
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr., 15 June 2022
Sec. Pediatric Rheumatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.849940
This article is part of the Research TopicPediatric UveitisView all 7 articles
 Mikhail M. Kostik1*
Mikhail M. Kostik1* Ekaterina V. Gaidar1
Ekaterina V. Gaidar1 Lubov S. Sorokina1
Lubov S. Sorokina1 Ilya S. Avrusin1
Ilya S. Avrusin1 Tatiana N. Nikitina1
Tatiana N. Nikitina1 Eugenia A. Isupova1
Eugenia A. Isupova1 Irina A. Chikova1
Irina A. Chikova1 Yuri Yu. Korin1
Yuri Yu. Korin1 Elizaveta D. Orlova1,2
Elizaveta D. Orlova1,2 Ludmila S. Snegireva1
Ludmila S. Snegireva1 Vera V. Masalova1
Vera V. Masalova1 Margarita F. Dubko1
Margarita F. Dubko1 Olga V. Kalashnikova1
Olga V. Kalashnikova1 Vyacheslav G. Chasnyk1
Vyacheslav G. Chasnyk1Objectives: Uveitis is the most frequent extra-articular manifestation of juvenile idiopathic arthritis (JIA). Our study is aimed to evaluate the possible difference in arthritis course depending on uveitis presence in patients with JIA, treated with biologics.
Methods: From our database of patients with JIA treated with biologics, we extracted patients to whom the first agent was administrated with or without MTX. The exclusion criteria included treatment with current systemic corticosteroids, infliximab, rituximab, observation period <3 years, and no missing data. After selection, 175 patients were eligible for analysis. We evaluated clinically significant flare with joint involvement (which required change of biologic or non-biologic DMARD) and time to flare. We compared two groups: (i) patients with uveitis (n = 32) and (ii) patients without uveitis (n = 143). For statistical analysis, we used Cox's regression models, the log-Rank test, x2 test, and the Mann–Whitney test.
Results: There was no difference in gender distribution and achievement of arthritis remission between groups. Patients in the non-uveitis group predominantly received etanercept (64.3%). In the uveitis group, the most prescribed biologic agent was adalimumab (71.9%). The presence of uveitis increased the risk of JIA flare, OR = 3.8 (95% CI: 1.7; 8.7), and the cumulative probability of joint flare, RR = 4.5 (95% CI: 1.7; 12.1), p =.003, after adjustment on methotrexate, RR = 3.1 (1.6; 6.), p =.0008. In the subgroup of patients treated with adalimumab, the absence of methotrexate increased the cumulative probability of flare [RR = 6.5 (95% CI: 1.4; 31.1), p = 0.02].
Conclusion: The presence of uveitis proved to be a risk factor in JIA flare. Methotrexate can decrease the cumulative flare probability. Further trials are required.
Juvenile idiopathic arthritis (JIA) is the most common pediatric rheumatic disease (1). JIA includes a heterogeneous group of chronic arthritis of unknown etiology that begins before 16 years (1). Uveitis is the most frequent extra-articular manifestation of JIA (2). JIA may have an unfavorable prognosis, leading to musculoskeletal system disability or visual impairment if adequate treatment is not prescribed (3–5). There are known predictors of uveitis in JIA, such as female gender, younger-onset age, ANA-positivity, oligoarticular course, and high CRP, but data about the features of the arthritis course in patients with concomitant uveitis are scarce (6, 7). Biological treatment is an option if previous therapy with methotrexate was ineffective or intolerable and can modify the course of arthritis and uveitis and their outcomes (8–10). The question about the benefits of combined treatment with biologics and methotrexate is still open. Meanwhile, the number of patients with JIA treated with biologics alone is growing due to methotrexate side effects correlated with treatment duration.
Most current studies aim to identify predictors of the development of uveitis in patients with JIA and its course, but there are limited studies to answer whether the presence of uveitis affects the severity of arthritis.
Written consent was obtained according to the Declaration of Helsinki. The local ethics Committee of Saint-Petersburg State Pediatric Medical University approved the protocol of this trial (protocol # 3/11 from 04.12.2012).
The study population was selected retrospectively from the database of patients with JIA treated with biologics. The 1997 International League of Rheumatology Association (ILAR) criteria for JIA subgroups were used to diagnose JIA and determine the type of arthritis (11). Diagnosis of uveitis and its anatomic classification (anterior, intermediate, posterior, or panuveitis) was performed according to the Standardization of Uveitis Nomenclature Workshop (SUN) criteria (12).
The inclusion criteria were as follows: (i) patients for whom the first biologic agent was administrated with or without methotrexate MTX; (ii) patients under a minimal observation period after biologic administration of 3 years; (iii) patients who developed uveitis de novo during the observation period were eligible only for group “uveitis” since uveitis was diagnosed.
The exclusion criteria were as follows: (i) systemic-onset JIA; (ii) acute anterior uveitis; (iii) treatment with current systemic corticosteroids, infliximab, and rituximab; and (iv) patients observed in our center irregularly or for <3 years. After selection, 175 patients were eligible for analysis.
In each patient, the following were evaluated: (i) clinical and demographic data: gender, age of the JIA onset, JIA category, and number of active joints; (ii) laboratory data: C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and antinuclear antibodies (ANA)-positivity; and (iii) treatment options: methotrexate (date of initiation and current status, whether the patient receives methotrexate or not), biologic agent (type, date of administration). The presence of uveitis was examined by an experienced ophthalmologist (TN, 30 years in uveitis), with the slit-lamp examination.
In each patient, we evaluated if a clinically significant flare with joint involvement (which required a change of biologic or non-biologic DMARD) occurred or not and the time to flare. The patients were divided into two groups: (i) patients with uveitis (n = 32) and (ii) patients without uveitis (n = 143). At least, a 3-year observation period was required to accurately select between “uveitis” and “no uveitis” groups because uveitis usually develops within the first 2 years after the JIA onset.
Each quantitative variable was checked with the Kolmogorov–Smirnov test, and no normal distribution was found. Descriptive statistics were reported in medians and interquartile ranges (IQRs) for continuous variables and absolute frequencies and percentages for categorical variables. We utilized the Mann–Whitney U-test to compare quantitative variables in two groups and the chi-square test to compare qualitative data, or the Fisher's exact test in case of expected frequencies <5. Odds ratio (OR) with a 95% confidence interval (CI) was calculated to assess the risk of JIA flares. Survival analysis with arthritis flare as the event of interest was conducted using the Kaplan–Meier method. The log-rank test compared survival curves. A Cox proportional hazards regression model tested factors significantly associated with time to achievement ID status and flares. A p < 0.05 was considered statistically significant. The software Statistica (release 10.0, StatSoft Corporation, Tulsa, OK, USA) was used for data analysis. The p < 0.05 were considered to indicate a significant difference.
The uveitis group has the following typical characteristics: younger age, less active joints, and ANA-positivity compared to the non-uveitis group. The female gender, ESR, CRP, and remission frequency were equal between the two groups. The patients with uveitis had more frequent flares during the observation period and a shorter time before the first significant flare (Table 1).
Etanercept (64.3%) was the main biological drug in the non-uveitis group and adalimumab (71.9%) in the uveitis group. Remission was observed in ~70% of the patients in both groups—seven children from the uveitis group and 18 from the non-uveitis group required the first biological drug change. In the uveitis group, adalimumab switched to infliximab and vice versa. In the non-uveitis group, etanercept was switched to adalimumab or tocilizumab.
Uveitis increases the risk of JIA flare, OR = 3.8 (95% CI: 1.7; 8.7), and the cumulative probability of flare, RR = 4.5 (95% CI: 1.7; 12.1), p = 0.003; log-rank test, p = 0.001 (Figure 1). After adjusting methotrexate treatment, uveitis is a risk factor in arthritis flare, RR = 3.1 (1.6; 6.), p = 0.0008. In the subgroup of the patients treated with adalimumab, the absence of methotrexate increases the cumulative probability of arthritis flare, RR = 6.5 (95% CI: 1.4; 31.1), p = 0.02; in the patients treated with adalimumab, uveitis increases the cumulative probability of arthritis flare.
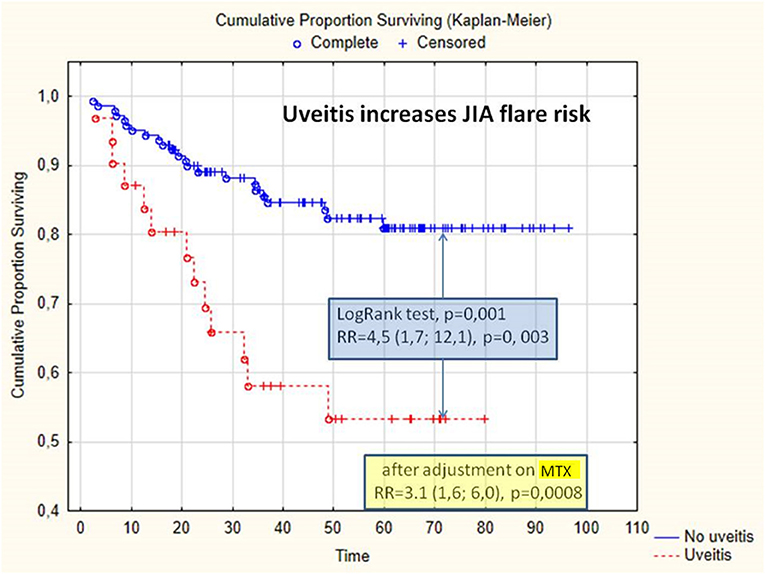
Figure 1. Cumulative proportion of survival without arthritis flare, depending on the presence of uveitis. JIA, juvenile idiopathic arthritis; MTX, methotrexate; RR, relative risk.
Uveitis might be a risk factor of more severe course of JIA. In previous studies, it was shown that the JIA category, e.g., oligoarthritis, is an independent risk factor of uveitis when combined with ANA-positivity and early age of the JIA onset (13–15). It is well-established that persistent oligoarthritis and extended oligoarthritis have the highest probability of developing uveitis, while, in systemic and RF-positive categories, uveitis occurs extremely rarely (5, 14, 15). The risk factors in acute anterior uveitis in enthesitis-related arthritis are male gender, ANA-positivity, and HLA B27 (2, 16, 17). The role of uveitis in the course of arthritis has not been previously described. Uveitis and arthritis might have similar pathogenesis related to immune cell activation and TNF-a hyperproduction (18, 19). Both arthritis and uveitis respond well to methotrexate and TNF-a inhibitors (adalimumab and infliximab) (20). Earlier prescription of methotrexate and biologics may decrease the incidence of uveitis (21–23). The combination of biologic and non-biologic DMARDs reduces the probability of active uveitis at any instance in time (22). The arthritis flare has a temporal association with uveitis flare (22). In our study, the absence of methotrexate increased the cumulative likelihood of an exacerbation rate in the subgroup of the patients receiving adalimumab. Immunogenicity of biologics, especially adalimumab, was associated with the production of antibodies against biologics. The protective role of methotrexate against the production of anti-adalimumab antibodies leads to preserving serum concentration of adalimumab and its efficacy (24, 25). The treatment efficacy of JIA-associated uveitis and arthritis with a combination of biologic and methotrexate has already been demonstrated in several studies (26–29).
According to our experience, we recommend starting methotrexate treatment early in patients with high risks of uveitis as well as continuing methotrexate in patients with JIA and uveitis, especially those who are treated with adalimumab, despite intolerance or adverse events occur.
The limitations of the study are related to its retrospective nature, the variation in times of uveitis development, and the absence of standard treatment protocol throughout the study. The use of various biologics may also influence the results. Different types of immunogenicity of adalimumab and etanercept may introduce bias into the results.
The results of our analysis indicate the need for ongoing cooperation between a pediatric rheumatologist and an ophthalmologist. Uveitis might be a hallmark of arthritis flare, and methotrexate therapy can reduce the likelihood of arthritis flare.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by The local Ethics Committee which approved the protocol of this trial of Saint-Petersburg State Pediatric Medical University (protocol # 3/11 from 04.12.2012). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
MK, EG, LSSo, and IA designed the study. MK, EG, LSSo, IA, TN, EI, IC, YK, EO, LSSn, VM, MD, OK, and VC collected and analyzed the data. MK, EG, IA, and LSSo wrote the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work was supported by Russian Science Foundation Grant 20-45-01005.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet. (2007) 369:767–78. doi: 10.1016/S0140-6736(07)60363-8
2. Angeles-Han ST, Ringold S, Beukelman T, Lovell D, Cuello CA, Becker ML et al. 2019 American college of rheumatology/arthritis foundation guideline for the screening, monitoring, and treatment of juvenile idiopathic arthritis-associated uveitis. Arthritis Care Res. (2019) 71:703–16. doi: 10.1002/acr.23871
3. Kolomeyer AM, Crane ES, Tu Y, Liu D, Chu DS. Adult patients with uveitis associated with juvenile idiopathic arthritis: a retrospective review. Can J Ophthalmol. (2017) 52:458–62. doi: 10.1016/j.jcjo.2017.01.011
4. Haasnoot AJ, Vernie LA, Rothova A, Doe P, Los LI, Schalij-Delfos NE, et al. Impact of juvenile idiopathic arthritis associated uveitis in early adulthood. PLoS ONE. (2016) 11:e0164312. doi: 10.1371/journal.pone.0164312
5. Angeles-Han ST, McCracken C, Yeh S, Jenkins K, Stryker D, Rouster-Stevens K, et al. Characteristics of a cohort of children with juvenile idiopathic arthritis and JIA-associated uveitis. Pediatr Rheumatol Online J. (2015) 13:19. doi: 10.1186/s12969-015-0018-8
6. Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Clin Immunol. (2020) 211:108322. doi: 10.1016/j.clim.2019.108322
7. Heiligenhaus A, Heinz C, Edelsten C, Kotaniemi K, Minden K. Review for disease of the year: epidemiology of juvenile idiopathic arthritis and its associated uveitis: the probable risk factors. Ocul Immunol Inflamm. (2013) 21:180–91. doi: 10.3109/09273948.2013.791701
8. Ramanan AV, Dick AD, Jones AP, McKay A, Williamson PR, Compeyrot-Lacassagne S, et al. SYCAMORE Study Group. Adalimumab plus methotrexate for uveitis in juvenile idiopathic arthritis. N Engl J Med. (2017) 376:1637–46. doi: 10.1056/NEJMoa1614160
9. Quartier P, Baptiste A, Despert V, Allain-Launay E, Koné-Paut I, Belot A, et al. ADJUVITE study group. ADJUVITE: a double-blind, randomised, placebo-controlled trial of adalimumab in early onset, chronic, juvenile idiopathic arthritis-associated anterior uveitis. Ann Rheum Dis. (2018) 77:1003–11. doi: 10.1136/annrheumdis-2017-212089
10. Cecchin V, Zannin ME, Ferrari D, Pontikaki I, Miserocchi E, Paroli MP, et al. Longterm safety and efficacy of adalimumab and infliximab for uveitis associated with juvenile idiopathic arthritis. J Rheumatol. (2018) 45:1167–72. doi: 10.3899/jrheum.171006
11. Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International league of associations for rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. (2004) 31:390–2.
12. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. (2005) 140:509–16. doi: 10.1016/j.ajo.2005.03.057
13. van Straalen JW, Giancane G, Amazrhar Y, Tzaribachev N, Lazar C, Uziel Y, et al. Paediatric Rheumatology International Trials Organisation (PRINTO). A clinical prediction model for estimating the risk of developing uveitis in patients with juvenile idiopathic arthritis. Rheumatology. (2021) 60:2896–905. doi: 10.1093/rheumatology/keaa733
14. Tappeiner C, Klotsche J, Sengler C, Niewerth M, Liedmann I, Walscheid K, et al. Risk factors and biomarkers for the occurrence of uveitis in juvenile idiopathic arthritis: data from the inception cohort of newly diagnosed patients with juvenile idiopathic arthritis study. Arthritis Rheumatol. (2018) 70:1685–94. doi: 10.1002/art.40544
15. Sim KT, Venning HE, Barrett S, Gregson RM, Amoaku WM. Extended oligoarthritis and other risk factors for developing JIA-associated uveitis under ILAR classification and its implication for current screening guideline. Ocul Immunol Inflamm. (2006) 14:353–7. doi: 10.1080/09273940600977233
16. Clarke SL, Sen ES, Ramanan AV. Juvenile idiopathic arthritis-associated uveitis. Pediatr Rheumatol Online J. (2016) 14:27. doi: 10.1186/s12969-016-0088-2
17. Moradi A, Amin RM, Thorne JE. The role of gender in juvenile idiopathic arthritis-associated uveitis. J Ophthalmol. (2014) 2014:461078. doi: 10.1155/2014/461078
18. Jiang Q, Li Z, Tao T, Duan R, Wang X, Su W. TNF-α in uveitis: from bench to clinic. Front Pharmacol. (2021) 12:740057. doi: 10.3389/fphar.2021.740057
19. Walters HM, Pan N, Lehman TJ, Adams A, Kalliolias GD, Zhu YS, et al. The impact of disease activity and tumour necrosis factor-α inhibitor therapy on cytokine levels in juvenile idiopathic arthritis. Clin Exp Immunol. (2016) 184:308–17. doi: 10.1111/cei.12782
20. Marelli L, Romano M, Pontikaki I, Gattinara MV, Nucci P, Cimaz R, et al. Long term experience in patients with JIA-associated uveitis in a large referral center. Front Pediatr. (2021) 9:682327. doi: 10.3389/fped.2021.682327
21. Rypdal V, Glerup M, Songstad NT, Bertelsen G, Christoffersen T, Arnstad ED, et al. Nordic study group of pediatric rheumatology. Uveitis in juvenile idiopathic arthritis: 18-year outcome in the population-based Nordic cohort study. Ophthalmology. (2021) 128:598–608. doi: 10.1016/j.ophtha.2020.08.024
22. Liebling EJ, Faig W, Chang JC, Mendoza E, Moore N, Ledesma Vicioso N, et al. The temporal relationship between juvenile idiopathic arthritis disease activity and uveitis activity. Arthritis Care Res. (2020). 74:349–54. doi: 10.1002/acr.24483
23. Walscheid K, Glandorf K, Rothaus K, Niewerth M, Klotsche J, Minden K, et al. Enthesitis-related arthritis: prevalence and complications of associated uveitis in children and adolescents from a population-based nationwide study in Germany. J Rheumatol. (2021) 48:262–9. doi: 10.3899/jrheum.191085
24. Ducourau E, Rispens T, Samain M, Dernis E, Dernis E, Le Guilchard F, et al. Methotrexate effect on immunogenicity and long-term maintenance of adalimumab in axial spondyloarthritis: a multicentric randomised trial. RMD Open. (2020) 6:e001047. doi: 10.1136/rmdopen-2019-001047
25. Bartelds GM, Wijbrandts CA, Nurmohamed MT, Stapel S, Lems WF, Aarden L, et al. Clinical response to adalimumab: relationship to anti-adalimumab antibodies and serum adalimumab concentrations in rheumatoid arthritis. Ann Rheum Dis. (2007) 66:921–6. doi: 10.1136/ard.2006.065615
26. Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. SYCAMORE Trial Management Group. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial). Trials. (2014) 15:14. doi: 10.1186/1745-6215-15-14
27. Lerman MA, Burnham JM, Chang PY, Daniel E, Foster CS, Hennessy S, et al. Response of pediatric uveitis to tumor necrosis factor-α inhibitors. J Rheumatol. (2013) 40:1394–403. doi: 10.3899/jrheum.121180
28. Lovell DJ, Brunner HI, Reiff AO, Jung L, Jarosova K, Němcová D, et al. Long-term outcomes in patients with polyarticular juvenile idiopathic arthritis receiving adalimumab with or without methotrexate. RMD Open. (2020) 6:e001208. doi: 10.1136/rmdopen-2020-001208
29. Brunner HI, Nanda K, Toth M, Foeldvari I, Bohnsack J, Milojevic D, et al. Paediatric rheumatology international trials organisation, and the pediatric rheumatology collaborative study group. Safety and effectiveness of adalimumab in patients with polyarticular course of juvenile idiopathic arthritis: STRIVE registry seven-year interim results. Arthritis Care Res. (2020) 72:1420–30. doi: 10.1002/acr.24044
Keywords: juvenile idiopathic arthritis, uveitis, flare, adalimumab, methotrexate
Citation: Kostik MM, Gaidar EV, Sorokina LS, Avrusin IS, Nikitina TN, Isupova EA, Chikova IA, Korin YY, Orlova ED, Snegireva LS, Masalova VV, Dubko MF, Kalashnikova OV and Chasnyk VG (2022) Uveitis Is a Risk Factor for Juvenile Idiopathic Arthritis' Significant Flare in Patients Treated With Biologics. Front. Pediatr. 10:849940. doi: 10.3389/fped.2022.849940
Received: 06 January 2022; Accepted: 12 May 2022;
Published: 15 June 2022.
Edited by:
Séverine Guillaume-Czitrom, Assistance Publique Hopitaux de Paris, FranceReviewed by:
Andrea Taddio, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyCopyright © 2022 Kostik, Gaidar, Sorokina, Avrusin, Nikitina, Isupova, Chikova, Korin, Orlova, Snegireva, Masalova, Dubko, Kalashnikova and Chasnyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail M. Kostik, a29zdC1taWtoYWlsQHlhbmRleC5ydQ==; bWlraGFpbC5rb3N0aWtAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.