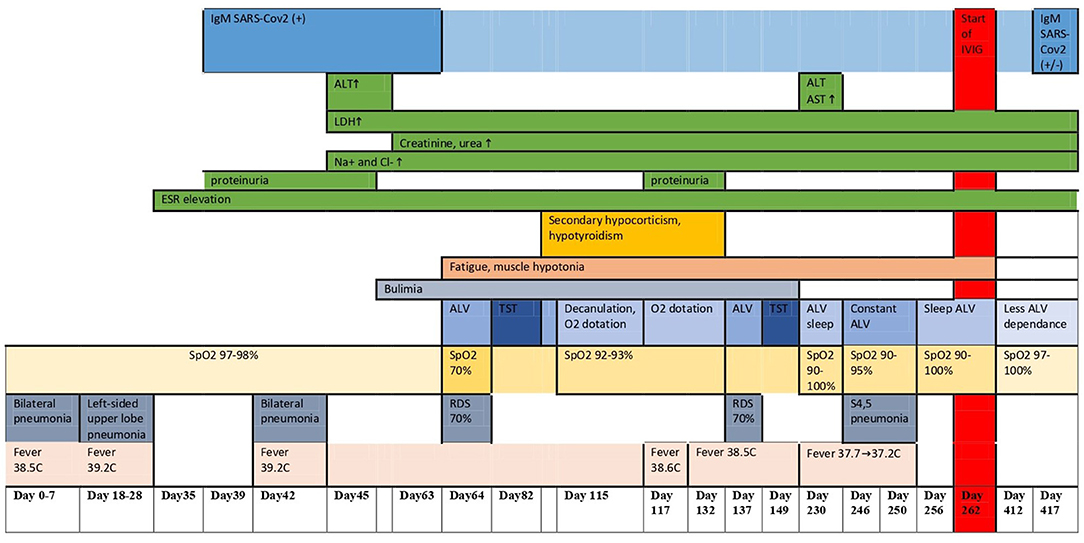
At 3.5 years, 3 weeks after her family contracted COVID-19, the girl had pneumonia (day 0). Nasal swab PCR for SARS-CoV-2 was not done at this timepoint. She recovered completely after a course of josamycin at 500 mg twice a day for 7 days. After 3 weeks, another episode of respiratory infection happened (day 18) (at the same time, half of the kindergarten group had respiratory symptoms, and a teacher had confirmed severe COVID-19 pneumonia). The patient had positive IgM against SARS-CoV-2, but the nasal swab PCR for SARS-CoV-2 was negative. 4 days of intravenous dexamethasone, accompanied with antibiotics, was given (Figures 1D,E). During the following 6 months (days 42, 64, and 137), she had three more episodes of respiratory distress (CT described the ground-glass opacities as affecting 70% of the lungs), requiring assisted lung ventilation through a tracheostomy tube, with febrile fever of up to 39.2°C and with good response to corticosteroids and antibiotics. After a while, the patient developed strabismus (days 64–80). The girl had constant hyperthermia within 37.6°C without laboratory signs of systemic inflammation.
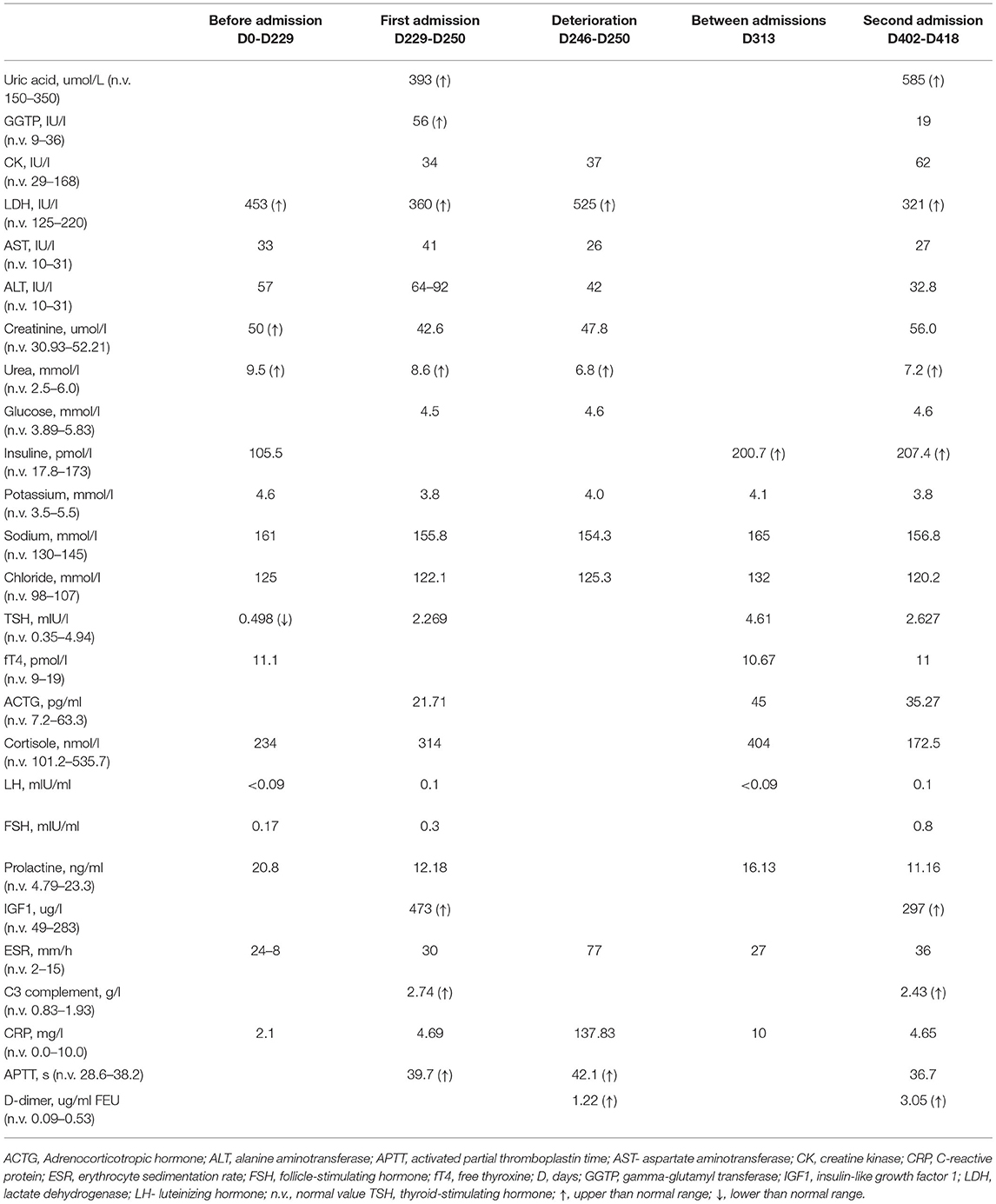
The patient had features of metabolic syndrome: increased insulin level −207.4 pmol/L (n.v. = 17.8–173), with normal levels of blood glucose and HbA1c, dyslipidemia—triglycerides at 6.3 mmol/L (n.v. = 0.0–1.69), VLDL at 2.89 mmol/L (n.v. = 0.1–1.0), HDL at 0.63 mmol/L (n.v. = 1.04–1.55), and hyperuricemia at 585 mmol/L (n.v. = 150–350) associated with obesity. She had elevated IGF1 at 473–297 mcg/L (n.v. = 49–283). By the provided documents, the girl used to have secondary hypocorticism and hypothyroidism, which resolved over time (days 80–132). Her endocrine function was not evaluated before the COVID-19 infection since she was considered healthy. Proteinuria was intermittent since day 39, and electrolyte disorders persisted since day 46 [hypernatremia 155.8–162 mmol/L (n.v. 130–145 mmol/l), hyperchloremia 116.9–129.6 mmol/L (n.v. 98–107 mmol/l)]. At around the same time, central hypoventilation and bulimia started (days 45–63), explaining the recurrent chest infections.
The antinuclear antibodies, anti-MPO, and anti-PR3 antibodies were negative; immunoglobulins A, M, and G were normal, while the IL6 (10.49 pg/ml, n.v. <7) and C3 complement components (2.74 g/l, n.v. 0.83–1.93) were slightly elevated.
SARS-CoV-2 IgM remained positive over time, decreasing to borderline after 1 year; the erythrocyte sedimentation rate (ESR) and D-dimer remained elevated: ESR, 30 → 70 → 36 mm/h (n.v. = 2–15) and D-dimer, 1.22 → 0.72 → 3.05 mcg/ml (n.v. = 0.09–0.53).
At first admission to our hospital (days 229–268), the girl had hypercapnia (tcCO2, 40–60 mmHg); SpO2 was 97–100% while she was awake and 92–96% during sleep on assisted lung ventilation, with episodic desaturations to 72–86%. Unfortunately, polysomnography was not available until day 412, when central hypoventilation was proven. On admission, 7.5 months after the disease onset, she continued being obese (BMI, 33.3 kg/m2) and required assisted lung ventilation through a tracheostomy tube while asleep. She could sit without support, hold hands above her head for more than 10 s, roll prone to supine and supine to prone, and stand for a short time with support. The results of the Denver Development Screening test were as follows: MQ, 0.32 (n.v. >0.75) and DQ = 0.7 (n.v. ≤ 0.7). Her behavior issues include short periods of “aggression” directed at other people. Cranial innervation was intact despite periodically alternating divergent strabismus (as reported by the mother, the child had transient ptosis at the onset of the disease). The muscle tone was diffusely reduced, without asymmetry, the muscle strength [according to the Medical Research Council (MRC) Scale] was reduced to three points, and the tendon reflexes were alive. There were no meningeal and cerebral symptoms; the coordinating tests were optimal.
A repeat MRI of the brain and hypothalamic–pituitary region with contrast showed mild post-hypoxic changes. In contrast, COVID-associated acute disseminated encephalomyelitis, corpus callosum lesion, brain tumor, post-hemorrhage lesion, and ischemic stroke were ruled out as well as known immune-mediated CNS disorders and primary disease CNS–angiitis. The neurophysiological study did not suggest acute inflammatory demyelinating polyneuropathy and inflammatory or congenital myopathy. The electroencephalography result during wakefulness showed age-appropriate bioelectrical activity.
The chest CT showed interstitial changes and atelectasis in both lungs, which are attributed to hypoventilation.
The echocardiography and electrocardiography (ECG) results were normal, with normal ejection fraction and no signs of pulmonary hypertension, systolic or diastolic dysfunction, or other cardiac issues. During 24-h ECG and arterial pressure monitoring, reduction of heart rate variability and diastolic arterial hypotension were detected. Diastolic arterial hypotension, reduction of heart rate variability, constant hyperthermia within 37.6°C, strabismus, and central hypoventilation were considered autonomic dysregulation.
The karyotyping showed a normal female karyotype (46, XX). The Sanger sequencing and multiplex ligation-dependent probe amplification (MLPA) technology using SALSA MLPA Probemix P318 kit (MRC Holland, The Netherlands) did not find mutations in the PHOX2B gene. As a result, polyalanine repeat expansion mutations, non-polyalanine repeat mutations, and whole-gene or exon-specific deletions were excluded. The results of FISH with locus-specific DNA probe Prader-Willi/Angelman SNRPN (15q11)/PML (15q24) was also normal. Whole-exome sequencing is in progress. The clinical dynamics, laboratory findings, and imaging allowed us to define the condition as a ROHHAD-like syndrome. The disease course is depicted in Figure 2 and Table 1.
Family History
The Caucasian girl of Russian nationality is from non-consanguineous marriage. Both parents are tall and obese (mother's height, 176 cm; weight, 141 kg; BMI, 45.5 kg/m2; father's height, 192 cm; weight, 117 kg; BMI, 31.7 kg/m2). The girl's grandparents, the girl's uncle from her mother's side, and an elder brother are obese, while her younger brother, who is 1.5 years old, is tall for his age but not obese yet. Most of the relatives developed diabetes mellitus type 2 after 40–45 years of age (Figure 3).
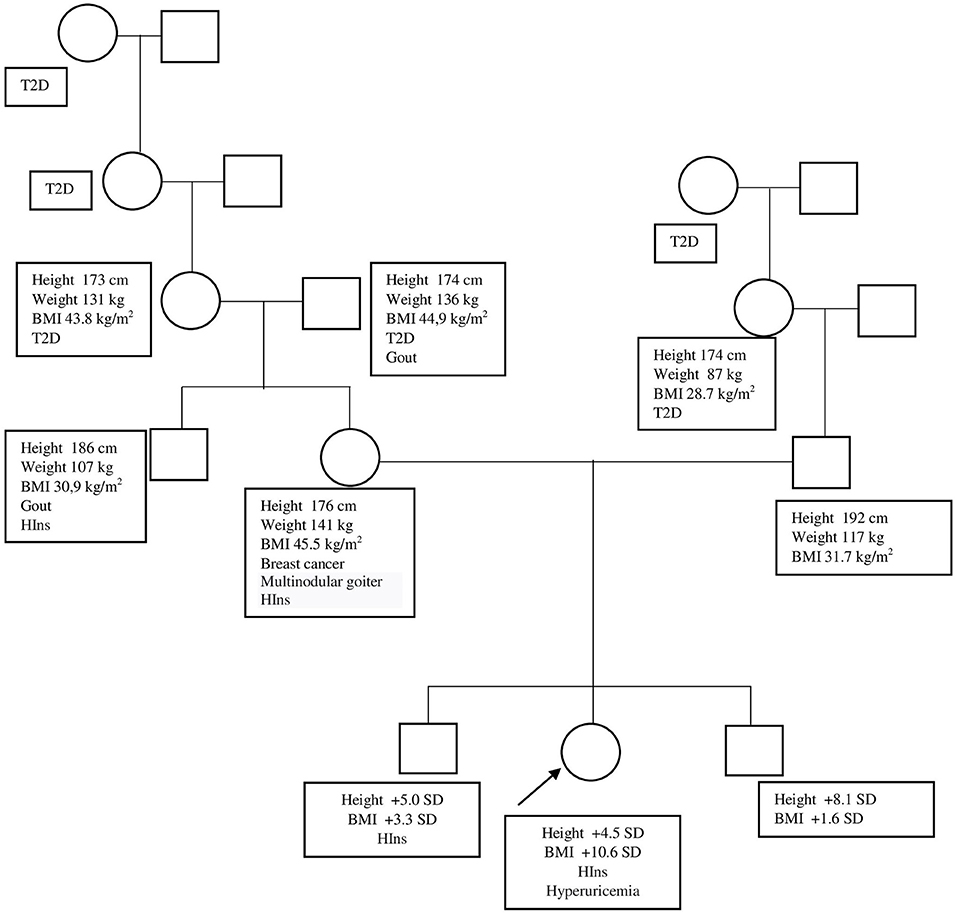
Figure 3. Patient's genealogic tree. BMI, body mass index; HIns, hyperinsulinism; SD, standard deviation; T2D, type 2 diabetes.
Therapy, Progress, and Outcomes
The anti-inflammatory treatment was started from day 262 [ibuprofen at 200 mg three times a day (11 mg/kg/day) for 2 months and monthly intravenous immunoglobulin (IVIG) at 1 g/kg of due weight, divided into two 2-h infusions once a day during 2 days] with dramatic clinical improvement.
After 5 months of therapy, the Denver Development Screening test results were MQ = 0.62 and DQ = 0.72. She had minor muscle hypotonia, and her tendon remained normal. Muscle strength (MRC Scale) was five points in the proximal upper limbs and lower limbs and reduced to four points in the distal upper limbs. She can now walk and run without support, do yoga and pony riding, and climb a small climbing slide. Though the girl remains obese, her BMI decreased (day 229 + 10.59 SD; day 412 + 8.4 SD). The requirement for ventilation decreased (assisted lung ventilation through a tracheostomy tube only during night sleep), and central hypoventilation persists during non-rapid eye movement sleep (Figure 4). The inflammation laboratory signs improved as well. Her BMI slightly decreased at 33.0 (+8.4 SD). However, her ESR remained elevated (36 mm/h).
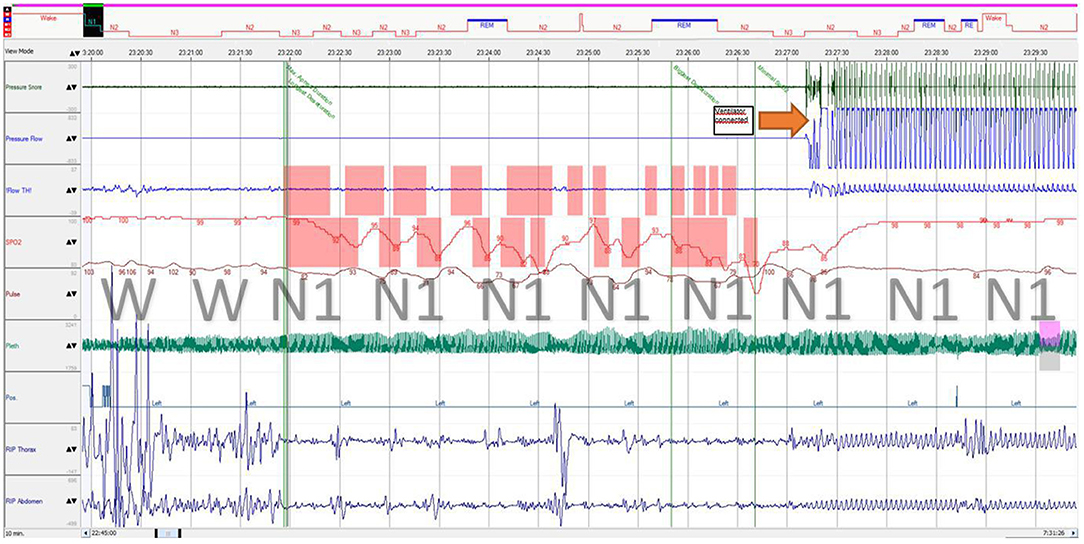
Figure 4. Polysomnography fragment of sleep onset with central breathing impairment followed by assisted ventilation. Immediately after sleep onset, the patient developed breathing movement amplitude decrease (both thoracic and abdominal) with a “periodic breathing”-like pattern associated with progressive desaturation and hypercapnia, which is considered central (airflow was not obtained from the tracheostomy tube during self-breathing). REM, stage rapid eye movement; N1, stage 1 non-rapid eye movement; N2, stage 2 non-rapid eye movement; N3, stage 3 non-rapid eye movement; RIP, respiratory inductance plethysmography; Flow TH, oral thermistor flow sensor; Pleth, photoplethysmography.
We planned to continue IVIG for another 3 months, following teleconsultation or in-hospital admission for clinical, instrumental, and laboratory evaluation and further treatment decisions (anti-inflammatory therapy discontinuation, prolongation, or escalation and assessment of ventilation needs).
Discussion
The girl with a family history of obesity and redundant height, who had the same trajectory of excessive weight and height gain from birth, presented with additional ROHHAD-like symptoms temporarily related to COVID-19 infection. Though other viruses were not searched for, we consider the initial episode as a COVID-19 probable case based on epidemiological, clinical, and serological data. The patient had typical features of the ROHHAD syndrome: central hypoventilation, electrolyte and endocrine disorders, and signs of autonomic dysregulation (febrile temperature without systemic inflammation, arterial hypotension, and strabismus). Considering the girl's physical development prior to the disease family history, we speculate that obesity and tallness might have a genetic nature and do not seem to be wholly associated with respiratory and hypothalamic dysfunctions. On the other hand, a gap between obesity and other symptoms has been reported in some ROHHAD cases (5), and the SARS-CoV-2 virus might have been triggered following ROHHAD symptom presentation. Thus, the diagnosis of classical ROHHAD syndrome seems doubtful. The fluctuating course of the disease with improvement on non-steroidal anti-inflammatory drugs and corticosteroid therapy maintenance of humoral activity (increased ESR and C3) allows assuming the permanent course of the inflammatory process with immune-mediated damage of the hypothalamic–pituitary region.
The amount of data on nervous system involvement in COVID-19 survivors is increasing. Neurologic symptoms can be divided into five major groups: (i) encephalopathies with delirium/psychosis and cognitive impairment; (ii) inflammatory CNS syndromes including encephalitis, acute disseminated encephalomyelitis, and isolated myelitis; (iii) ischemic stroke due to a pro-thrombotic state; (iv) peripheral neurological disorders, including Guillain–Barré syndrome; and (v) other uncategorized syndromes, including autonomic dysfunction with fever, dyspnea, fatigue, and syncope, like in our case (1–3, 25–28). Some of the neurologic symptoms manifested immediately after or even before the lung disease, while in other cases, neurologic symptoms were developed about a month after the disease onset or even later (29). Interestingly, only some of the patients with symptoms of encephalitis had MRI changes and markers of inflammation in the cerebrospinal fluid, which made the diagnosis of COVID-19-associated CNS involvement difficult (1, 29–33). As with other neurotropic viruses, the fundamental question for SARS-CoV-2 infection concerns the relative contribution of viral infection vs. host response to the subsequent damage (26).
We could not find data of any single case of detection of SARS-CoV-2 PCR from cerebrospinal fluid (CSF) samples in pediatric patients. Concerns on the presence of SARS-CoV-2 in CSF include the absence of validated tests and appropriate timing of lumbar puncture (33).
In the analysis of CSF of adult patients with SARS-CoV-2 infection and neurological manifestations, SARS-CoV-2 RNA in CSF was detected in 2 of 58 cases (34).
The delayed time of presentation and the presence of autoantibodies let us speculate that, at least, in some cases the virus does not affect CNS itself but promotes a secondary autoinflammatory process, similar to Guillain–Barré syndrome, after COVID-19 infection (30, 35, 36).Since April 2020, a new multisystem inflammatory syndrome in children (MIS-C) was related to SARS-CoV-2 infection (37). More than 186 patients with MIS-C were described so far (38). The majority had cardiovascular system involvement and respiratory failure. Most of them had inflammatory laboratory picture (elevated ESR, C-reactive protein, D-dimer, and ferritin levels, anemia, thrombocytopenia, neutrophilia) (38). Neurological issues were described as well (33, 37). In their systematic review of neurological complications in pediatric patients with SARS-CoV-2 infection, Siracusa et al. (37) showed that most of the cases of CNS involvement in COVID-19 patients, including headache, altered mental status, seizure, muscular weakness, and meningism, happened in the course of the MIS-C. The minority of neurological issues were secondary to cerebrovascular involvement, and only sporadic cases had other reasons. Presuming the inflammatory pathogenesis of the condition, immunosuppressive treatment, including glucocorticosteroids, intravenous immunoglobulin, and anakinra, was tried with good effect (33), just as it happened in our clinical case. Nevertheless, unlike our case, only a short course of immunosuppressive treatment was enough (37).
According to MRI, the temporal lobes, cerebellum, thalami, hippocampus, and pons can be involved (1, 29, 31, 32). However, no reports of hypothalamus or hypophysis involvement have been previously published.
The SARS-CoV-2 virus, causing COVID-19, enters in pneumocytes through binding with angiotensin-converting enzyme 2 (ACE2) receptors. The ACE2 receptors are located in different tissues, including the lungs, pancreas, thyroid, testis, ovary, adrenal glands, pituitary, and hypothalamus. Theoretically, all the abovementioned tissues might be targeted by the SARS-CoV-2 virus with inflammation development and signs of organ involvement (32). SARS-CoV-2 has a lot in common with SARS-CoV, which is tropic to the hippocampus and hypothalamic region. Viral particles were found in endothelial cells and neural tissue in the autopsies of SARS-CoV patients (39). However, in SARS-CoV survivors, only central hypercorticism and central hypothyroidism have already been described (29, 32). Hypothalamic involvement might be presented with central diabetes insipidus, hypopituitarism, hyperprolactinemia, follicle-stimulating, luteinizing, adrenocorticotropic, thyroid-stimulating, and growth hormone deficiencies, and disorders of temperature regulation, sleep rhythm, emotions, and behavior (31). The clinical course of hypothalamitis perfectly corresponds to the classical ROHHAD syndrome. The direct viral involvement of the hypothalamic–pituitary region by SARS-CoV-2 could also be supposed, though it cannot be confirmed in the described case.
Though the etiology and pathogenesis of ROHHAD syndrome remain unknown, an autoimmune theory is widely discussed (6–9, 11–13, 16–24). There are three findings to support this theory: (i) presence of oligoclonal bands (8, 17, 24), B-lymphocytes (13), and specific anti-hypothalamus and anti-pituitary autoantibodies in the CSF (9); (ii) lymphocytic infiltration in the hypothalamus and midbrain (19, 23); and (iii) the association with other autoimmune disorders like celiac disease (13) and autoimmunity-predisposing HLA alleles (DQB1*0201, DQB1*0202, or DQB1*0302) (18).
However, all these findings were discovered in some, but not all, ROHHAD patients, which interfere with the heterogeneity of this syndrome. On the other hand, with COVID-19 encephalitis, MRI and CSF changes were not always present either (1, 29–32). In some COVID-19 encephalitis cases as well, some of the ROHHAD patients were treated with corticosteroids and IVIG with temporary improvement. However, the best results were obtained with high-dose cyclophosphamide treatment (4, 9, 13, 16, 17, 21, 24, 40, 41).
In our case, the timing of the autonomic dysregulation and endocrine disorders, which is approximately 4 weeks after the possible COVID-19 infection, may indicate an immune-mediated mechanism of the disease. The effectiveness of the anti-inflammatory therapy also support this theory.
The limitations of this case report can be considered as the absence of the initial polysomnography data, which does not allow us to indicate the timing of central hypoventilation onset, and the absence of the CSF analysis data (lumbar puncture was not done at the local hospital and was considered to be irrational at 7 months after symptom onset).
Conclusion
This case report not only expands available data on the clinical manifestations of COVID-19 in a pediatric population but can also help to understand ROHHAD syndrome's nature and impact on its treatment strategies. Some patients with ROHHAD syndrome might have a viral or immune-mediated nature, so immune-modulating therapy (especially IVIG) might be a promising option. The case may not be conclusively attributed to COVID-19 infection and, at the same time, is rather a ROHHAD-like than ROHHAD syndrome itself. The case presented seems to be a subject for further understanding and follow-up.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was financially supported by the Ministry of Science and Higher Education of the Russian Federation (agreement number 075-15-2020-901).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor OK declared past co-authorships with one of the authors MK and the absence of any ongoing collaboration with any of the authors.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain J Neurol. (2020) 143:3104–20. doi: 10.1093/brain/awaa240
2. Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. (2020) 70:311–22. doi: 10.33588/rn.7009.2020179
3. Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID': rationale, physiology and management strategies. Clin Med Lond Engl. (2021) 21:e63–7. doi: 10.7861/clinmed.2020-0896
4. Sanklecha M, Sundaresan S, Udani V, ROHHAD. syndrome: the girl who forgets to breathe. Indian Pediatr. (2016) 53:343–4. doi: 10.1007/s13312-016-0849-5
5. Ize-Ludlow D, Gray JA, Sperling MA, Berry-Kravis EM, Milunsky JM, Farooqi IS, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation presenting in childhood. Pediatrics. (2007) 120:e179–88. doi: 10.1542/peds.2006-3324
6. Chew HB, Ngu LH, Keng WT. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation and autonomic dysregulation (ROHHAD): a case with additional features and review of the literature. Case Rep. (2011) 2011:bcr0220102706–bcr0220102706. doi: 10.1136/bcr.02.2010.2706
7. Barclay SF, Rand CM, Gray PA, Gibson WT, Wilson RJA, Berry-Kravis EM, et al. Absence of mutations in HCRT, HCRTR1 and HCRTR2 in patients with ROHHAD. Respir Physiol Neurobiol. (2016) 221:59–63. doi: 10.1016/j.resp.2015.11.002
8. Siraz ÜG, Okdemir D, Direk G, Akin L, Hatipoglu N, Kendirci M, et al. A rare cause of hypothalamic obesity, rohhad syndrome: 2 cases. J Clin Res Pediatr Endocrinol. (2018) ; doi: 10.4274/jcrpe.0027 (accessedNovember 29, 2021).
9. Giacomozzi C, Guaraldi F, Cambiaso P, Niceta M, Verrillo E, Tartaglia M, et al. Anti-Hypothalamus and anti-pituitary auto-antibodies in ROHHAD syndrome: additional evidence supporting an autoimmune etiopathogenesis. Horm Res Paediatr. (2019) 92:124–32. doi: 10.1159/000499163
10. Lee JM, Shin J, Kim S, Gee HY, Lee JS, Cha DH, et al. Rapid-onset obesity with hypoventilation, hypothalamic, autonomic dysregulation, and neuroendocrine tumors (ROHHADNET) syndrome: a systematic review. BioMed Res Int. (2018) 2018:1–17. doi: 10.1155/2018/1250721
11. Rand CM, Patwari PP, Rodikova EA, Zhou L, Berry-Kravis EM, Wilson RJA, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation: analysis of hypothalamic and autonomic candidate genes. Pediatr Res. (2011) 70:375–8. doi: 10.1203/PDR.0b013e318229474d
12. Jalal Eldin AW, Tombayoglu D, Butz L, Affinati A, Meral R, Ontan MS, et al. Natural history of ROHHAD syndrome: development of severe insulin resistance and fatty liver disease over time. Clin Diabetes Endocrinol. (2019) 5:9. doi: 10.1186/s40842-019-0082-y
13. Patwari PP, Rand CM, Berry-Kravis EM, Ize-Ludlow D, Weese-Mayer DE. Monozygotic twins discordant for ROHHAD phenotype. Pediatrics. (2011) 128:e711–5. doi: 10.1542/peds.2011-0155
14. Aljabban L, Kassab L, Bakoura NA, Alsalka MF, Maksoud I. Rapid-onset obesity, hypoventilation, hypothalamic dysfunction, autonomic dysregulation and neuroendocrine tumor syndrome with a homogenous enlargement of the pituitary gland: a case report. J Med Case Reports. (2016) 10:328. doi: 10.1186/s13256-016-1116-z
15. Barclay SF, Rand CM, Borch LA, Nguyen L, Gray PA, Gibson WT, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD): exome sequencing of trios, monozygotic twins and tumours. Orphanet J Rare Dis. (2015) 10:103. doi: 10.1186/s13023-015-0314-x
16. Jacobson LA, Rane S, McReynolds LJ, Steppan DA, Chen AR, Paz-Priel I. Improved behavior and neuropsychological function in children with ROHHAD after high-dose cyclophosphamide. Pediatrics. (2016) 138:e20151080. doi: 10.1542/peds.2015-1080
17. Chow C, Fortier MV, Das L, Menon AP, Vasanwala R, Lam JCM, et al. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation (ROHHAD) syndrome may have a hypothalamus–periaqueductal gray localization. Pediatr Neurol. (2015) 52:521–5. doi: 10.1016/j.pediatrneurol.2014.11.019
18. Pontual LD, Trochet D, Caillat-Zucman S, Shenab OAA, Bougneres P, Crow Y, et al. Delineation of late onset hypoventilation associated with hypothalamic dysfunction syndrome. Pediatr Res. (2008) 64:689–94. doi: 10.1203/PDR.0b013e318187dd0e
19. North KN, Ouvrier RA, McLean CA, Hopkins IJ. Idiopathic hypothalamic dysfunction with dilated unresponsive pupils: report of two cases. J Child Neurol. (1994) 9:320–5. doi: 10.1177/088307389400900320
20. Armangue T, Petit-Pedrol M, Dalmau J. Autoimmune encephalitis in children. J Child Neurol. (2012) 27:1460–9. doi: 10.1177/0883073812448838
21. Abaci A, Catli G, Bayram E, Koroglu T, Olgun HN, Mutafoglu K, et al. A case of rapid-onset obesity with hypothalamic dysfunction, hypoventilation, autonomic dysregulation, and neural crest tumor: rohhadnet syndrome. Endocr Pract. (2013) 19:e12–6. doi: 10.4158/EP12140.CR
22. Cemeroglu AP, Eng DS, Most LA, Stalsonburg CM, Kleis L. Rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome and celiac disease in a 13-year-old girl: further evidence for autoimmunity? J Pediatr Endocrinol Metab. (2016) ;29. doi: 10.1515/jpem-2015-0129 (accessed November 29, 2021).
23. Sethi K, Lee YH, Daugherty LE, Hinkle A, Johnson MD, Katzman PJ, et al. ROHHADNET syndrome presenting as major behavioral changes in a 5-year-old obese girl. Pediatrics. (2014) 134:e586–9. doi: 10.1542/peds.2013-2582
24. Sartori S, Priante E, Pettenazzo A, Marson P, Suppiej A, Benini F, et al. Intrathecal synthesis of oligoclonal bands in rapid-onset obesity with hypothalamic dysfunction, hypoventilation, and autonomic dysregulation syndrome: new evidence supporting immunological pathogenesis. J Child Neurol. (2014) 29:421–5. doi: 10.1177/0883073812469050
25. Roy D, Ghosh R, Dubey S, Dubey MJ, Benito-León J, Kanti Ray B. Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can J Neurol Sci J Can Sci Neurol. (2021) 48:9–24. doi: 10.1017/cjn.2020.173
26. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, et al. Neurological associations of COVID-19. Lancet Neurol. (2020) 19:767–83. doi: 10.1016/S1474-4422(20)30221-0
27. Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19–associated encephalitis mimicking glial tumor. World Neurosurg. (2020) 140:46–8. doi: 10.1016/j.wneu.2020.05.194
28. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. (2020) 88:945–6. doi: 10.1016/j.bbi.2020.04.017
29. McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. (2020) 109:94. doi: 10.1016/j.pediatrneurol.2020.04.013
30. Grimaldi S, Lagarde S, Harlé JR, Boucraut J, Guedj E. Autoimmune encephalitis concomitant with SARS-CoV-2 infection: insight from 18F-FDG PET imaging and neuronal autoantibodies. J Nucl Med Off Publ Soc Nucl Med. (2020) 61:1726–9. doi: 10.2967/jnumed.120.249292
31. Wei Q, Yang G, Lue Z, Dou J, Zang L, Li Y, et al. Clinical aspects of autoimmune hypothalamitis, a variant of autoimmune hypophysitis: experience from one center. J Int Med Res. (2019) 48:0300060519887832. doi: 10.1177/0300060519887832
32. Pal R, Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. (2020) 2:1–5. doi: 10.1007/s40618-020-01276-8
33. Lin JE, Asfour A, Sewell TB, Hooe B, Pryce P, Earley C, et al. Neurological issues in children with COVID-19. Neurosci Lett. (2021) 743:135567. doi: 10.1016/j.neulet.2020.135567
34. Espíndola OM, Brandão CO, Gomes YCP, Siqueira M, Soares CN, Lima MASD, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. (2021) 102:155–62. doi: 10.1016/j.ijid.2020.10.044
35. Davido B, Seang S, Tubiana R, de Truchis P. Post–COVID-19 chronic symptoms: a postinfectious entity? Clin Microbiol Infect. (2020) 26:1448–9. doi: 10.1016/j.cmi.2020.07.028
36. Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. (2021) 268:751–7. doi: 10.1007/s00415-020-10108-x
37. Siracusa L, Cascio A, Giordano S, Medaglia AA, Restivo GA, Pirrone I, et al. Neurological complications in pediatric patients with SARS-CoV-2 infection: a systematic review of the literature. Ital J Pediatr. (2021) 47:123. doi: 10.1186/s13052-021-01066-9
38. Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, et al. Multisystem inflammatory syndrome in US. children and adolescents. N Engl J Med. (2020)383:334–46. doi: 10.1056/NEJMoa2021680
39. Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis Off Publ Infect Dis Soc Am. (2005) 41:1089–96. doi: 10.1086/444461
40. Ibáñez-Micó S, Marcos Oltra AM, de Murcia Lemauviel S, Ruiz Pruneda R, Martínez Ferrández C, Domingo Jiménez R. Síndrome ROHHAD (obesidad de rápida progresión, disfunción hipotalámica, hipoventilación y disregulación autonómica). Presentación de un caso y revisión de la literatura Neurología. (2017) 32:616–22. doi: 10.1016/j.nrl.2016.04.008
Keywords: ROHHAD-syndrome, obesity, central hypoventilation, hypothalamus dysfunction, hypocorticism, autonomic dysregulation, COVID-19, SARS-CoV-2
Citation: Artamonova IN, Petrova NA, Lyubimova NA, Kolbina NY, Bryzzhin AV, Borodin AV, Levko TA, Mamaeva EA, Pervunina TM, Vasichkina ES, Nikitina IL, Zlotina AM, Efimtsev AY and Kostik MM (2022) Case Report: COVID-19-Associated ROHHAD-Like Syndrome. Front. Pediatr. 10:854367. doi: 10.3389/fped.2022.854367
Received: 13 January 2022; Accepted: 18 February 2022;
Published: 31 March 2022.
Edited by:
Ozgur Kasapcopur, Istanbul University-Cerrahpasa, TurkeyReviewed by:
Zeynep Siklar, Ankara University, TurkeyOvgu Kul Cinar, Great Ormond Street Hospital for Children NHS Foundation Trust, United Kingdom
Copyright © 2022 Artamonova, Petrova, Lyubimova, Kolbina, Bryzzhin, Borodin, Levko, Mamaeva, Pervunina, Vasichkina, Nikitina, Zlotina, Efimtsev and Kostik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail M. Kostik, a29zdC1taWtoYWlsQHlhbmRleC5ydQ==; bWlraGFpbC5rb3N0aWtAZ21haWwuY29t
 Irina N. Artamonova1
Irina N. Artamonova1 Natalia A. Petrova
Natalia A. Petrova Natalia Yu Kolbina
Natalia Yu Kolbina Elena S. Vasichkina
Elena S. Vasichkina Mikhail M. Kostik
Mikhail M. Kostik