
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 01 March 2022
Sec. Pediatric Surgery
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.853517
This article is part of the Research TopicCurrent Advances in Pediatric SurgeryView all 32 articles
Congenital abdominal aortic aneurysm is a rare disease with unknown etiology, and the common symptoms are abdominal pulsatile mass and pain caused by aneurysm rupture. The disease has a high mortality rate and fewer reports of surgical treatment. Here, we present a case of an idiopathic congenital abdominal aortic aneurysm. A 4-year-old boy had an abdominal pulsatile mass, and computed tomography angiography revealed an isolated infrarenal abdominal aortic aneurysm. To prevent rupture of the aneurysm, we repaired the aneurysm with artificial graft transplantation. No genetic mutation of the known congenital aneurysmal diseases was found in the whole-exome sequencing of the patient and his parents. There was no graft obstruction, and the patient grew well 40 months after surgery. Open surgery is the best treatment for idiopathic congenital abdominal aortic aneurysms. Surgical details such as timing and graft selection need to be further explored.
Abdominal aortic aneurysms in children are very rare. The common causes are congenital connective tissue disorders, vasculitis, traumatic umbilical artery intubation, and infection, while congenital abdominal aortic aneurysms (cAAAs) is rarely reported and has an unknown etiology and high mortality. This article reports the case of a 4-year-old child with isolated cAAA and the results of a 40-month follow-up of open surgery.
In June 2018, a 4-year-old boy was hospitalized because of the discovery of a left abdominal pulsatile mass for 2 months. Computed tomography angiography (CTA) revealed an isolated infrarenal AAA with a maximum diameter of 67 mm (Figure 1). The patient was 112 cm tall and weighs 23 kg and he had no family history of aneurysmal disease, connective tissue disorders, a history of trauma, umbilical cannulation, and infection. Laboratory tests revealed no abnormalities, and blood pressure was normal.
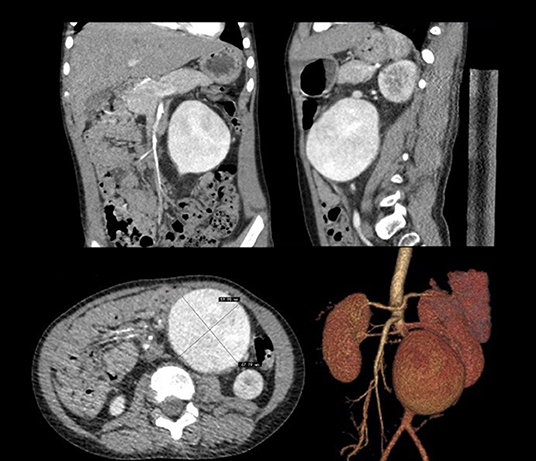
Figure 1. Computerized tomography angiography demonstrated isolated infrarenal abdominal aortic aneurysm.
In July 2018, we repaired the AAA using open surgery to prevent aneurysm rupture. When we blocked the blood flow at both ends of the aneurysm and opened the aneurysm through a midline incision, we found that the intima of the aneurysm was smooth without thrombus. We then used a 10-mm Dacron aorto-aortic tube graft to replace the AAA; the graft was oversized by 6 cm and formed a “C” shape to allow aortic growth. The aneurysmal sac was wrapped around the graft to avoid aortoduodenal fistula. The patient recovered well after the operation. Whole-exome sequencing of the boy and his parents revealed no genetic mutations of the known congenital aneurysmal diseases. The patients had frequent follow-ups outside the hospital, and at 40 months post-operative follow up without any antiplatelet drugs, the patient was 140 cm tall and weighs 43 kg, and the CTA revealed that the graft blood flow was unobstructed (Figure 2).
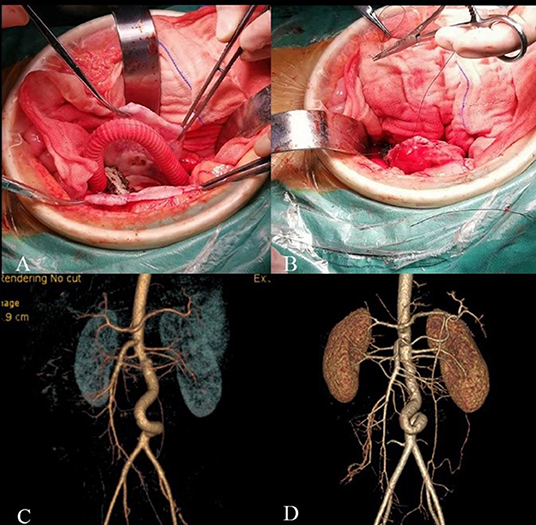
Figure 2. Intraoperative and follow-up images. (A) A 10 mm Dacron aorto-aortic tube graft replace the AAA with a “C” shape. (B) The aneurysmal sac was wrapped around the graft. (C) CTA 7 days after surgery. (D) CTA 40 months after surgery showed twisted but unobstructed graft.
AAA is more common in the elderly with arteriosclerosis, and is rare in children and infants, and is commonly caused by congenital connective tissue disorders, vasculitis, umbilical cannulation, and infection, while cAAA is extremely rare and has an unknown etiology. One relevant hypothesis is that cAAA results from a developmental defect during embryogenesis that creates a focal narrowing of the abdominal aorta, which leads to poststenotic turbulent blood flow and subsequent aneurysm formation (1). There are no epidemiological data related to cAAA, and as of December 2021, only 31 cases have been reported (Table 1) (2–8). There are fifteen patients diagnosed before 1 year of age, eight patients were diagnosed after 1 year of age, and the remaining eight patients were diagnosed at 19–30 weeks of gestation. The male-to-female ratio was 18:10, 20 of 31 patients had infrarenal AAA, and 19 of 30 patients had other aneurysms. The reason for admission of patients is usually abdominal pulsatile mass or rupture of aneurysm; suspected diseases should receive vital imaging examination, such as ultrasound, which can provide a clear diagnosis.
The histopathological changes in the intima of cAAA include calcifications, thromboses, and ulcerations and ruptures of the layers (2, 9). Molecular genetic defects considered to be associated with AAA, Marfan's syndrome, and Loeys-Dietz syndrome are caused by mutations in the genes encoding TGF-β2 or TGF-β receptor (TGFBR) I or II. Mutations in the fibrillin-1(FBN1) gene have also been found to be associated with the occurrence of coronary aneurysms (10–13). Unfortunately, no similar genetic or molecular changes have been found in cAAA, including this case.
The mortality caused by cAAA rupture and renal failure was 30.76% (2). There is no universal approach to the management of cAAA. Although steroids, cyclophosphamide, antihypertensive drugs, non-steroidal anti-inflammatory drugs, and statins have certain curative effects, the reported mortality of conservative treatment is still as high as 57.14% (4/7). It is still unclear how to judge the diameter of aneurysms in the intervention, and the uncertainty of children's activity cannot refer to the surgical standards of adults. Surgical repair after diagnosis should be considered. Endovascular aneurysm repair (EVAR) is not feasible in infants or children because of the lack of an appropriate endograft and the impact on patients' growth and development. Artificial grafts and allografts were most frequently selected for revascularization, and in the past, 13 cases were reported using Dacron graft or polytetrafluoroethylene (PTFE) graft, 4 cases of allografts, and 1 case of native vessels. Although allografts have the advantages of high long-term patency and low risk of postoperative graft infection, there are difficulties with the long-term use of immune-suppressants and allograft sources. Malikov reported a successful case of revascularization with native iliac vessels (14).
The common complications of artificial vascular grafts are graft stenosis and obstruction. The diameter of the artificial graft should be considered to match with the artery and ensure blood supply to the lower limbs. At present, the reported diameter is mostly between 8 and 12 mm, which is easier for older children to choose. There is a high risk of synthetic vascular graft occlusion with a diameter of <6 mm for a neonatal patient; it may, therefore, be more appropriate to delay surgery for smaller aneurysms to produce better results and prevent the need for follow-up surgery (15). It is necessary to reserve appropriate length for artificial grafts to adapt to the patient's growth; however, most reports do not indicate the specific appropriate length. Dueppers et al. (16) reserved 4-cm graft to meet the growth needs of children. We considered the patient's age, preoperative aortic diameter and adult physique estimated from his parents' physique, and decided to use 10-mm Dacron graft to reconstruct the diseased artery with a 6-cm long graft reserved to form a “C” shape; our length selection principle is to keep a certain length on the premise of avoiding angulation.
The average follow-up time of the 16 patients who underwent surgical repair with follow-up records was 19 months. The longest follow-up time with artificial grafts under the age of 18 years was 26 months. Of these 16 patients, two died of infection during follow-up, an anastomotic stenosis of allografts occurred 7 days after surgery, and one PTFE graft was completely occluded 13 months after the operation. The graft patency rate was 93.3% (14/15). There is no literature recommending the routine use of anticoagulant or antiplatelet drugs for AAA patients with reconstructed branches. LeNguyen et al. (8) continued to use low-molecular-weight heparin for patients with anastomotic stenosis, resulting in graft patency at 1-year follow-up.
During the 40-month follow-up in our case, the artificial graft was twisted, but there was no obvious angulation and it remained unobstructed. The patient's physical development was not affected. We will continue to follow up the patient to observe graft patency.
CAAA is rare and has unknown etiology, and for patients with confirmed aneurysms, it is suggested to improve the systemic examination and long-term follow-up to exclude other lesions. Due to the high mortality rate, the long-term results of open repair in children are still unclear. The patency of grafts also requires long-term follow-up observations and necessary drug adjuvant treatment. In cases of complications, timely and effective interventions are necessary.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Medical Ethical Committee of the First Affiliated Hospital of Zhengzhou University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
ZZ was wrote the manuscript and was assistant in surgery. KM and YY were assistant in surgery and participate in editing the articles. ZH and ZL designed the operation and revising the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by National Natural Science Foundation of China (8187020205), and its main expenditure is layout fee.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lee BB, Laredo J, Lee TS, Huh S, Neville R. Terminology and classification of congenital vascular malformations. Phlebology. (2007) 22:249–52. doi: 10.1177/026835550702200605
2. Wang Y, Tao Y. Diagnosis and treatment of congenital abdominal aortic aneurysm: a systematic review of reported cases. Orphanet J Rare Dis. (2015) 10:4. doi: 10.1186/s13023-015-0225-x
3. Bansal A, Mitra A, Bisoi AK, Agarwala S. Surgical repair of congenital abdominal aortic aneurysm in a 1-year-old child with literature review. J Indian Assoc Pediatr Surg. (2017) 22:176–8. doi: 10.4103/jiaps.JIAPS_258_16
4. Sirisabya A, Trinavarat P, Namchaisiri J, Punnahitanonda S, Thaithumyanon P. Congenital abdominal aortic aneurysm in a term neonate: a case report. Nephron Clin Pract. (2017) 11:163–7. doi: 10.5372/1905-7415.1102.548
5. Kuboi T, Miyagi M, Kondo S, Arioka M, Yamato S, Sadamura T, et al. Congenital abdominal aortic aneurysm discovered incidental to a lower back mass. Pediatr Int. (2018) 60:98–9. doi: 10.1111/ped.13458
6. Higuchi K, Furukawa K, Nakamura E, Imamura H, Gi T, Nakamura K. Congenital abdominal aortic aneurysm in a four year old girl. EJVES Vasc Forum. (2020) 48:12–8. doi: 10.1016/j.ejvsvf.2020.05.004
7. Tanga CF, Fakhoury E, Ham PB III, Dosluoglu HH, Harris LM. Ruptured abdominal aortic aneurysm in an 11-year-old with multiple peripheral artery aneurysms. J Vasc Surg Cases Innov Tech. (2020) 6:539–42. doi: 10.1016/j.jvscit.2020.07.015
8. Le-Nguyen A, Joharifard S, Cote G, Borsuk D, Ghali R, Lallier M. Neonatal microsurgical repair of a congenital abdominal aortic aneurysm with a cadaveric graft. Eur J Pediatr Surg Rep. (2021) 9:e23–7. doi: 10.1055/s-0041-1723019
9. Ye C, Yin H, Lin Y, Zhou L, Ye R, Li X, et al. Abdominal aorta aneurysms in children: single-center experience of six patients. Ann Thorac Surg. (2012) 93:201–5. doi: 10.1016/j.athoracsur.2011.08.038
10. Gentilini D, Oliveri A, Fazia T, Pini A, Marelli S, Bernardinelli L, et al. NGS analysis in Marfan syndrome spectrum: Combination of rare and common genetic variants to improve genotype-phenotype correlation analysis. PLoS ONE. (2019) 14:e0222506. doi: 10.1371/journal.pone.0222506
11. Mariucci E, Bonori L, Lovato L, Graziano C, Ciuca C, Pacini D, et al. Coronary artery aneurysms in patients with marfan syndrome: frequent, progressive, and relevant. Can J Cardiol. (2021) 37:1225–31. doi: 10.1016/j.cjca.2021.03.002
12. Forte A, Galderisi U, Cipollaro M, De Feo M, Della Corte A. Epigenetic regulation of TGF-β1 signalling in dilative aortopathy of the thoracic ascending aorta. Clin Sci. (2016) 130:1389–405. doi: 10.1042/CS20160222
13. Verstraeten A, Perik M, Baranowska AA, Meester JAN, Van Den Heuvel L, Bastianen J, et al. Enrichment of rare variants in Loeys-Dietz Syndrome Genes in spontaneous coronary artery dissection but not in severe fibromuscular dysplasia. Circulation. (2020) 142:1021–4. doi: 10.1161/CIRCULATIONAHA.120.045946
14. Malikov S, Delarue A, Fais PO, Keshelava G. Anatomical repair of a congenital aneurysm of the distal abdominal aorta in a newborn. J Vasc Surg. (2009) 50:1181–4. doi: 10.1016/j.jvs.2009.05.022
15. Barral X, de Latour B, Vola M, Lavocat MP, Fichtner C, Favre JP. Surgery of the abdominal aorta and its branches in children: late follow-up. J Vasc Surg. (2006) 43:1138–44. doi: 10.1016/j.jvs.2006.01.033
Keywords: aortic aneurysm, aortic diseases, congenital, children, surgical treatment
Citation: Zhou Z, Yue Y, Ma K, Hua Z and Li Z (2022) Congenital Abdominal Aortic Aneurysm: A Case Report and Literature Review. Front. Pediatr. 10:853517. doi: 10.3389/fped.2022.853517
Received: 12 January 2022; Accepted: 07 February 2022;
Published: 01 March 2022.
Edited by:
Juan A. Tovar, University Hospital La Paz, SpainReviewed by:
Mario Carminati, IRCCS San Donato Polyclinic, ItalyCopyright © 2022 Zhou, Yue, Ma, Hua and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaohui Hua, aHVhemhhb2h1aXNmeUAxNjMuY29t; Zhen Li, bGl6aGVuMTAyOUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.