
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 04 April 2022
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.846273
This article is part of the Research Topic The Broader Aspects of Non-Alcoholic Fatty Liver Disease in Children View all 5 articles
Background: The spectrum of non-alcoholic fatty liver disease (NAFLD) ranges from isolated hepatic steatosis to non-alcoholic steatohepatitis to fibrosis. We aimed to introduce useful biomarkers released during liver inflammation and fibrogenesis that are easy to use in outpatient clinic and adjust to children to evaluate each NAFLD stage without biopsy.
Methods: This prospective study included 60 patients aged under 19 years whose alanine aminotransferase (ALT) levels were elevated from March 2021. All patients were proven to have NAFLD by ultrasonography and laboratory work-up to exclude other causes of hepatitis. Fibroscan and additional laboratory tests for biomarkers [procollagen type1 amino-terminal propeptide (P1NP), osteocalcin, interleukin-6 (IL-6), and Mac-2 binding protein glycosylated isomer (M2BPGi)] were performed. Fibroscan-AST (FAST) score was used for the comparison of steatohepatitis and liver stiffness measurement (kPa) was used for the comparison of advanced fibrosis.
Results: The biomarker that showed a significant difference between the FAST-positive and negative groups was the P1NP/osteocalcin ratio with a p-value of 0.008. The area under receiver operating characteristic (AUROC) of P1NP/osteocalcin ratio*ALT values (values obtained through multivariate analysis) was 0.939 with the cut-off value of 305.38. The biomarkers that showed a significant difference between the LSM-positive and negative groups were IL-6 and M2BPGi with a p-values of 0.005 and <0.001. AUROC of IL-6 *AST values (values obtained through multivariate analysis) was 0.821 with the cut-off value of 228.15. M2BPGi showed a significant linear relationship with LSM in Pearson correlation analysis (Pearson correlation coefficient = 0.382; p = 0.003). The diagnostic capability of M2BPGi to evaluate advanced fibrosis showed an acceptable result (AUROC = 0.742; p = 0.022).
Conclusions: Non-invasive biomarkers can be used to predict each stage of NAFLD in children. The measurements of P1NP, IL-6 or M2BPGi along with the basic chemistry tests would help determine the stage of NAFLD they correspond to at the time of initial diagnosis and predict responsiveness after the treatment.
The non-alcoholic fatty liver disease (NAFLD) is asymptomatic to the most patients and discovered incidentally from laboratory examinations due to the elevated liver enzymes. Therefore, the disease is sometimes found after progression. The spectrum of NAFLD according to the progression of the disease is from isolated hepatic steatosis to non-alcoholic steatohepatitis to fibrosis to cirrhosis (1). Although the definitive test for diagnosis of NAFLD and its stage is liver biopsy, several studies have introduced non-invasive imaging techniques and scoring systems to assess the stage of NAFLD (2–6). With the aid of advances in imaging and scoring systems, we are one step closer to assessment of the NAFLD stage without liver biopsy.
Moving forward with these imaging modalities and scoring systems, several studies have begun exploring novel biomarkers that are more convenient for the assessment of the stage of NAFLD. Notably, the aminotransferase levels may fluctuate over time and sometimes be normal when they are followed-up. Unfortunately, normalized aminotransferase levels do not exclude the progression to fibrosis or cirrhosis (7). Representatively, Mac-2 binding protein glycosylated isomer (M2BPGi) is a hematological biomarker that predicts high-grade fibrosis and cirrhosis (8, 9). Besides that, the extracellular matrix (ECM) component, hyaluronic acid, amino-terminal peptide of type III collagen, and tissue inhibitor of metalloproteinase-1 are the introduced biomarkers to predict fibrosis (10, 11).
All the studies on these biomarkers were conducted in adult patients with NAFLD and focused only on the evaluation of advanced fibrosis in the NAFLD stage. Additionally, unlike adults, pediatric patients develop steatohepatitis rather than progress to cirrhosis. Therefore, the research on biomarkers in children should be focused on the stages of steatohepatitis and fibrogenesis. In addition, recent studies have found an increased risk of cardiovascular disease in pediatric patients with NAFLD (12, 13). Although it can be subclinical at young age, atherosclerosis and cardiac dysfunction may occur (12). Therefore, it is an important factor to evaluate metabolic (dysfunction) associated fatty liver disease (MAFLD) in patients with confirmed liver steatosis, suggesting that it is important to detect NAFLD at an early stage in children (14, 15).
When inflammation occurs in liver cells, inflammatory cytokines, such as TNF-alpha, interleukin-6 (IL-6), and IGF are generated. Hepatic stellate cells are also converted to myofibroblasts, and collagen, glycoproteins, and proteoglycans are formed as ECM. This study aims to introduce useful biomarkers released during liver inflammation and fibrogenesis, which are easy to use in the outpatient clinic and to adjust to children to evaluate each stage of NAFLD without biopsy.
We prospectively enrolled 60 pediatric patients with NAFLD (age <19 years). The initial screening for the study enrollment included patients who visited the out-patient clinic with the chief complaint of elevated alanine aminotransferase (ALT) levels and the body mass index (BMI) >23.0 (Figure 1). They were also screened with questions, such as previous history of inborn errors of metabolism, other liver diseases and whether or not the patients had been administered drugs metabolized by the liver, and showed no signs of infection when they underwent laboratory testing. The patients who met the criteria underwent laboratory tests to exclude other causes of hepatitis and metabolic disease leading to hepatic steatosis as follows: Hepatitis A, B, and C virus, Epstein–Barr virus, levels of creatinine kinase, ceruloplasmin, fasting glucose, ammonia, and fluorescent antinuclear antibody. They also underwent ultrasonography to check for the presence of focal lesion or anatomical abnormalities in the hepatobiliary system and simultaneously check for the presence of fatty liver. Among patients for whom other causes of hepatitis and fatty liver were excluded, ultrasonography screening was used for confirmation; patients with a chest circumference of 75 cm or more were enrolled in the study as a condition for fibroscan examination. Of the 67 patients who were initially recruited, 7 patients dropped out because their chest circumference was <75 cm. Finally, total of 60 patients were included in the study. These 60 patients underwent additional blood tests including biomarkers after fasting and fibroscan to evaluate the stage of NAFLD (Figure 1).
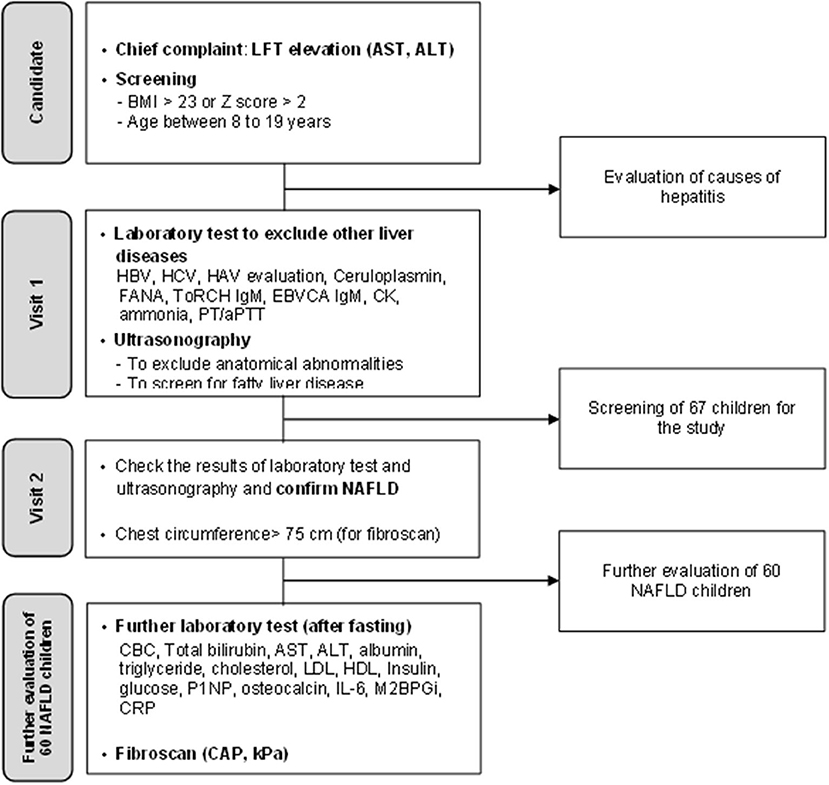
Figure 1. A flowchart illustrating the screening process for non-alcoholic fatty liver disease and enrollment process in this study.
The purpose of this study was to evaluate the diagnostic value of the two measured biomarkers: IL-6 (inflammatory cytokine) and P1NP (ECM product) to assess each stage of NAFLD. The significant differences were compared in patients with steatosis by classifying them into three groups according to their grade of fatty liver on ultrasonography. To evaluate the significant difference in patients with steatohepatitis (in the process of fibrogenesis), we divided the positive and the negative group with the fibroscan-AST (FAST) score and compared them. Advanced fibrosis was compared by dividing the groups based on the liver stiffness measurement (LSM) value obtained through fibroscan.
The second objective of this study was to find out their cut-off values to find meaningful biomarkers. The third objective of this study was to evaluate whether M2BPGi has the diagnostic capability to assess NAFLD with advanced fibrosis in children. All the methods of this study were performed in accordance with the relevant guidelines and regulations and were approved by the Clinical Research Ethics Committee of Samsung Medical Center. We have undergone appropriate written informed consent procedures for analysis of clinical data (IRB File No: SMC 2021-04-064).
Clinical data, such as age, sex, and weight (kg) were recorded. BMI was recorded as z-score because the patients were children. Venous blood samples were obtained after fasting over 8 h. The biochemical variables were measured using automated analyzer at the laboratory in the hospital.
We recorded the serum levels for the following: total bilirubin, aspartate aminotransferase (AST), ALT, triglyceride, cholesterol, low density lipoprotein, albumin, platelets, fasting plasma glucose, insulin, c-reactive protein, IL- 6, P1NP, osteocalcin, and M2BPGi.
The values of serum total procollagen type 1 amino-terminal propeptide (P1NP), osteocalcin, and IL-6 were measured using chemiluminescence enzyme immunoassay with the products of Roche Elecsys® (Roche, Basel, Switzerland). P1NP is a representative bone formation marker and is used in the treatment of osteoporosis patients (16). In children and adolescents with rapid bone formation, the standard of normal values is different and higher than that of adults. As the bone formation is rapid, the normal level of P1NP is higher in children and adolescents than in adults. Data regarding osteocalcin, another bone formation marker, were also collected to correct the P1NP values (17). Therefore, in this paper, the value obtained by dividing P1NP by osteocalcin was used as a variable in analysis. In addition, since ALP is a test that can obtain values more easily than osteocalcin, P1NP corrected by ALP was also evaluated. The serum M2BPGi level was measured using chemiluminescence enzyme immunoassay with the HISCL™ M2BPGi™ reagent kit (Sysmex, Kobe, Japan).
The presence of impaired fasting glycemia or diabetes was evaluated by a homeostatic model assessment for insulin resistance, which was calculated as follows: fasting insulin (microU/L) * fasting glucose (nmol/L)/22.5. A homeostatic model assessment for insulin resistance value of 3.8 or higher was considered as insulin resistance (18, 19). The FAST score, which is the only non-invasive scoring system to evaluate steatohepatitis status, was calculated as follows: e−1.65+1.07 × In(LSM)+2.66*10−8 × CAP3−63.3 × AST−1/1+e−1.65+1.07 × In(LSM)+2.66*10−8 × CAP3−63.3 × AST−1.
The FAST score of 0.35 or higher is considered as steatohepatitis (20). The fibrosis-4 index is a representative scoring system evaluating advanced fibrosis in NAFLD and is calculated as follows: age (years)*AST (U/L)/platelet count (*109/L) (U/L) (21).
The three trained radiology professors evaluated the degree of fatty liver according to the following four grades: (1) grade 0 (absence of steatosis, with normal liver echogenicity); (2) grade 1 (mild steatosis, the liver had a higher echogenicity than the right renal cortex; however, the echogenic wall of the main portal vein was preserved); (3) grade 2 (moderate steatosis, impaired echogenicity of the main portal vein wall); and (4) grade 3 (severe steatosis, impaired visualization of the posterior hepatic parenchyma or the diaphragm) (22, 23). The presence of hepatomegaly and splenomegaly was also evaluated.
Fibroscan (Echosens, Paris, France) was performed by trained nurses. The machine model of fibroscan in the center was 502 TOUCH, with M and XL probes. An automatic probe selection tool was embedded in the software; however, most patients (54/60, 90.0%) were tested using the M probe because most patients were aged under 18 years. The LSM and controlled attenuation parameter (CAP) were evaluated using vibration controlled transient elastography technology. LSM measures the speed of shear wave and expresses the degree of liver fibrosis numerically (unit: kPa). CAP measures the amount of attenuation of ultrasound transmitted into the liver and expresses the degree of fat distribution in the liver numerically (unit: dB/m). Eddewes et al. (24) presented the cut-off values of LSM and CAP in patients with NAFLD through a comparison with biopsy. For LSM, more than 8.2 kPa was evaluated as fibrosis stage 2 or higher (advanced fibrosis), and for CAP, more than 302 kPa was evaluated as steatosis stage 1 or higher (24).
For descriptive statistics, the continuous variables were expressed as mean (standard deviation) and categorical variables were expressed as an absolute number with percentages. In Table 1, one-way analysis of variance test was performed to analyze the mean value comparison between the three groups classified according to the fatty liver grades of ultrasonography. In Table 2, the t-test and the Mann–Whitney test were performed to analyze the mean value comparison between the two groups classified as positive and negative for the FAST score. In Table 3, the t-test and the Mann–Whitney test were performed to analyze the mean value comparison between the two groups classified as positive and negative for LSM (kPa) values. Univariable and multivariable analyses of the associations between steatohepatitis or fibrosis and other factors were performed using a logistic regression model (Table 3). Multivariable analysis was performed by selecting variables with p < 0.1 in univariate analysis. The diagnostic capabilities were evaluated using the receiver operating characteristic (ROC) curve using the items evaluated based on the results of the logistic regression analysis (Figures 2, 3). The diagnostic performances of the biomarkers were expressed as the diagnostic sensitivity, specificity, and area under the ROC (AUROC) curve. Youden's J statistic was computed to identify the cut-off values. In the analysis to evaluate the correlation between LSM and M2BPGi, the Pearson correlation analysis was used (Figure 4).
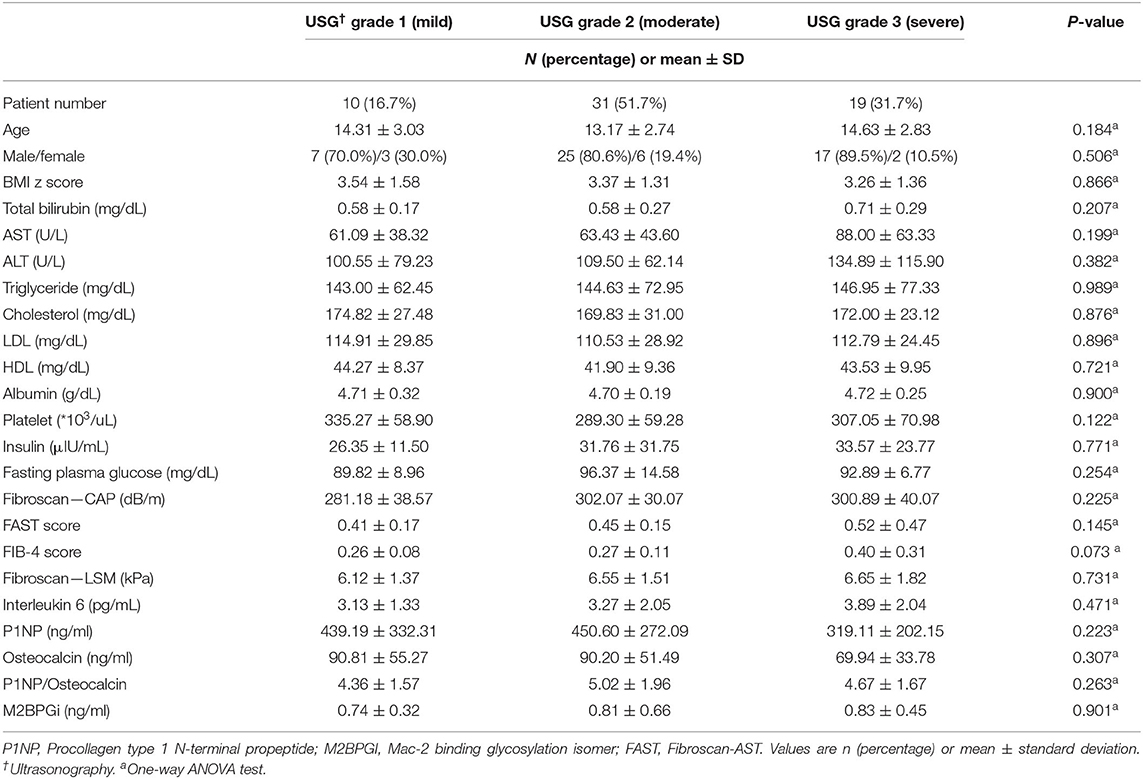
Table 1. Comparison of the clinical characteristics, laboratory results, and biomarkers released during liver inflammation and fibrogenesis in the patients' groups divided according to the grade of ultrasonography.
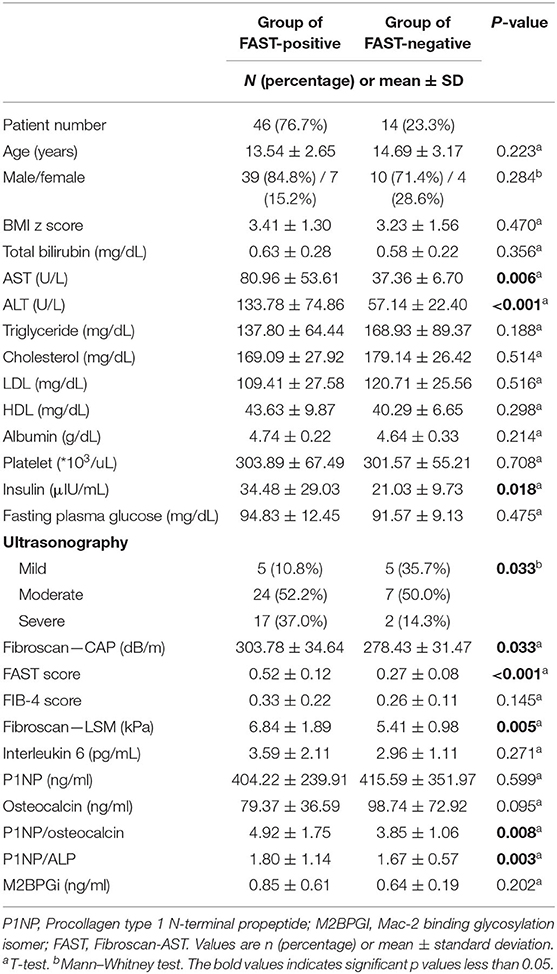
Table 2. Comparison of clinical characteristics, laboratory results, and biomarkers released during liver inflammation and fibrogenesis in patients who had positive and non-positive FAST scores [comparison between patients with steatohepatitis (early fibrosis) and patients with non-steatohepatitis].
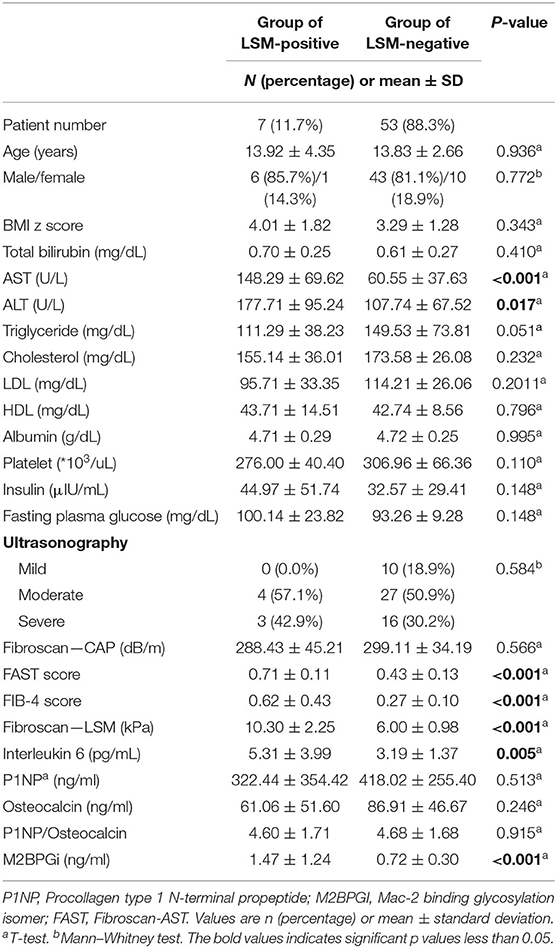
Table 3. Comparison of the clinical characteristics, laboratory results, and biomarkers released during liver inflammation and fibrogenesis in patients who had positive and non-positive FAST scores (comparison between patients with advanced fibrosis and with non-advanced fibrosis).
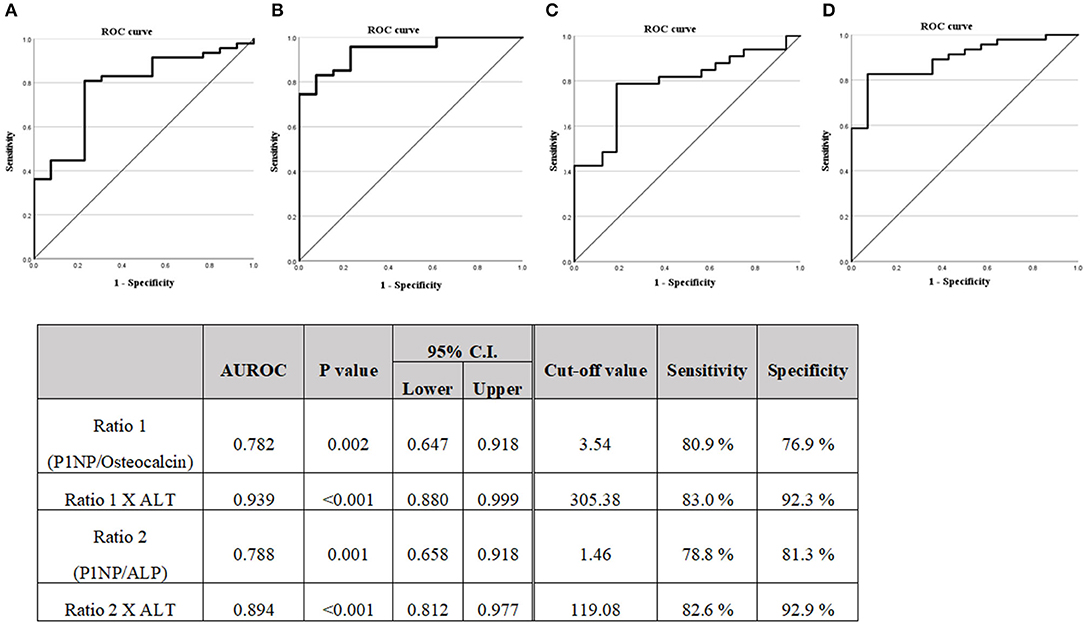
Figure 2. (A) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum P1NP/Osteocalcin ratio values for assessing the steatohepatitis. (B) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum P1NP/Osteocalcin ratio X ALT values for assessing steatohepatitis. (C) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum P1NP/ALP ratio values for assessing steatohepatitis. (D) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum P1NP/ALP ratio X ALT values for assessing steatohepatitis. P1NP, Procollagen type 1 N-terminal propeptide.
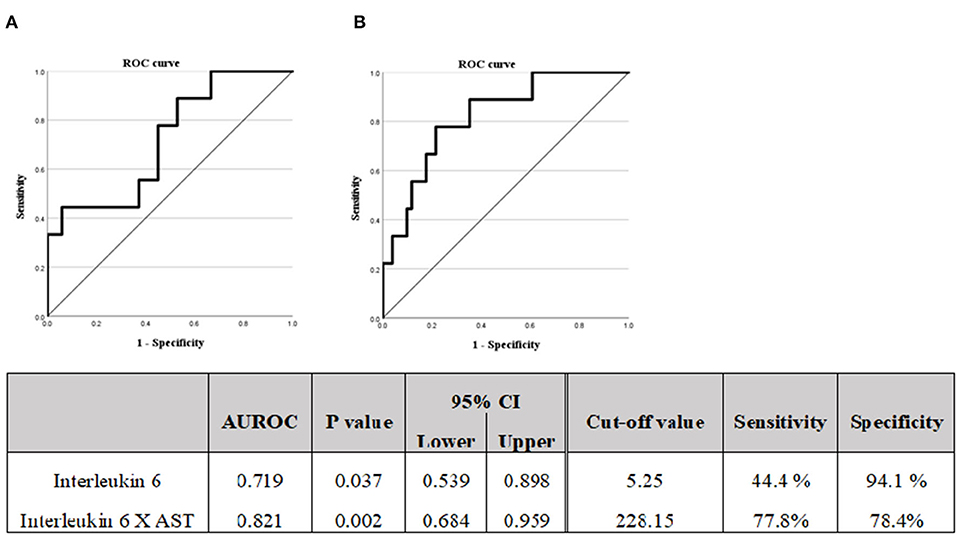
Figure 3. (A) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum interleukin 6 values for assessing the advanced liver fibrosis. (B) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum Interleukin 6 X AST values for assessing advanced liver fibrosis. M2BPGi, Mac-2 binding glycosylation.
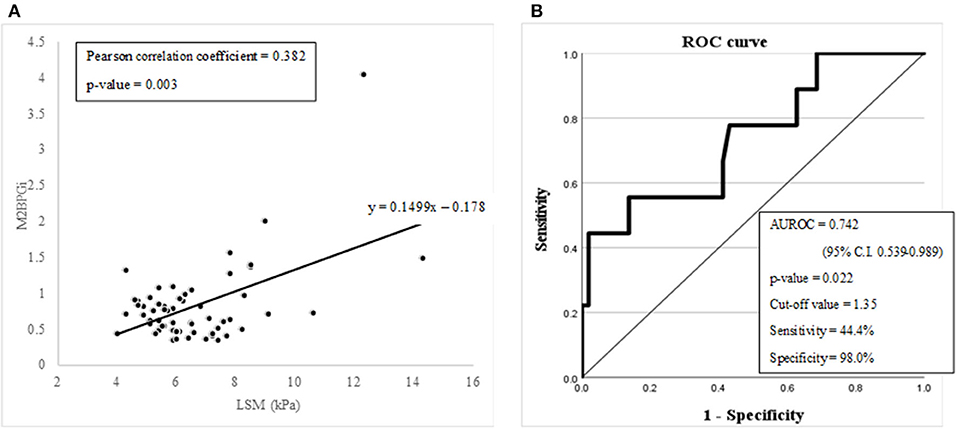
Figure 4. (A) A graph showing the linear correlation between M2BPGi and LSM (kPa) with Pearson correlation analysis. (B) Diagnostic capabilities with the area under the receiver operating characteristic curve of the serum M2BPGi values for assessing advanced liver fibrosis.
All of the above statistical analysis were conducted using SPSS version 27 (IBM Corporation, Armonk, NY, USA). A p-value <0.05 was considered as statistically significant.
The mean age of the patients (n = 60) was 13.84 years, and the majority of the patients were male (81.7%) (Table 4). The mean value of BMI evaluated by the Z score was 3.37, indicating obesity (25). Six patients with insulin resistance assessed by a homeostatic model assessment for insulin resistance value, accounted for 10.0% of all patients. Two patients were diagnosed with type 2 diabetes. No patient had bilirubin level higher than normal; additionally, ALT elevation (mean value: 70.78 U/L) was more prominent than AST elevation (mean value: 115.90 U/L). Although the test was performed after fasting, the triglyceride level was 145.07 mg/dL, which was high when compared to the normal values considering the age. The cholesterol and low-density lipoprotein levels were 171.43 and 112.05 mg/dL, respectively.
The degree of fatty liver confirmed by ultrasonography was 31/60 (51.7%) in the moderate stage, accounting for more than half the cases. The quantified steatosis, CAP obtained through fibroscan was confirmed with the mean value of 297.87 dB/m. The FAST score for evaluating steatohepatitis had the mean value of 0.47. LSM, which evaluates fibrosis, showed the mean value of 6.57 kPa.
The clinical characteristics, laboratory results, and biomarker values were compared by dividing the patients into three groups according to their grade (mild, moderate, and severe) of fatty liver on ultrasonography (Table 1). Among 60 patients, 10 patients (16.7%) were identified as having mild fatty liver, 31 patients (51.7%) were identified as having moderate fatty liver, and 19 patients (31.7%) were identified as having severe fatty liver on ultrasonography. No statistically significant differences were observed between the three groups in clinical characteristics, laboratory results, scores, and biomarkers. However, when looking at the mean value of each group, there are items worth interpreting, although there is no statistical significance. In the laboratory results, the mean levels of AST, ALT, triglyceride, and insulin were observed to increase as the fatty liver grade increases. It was expected that the CAP test result would show a statistically significant difference between groups as it had a linear relationship with the degree of fatty liver evaluated by ultrasonography; however, it did not show a significant difference (p-value: 0.225).
The clinical characteristics, laboratory results, and biomarker values were compared by dividing the patients into two groups: FAST-positive group (FAST score of 0.35 or higher) and FAST-negative group (FAST score of <0.35) (Table 2). Among 60 patients, 47 patients (76.7%) were identified as the FAST-positive group and 14 patients (23.3%) were identified as the FAST-negative group. The mean value of the FAST score of FAST-positive group was 0.52 and that of FAST-negative group was 0.27. No significant differences were observed between the two groups in clinical characteristics, such as age, sex, and BMI z score. In the laboratory results, the AST, ALT, and insulin levels showed a significant difference (p-value 0.006, <0.001, and 0.018, respectively) between the two groups, and all three values showed significantly higher values in the FAST-positive group (AST: 80.96 vs. 37.36, ALT: 133.78 vs. 57.14, and insulin: 34.48 vs. 21.03).
There was also a statistically significant difference in the ratio of mild, moderate, and severe fatty liver confirmed by ultrasonography. In the FAST-positive group, the percentage of patients in the severe status was 37% and was significantly higher than 14.3% in the negative group. CAP and LSM showed a significant difference between the two groups with p-values of 0.033 and 0.005, respectively; additionally, the FAST-positive group showed a higher mean value (CAP: 303.78 vs. 278.43, LSM: 6.84 vs. 5.41). Among the biomarkers (IL-6, P1NP, osteocalcin, and M2BPGi), P1NP/osteocalcin ratio and P1NP/ALP ratio (corrected ECM product) showed significant differences with p-values of 0.008 and 0.003, respectively. Pearson's correlation between osteocalcin and ALP showed a significant correlation with a p-value < 0.001.
A logistic regression analysis was performed on the basis of the results of Table 2, the P1NP/osteocalcin ratio and ALT were evaluated as suitable factors in the regression formula with statistical significance (Table 5A). For the P1NP/osteocalcin ratio, the odds ratio was 1.850, with a p-value of 0.018; additionally, for ALT, the odds ratio was 1.078, with a p-value of 0.022. Based on these results, whether the P1NP/osteocalcin ratio had a diagnostic capability in predicting steatohepatitis (early fibrosis) or not was evaluated using the ROC curve (Figure 2). Even the P1NP/osteocalcin ratio alone, with AUROC of 0.782, p-value of 0.002, and cut-off value of 3.54 or higher, had a diagnostic capability to evaluate steatohepatitis (early fibrosis); however, the diagnostic capability for assessing the steatohepatitis was higher when the ratio was multiplied by ALT. The AUROC of P1NP/osteocalcin ratio*ALT value was 0.939 and the p-value was <0.001. The cut-off value was 305.38. P1NP/ALP ratio evaluation through AUROC curve also showed a diagnostic capability, with AUROC of 0.788, p-value of 0.001, and cut-off value of 1.46 or higher. Similarly, the P1NP/ALP ratio showed better diagnostic capability when the ratio was multiplied by ALT, with AUROC of 0.894, p-value of <0.001, and cut-off value of 119.08 or higher.
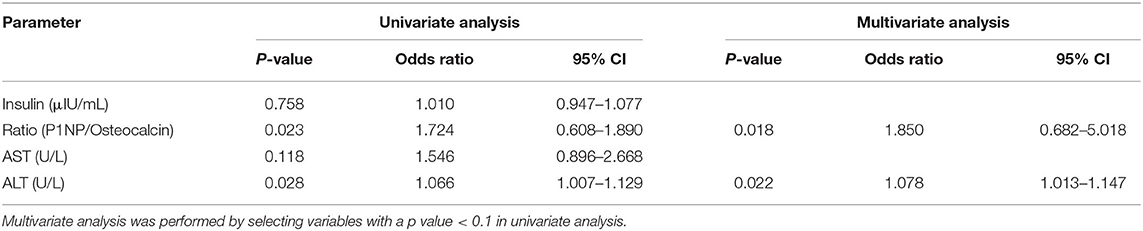
Table 5A. Univariate and logistic multivariate analyses of the association between steatohepatitis (early fibrosis) assessed by the FAST score and other factors.
The clinical characteristics, laboratory results, and biomarker values were compared by dividing the patients into two groups with LSM-positive group (LSM of 8.2 kPa or higher), and LSM-negative group (LSM of <8.2 kPa) (Table 3). Among 60 patients, 7 patients (11.7%) were identified as the LSM-positive group and 53 patients (88.3%) were identified as the LSM-negative group. The mean value of LSM of the LSM-positive group was 10.30 and that of the LSM-negative group was 6.00. No significant differences were observed between the two groups in clinical characteristics, such as age, sex, and BMI z score. In the laboratory results, the AST and ALT levels showed a significant difference (p-value <0.001 and 0.017, respectively) between the two groups, and the two values showed significantly higher values in the LSM-positive group (AST: 148.29 vs. 60.55 and ALT: 177.71 vs. 107.74).
The FAST score and fibrosis-4 score showed a significant difference between the two groups with both p-values of <0.001; additionally, the LSM-positive group showed higher mean value (FAST score: 0.71 vs. 0.43, fibrosis-4 score: 0.62 vs. 0.27). Among the biomarkers (IL-6, P1NP, osteocalcin, and M2BPGi), only IL-6 and M2BPGi showed significant differences with p-values of 0.005 and <0.001, respectively.
A logistic regression analysis was performed based on the results of Table 3; the IL6 and AST were evaluated as suitable factors in the regression formula with statistical significance (Table 5B). For the IL-6, the odds ratio was 1.844, with a p-value of 0.010, and for ALT, the odds ratio was 1.010, with a p-value of 0.045. Based on these results, whether the IL-6 has a diagnostic capability in predicting advanced fibrosis was evaluated using the ROC curve (Figure 3). The AUROC of IL-6 as 0.719, p-value of 0.037, and cut-off value of 5.25 or higher had a diagnostic capability to evaluate advanced fibrosis; however, the diagnostic capability for assessing advanced fibrosis was higher when the ratio was multiplied by AST. The AUROC of IL-6* AST value was 0.821 (p = 0.002) and the cut-off value was 228.15.
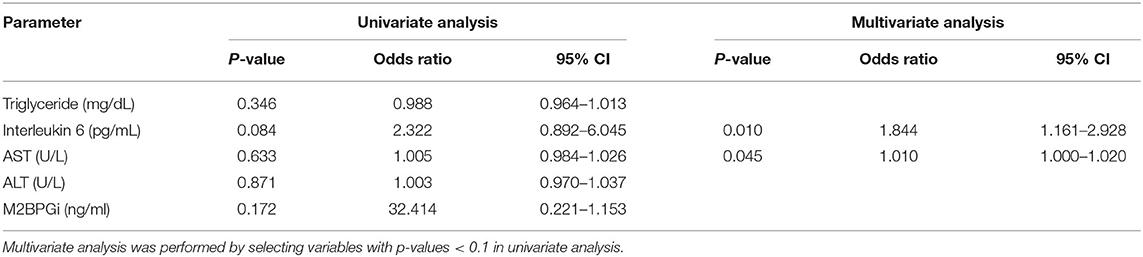
Table 5B. Univariate and multivariate analyses of the association between advanced fibrosis assessed by liver stiffness measurement (kPa) and other factors.
M2BPGi, which has already proven its diagnostic value for advanced fibrosis or cirrhosis in chronic liver disease, was evaluated to check whether it can be utilized in pediatric NAFLD. LSM (kPa) and M2BPGi showed a significant linear relationship in the Pearson correlation analysis (Pearson correlation coefficient = 0.382 with a p-value of 0.003) (Figure 4A). The diagnostic capability evaluation of advanced fibrosis with AUROC also showed an acceptable result (AUROC = 0.742 with a p-value of 0.022) (Figure 4B).
We prospectively studied the diagnostic capability of biomarkers for predicting each stage of NAFLD in children and adolescents with fatty liver disease (Figure 5). Biomarkers could be used to determine the status after treatment because NAFLD may not be completely resolved even if AST and ALT are normalized. In a series of processes from steatosis to steatohepatitis to fibrosis and to cirrhosis, inflammatory cytokines and ECM products are formed. This study evaluated each stage of NAFLD by measuring these substances.
Our major findings were as follows. First, previous studies evaluated that amino-terminal propeptide of type III procollagen (PIIINP) could be used as a biomarker, but in this study, P1NP, a popular test, as it is relatively convenient to measure and is frequently performed in osteoporosis patients, was evaluated as a biomarker and its diagnostic capability was confirmed. Second, previous studies evaluated that interleukin such as IL-8 and IL-18 could be secreted during proinflammatory or inflammatory processes and used as biomarkers, but this study evaluated fibrosis stage with IL-6, which is secreted during the regeneration process after an inflammatory response. Third, this study initially evaluated that M2BPGi, a known biomarker to predict advanced fibrosis or cirrhotic changes in chronic liver disease, has the diagnostic capability in pediatric NAFLD. Taken together, the measurements of P1NP, IL-6 or M2BPGi along with the basic chemistry tests would help determine the stage of NAFLD they correspond to at the time of initial diagnosis and predict responsiveness after the treatment.
Our first major finding is the usefulness of the P1NP/osteocalcin ratio or P1NP/ALP ratio to evaluate steatohepatitis (in the process of fibrogenesis). In the case of PIIINP, a procollagen known as a biomarker, it is difficult to measure because it is not a widely used test, so we tried to evaluate other ECM products. Among various ECM products, P1NP, one of procollagen, was selected as an evaluation biomarker because it is widely used to evaluate the treatment response of osteoporosis patients. The advantage of P1NP is that results can be obtained by collecting blood at most hospitals in Korea, and the price is lower than that of other collagens (about 33.1 U.S. $ without insurance adjustment). Additionally, the results can be obtained within 2 h on the same day.
Some other studies have also suggested that P1NP is formed in the liver during fibrogenesis (26–28). These are recent studies and includes adult patients with NAFLD. An animal using mice with bile duct ligation-induced fibrosis also confirmed P1NP formation during fibrogenesis (29). We think that inflammation (steatohepatitis) and fibrogenesis (early fibrosis) are mixed because they are a series of processes. As a result of our study, P1NP was significantly evaluated on the basis of the FAST score (steatohepatitis); thus, we believe that P1NP predicts the stage of inflammation with early fibrosis rather than advanced fibrosis. However, as the normal levels of P1NP in children and adolescent patients are different because it is bone-related factor, we planned to correct P1NP using osteocalcin or ALP. As can be seen from the results, the statistical significance could not be confirmed with P1NP alone; however, the statistical significance was confirmed after correction by osteocalcin or ALP.
Hepatocyte injury in hepatitis results in the leakage of enzymes into the circulation. Therefore, both AST and ALT act as important liver function tests that are elevated in patients with NAFLD. However, ALT is more specific for liver damage since it is found primarily in the liver; however, the AST is also found in several other organs. Therefore, in our study, both AST and ALT were statistically significant in an early liver injury situation; nevertheless, the p-value of ALT (<0.001) was lower than the p-value of AST (0.006), and ALT was selected in the logistic regression analysis in Table 5A. Therefore, based on the results of the logistic regression analysis, it was confirmed that the diagnostic capability using the AUROC curve was further increased when the above-mentioned P1NP/osteocalcin ratio and ALT were multiplied (Figure 3).
Our second major finding is the usefulness of the IL-6, an inflammatory cytokine, to evaluate advanced fibrosis (30). IL-6 is involved in immune, hematopoietic, and inflammatory responses by acting on T cells, hepatocytes, and hematopoietic stem cells. IL-6 increases rapidly even during infections, inflammation, and trauma, and shows high serum concentrations in rheumatoid arthritis and autoimmune diseases. We initially checked whether there are other infections of inflammatory signs currently through the questionnaire. We initially hypothesized that IL-6 might be involved in the early inflammatory stage of NAFLD because it is an inflammatory cytokine. However, herein, it was meaningfully evaluated in the analysis of the advanced stage of fibrosis. Considering the reasons for the result, other studies demonstrated that a strong activation of the transcription factors has been described through animal studies using mice, resulting in the enhanced transcription of their target genes after the increase in the IL-6 serum levels (31, 32). Through these processes, IL-6 maintains adequate levels of anti-apoptotic factors and is an important mediator of liver regeneration (31–33). We believe that patients with elevated IL-6 levels in this study are in the process of regeneration as a late response after an injury. Therefore, it is considered to be highly measured in patients with advanced fibrosis who have had inflammation for a long time. As a result, the value of IL-6 was significantly higher in patients who were LSM-positive (Table 3). The advantage of IL-6 is that the results can be obtained by collecting blood at most hospitals in Korea; additionally, the price is low compared to other inflammatory cytokines (~72.8 USD without insurance adjustment). Moreover, the results can be obtained within 1 week. In the case of TNF, it takes a month to obtain the test results and the cost is three times that of IL-6.
The fact that AST showed more significant difference than ALT in patients with advanced fibrosis can be explained in the same context. Several studies have been conducted on the AST/ALT ratio in patients with NAFLD. Although this ratio is used to distinguish fibrosis from alcoholic fatty liver, it is also used to differentiate between early and advanced fibroses. The AST/ALT ratio, with a value >0.8, is considered to be associated with advanced fibrosis (34, 35). Therefore, in our study, it seems that AST, not ALT, was evaluated as a significant factor in the logistic regression analysis performed on the basis of LSM positivity. Therefore, based on the results of a logistic regression analysis, it was confirmed that the diagnostic capability with the AUROC curve was further increased when the above-mentioned IL-6 and AST were multiplied (Figure 4).
Our third major finding is that M2BPGi has a diagnostic capability even in pediatric NAFLD. M2BPGi has proven to be an appropriate biomarker to evaluate advanced fibrosis or cirrhotic changes in chronic liver disease. Regarding adults, there are various articles evaluating the diagnostic capability of patients with NAFLD (36–39). However, regarding children, there are only articles evaluating biliary atresia or multiple diseases together (40–43). It is of great significance that we evaluated the diagnostic capability of M2BPGi for assessing the advanced fibrosis status in pediatric patients with NAFLD for the first time. Although the AUROC value did not exceed 0.8, it showed a cut-off value (1.35 ng/ml) similar to that of the F3 state (1.57 ng/ml) in the adult study with a statistically significant p-value (38). Therefore, we concluded that M2BPGi can be applied to diagnose an advanced fibrosis status in pediatric NAFLD.
To correct the errors that occur when dividing by only one criterion FAST score or LSM, an analysis of subgroups was also conducted using both FAST score and LSM. Supplementary Figure 1 shows the results of biomarker comparisons between the group with both negative FAST scores and LSM, and the group with only positive FAST scores (LSM is negative). In this subgroup, the group with only positive FAST score can be considered specific for steatohepatitis (early fibrosis). As can be seen in the figure, only the P1NP/osteocalcin ratio was statistically significantly evaluated (p-value = 0.043). Supplementary Figure 2 shows the biomarker results compared by dividing the group with both positive FAST scores and LSM, and the group with only positive FAST scores (LSM is negative). In this subgroup, the group with both positive LSM and FAST scores can be considered specific for advanced fibrosis. As can be seen in the figure, M2BPGi and IL-6 show a statistically significant difference for these subgroups (p-value = 0.002, 0.001, respectively).
The major limitation of this study is that liver biopsy was not performed and not directly compared to the histologic grade because the most accurate NAFLD diagnosis method is still a biopsy. However, the importance of non-invasive diagnoses is emerging in adults; additionally, non-invasiveness is particularly important in children. We conducted the study on the basis of recent papers with high reliability in the criteria for determining positive and negative results. Another limitation of this study is that information on pubertal status was not collected. Since previous studies have found that steatosis, portal inflammation, and fibrosis are less severe during and after puberty than before puberty in subjects with NAFLD, a possible association between puberty status and NAFLD should have considered.
We believe that by evaluating the stage of NAFLD, a more systematic approach for MAFLD and appropriate treatment intervention will be possible. Appropriate treatment at each stage can be suggested by utilizing dietary modifications, exercise, and medications.
In conclusion, non-invasive biomarkers can be used to predict each stage of NAFLD in children. The value of P1NP/osteocalcin or ALP ratio* ALT has a diagnostic capability to assess the stage of steatohepatitis or in the process of fibrogenesis of NAFLD. The value of IL-6 * AST has a diagnostic capability to assess the stage of advanced fibrosis of NAFLD. M2BPGi, a known biomarker to predict advanced fibrosis or cirrhotic changes in chronic liver disease, has shown a diagnostic capability even in pediatric NAFLD. Our findings suggest that measuring P1NP, IL-6 or M2BPGi along with the basic chemistry tests would help determine the stage of NAFLD they correspond to at the time of initial diagnosis and predict responsiveness after the treatment. A more systematic approach for MAFLD and appropriate treatment intervention will be possible by evaluating the stage of NAFLD.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by Name of the Ethics Committee and reference: Samsung Medical Center IRB File No.: 2021-02-121. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
MK and YC: conception or design. YK and EK: acquisition, analysis, or interpretation of data. YK and MK: drafting the work or revising and final approval of the manuscript. All authors read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.846273/full#supplementary-material
1. Dowman JK, Tomlinson J, Newsome P. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Therap. (2011) 33:525–40. doi: 10.1111/j.1365-2036.2010.04556.x
2. Lăpădat A, Jianu I, Ungureanu B, Florescu L, Gheonea D, Sovaila S, et al. Non-invasive imaging techniques in assessing non-alcoholic fatty liver disease: a current status of available methods. J Med Life. (2017) 10:19. doi: 10.25122/jml-2017-0019
3. Pais R, Lupşor M, Poantă L, Silaghi A, Rusu M, Badea R. Liver biopsy versus noninvasive methods–fibroscan and fibrotest in the diagnosis of non-alcoholic fatty liver disease: a review of the literature. Rom J Intern Med. (2009) 47:331–40.
4. Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. (2016) 46:862–70. doi: 10.1111/hepr.12647
5. Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int. (2011) 31:461–73. doi: 10.1111/j.1478-3231.2011.02451.x
6. Long MT, Gandhi S, Loomba R. Advances in non-invasive biomarkers for the diagnosis and monitoring of non-alcoholic fatty liver disease. Metabolism. (2020) 111:154259. doi: 10.1016/j.metabol.2020.154259
7. Wyllie R, Hyams JS, Kay M. Pediatric Gastrointestinal and Liver Disease E-Book. Amsterdam: Elsevier Health Sciences (2020).
8. Shirabe K, Bekki Y, Gantumur D, Araki K, Ishii N, Kuno A, et al. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: more than a biomarker of liver fibrosis. J Gastroenterol. (2018) 53:819–26. doi: 10.1007/s00535-017-1425-z
9. Tamaki N, Kurosaki M, Loomba R, Izumi N. Clinical utility of Mac-2 binding protein glycosylation isomer in chronic liver diseases. Ann Lab Med. (2021) 41:16–24. doi: 10.3343/alm.2021.41.1.16
10. Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JMB, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastroint Liver Physiol. (2015) 308:G807–30. doi: 10.1152/ajpgi.00447.2014
11. Villesen IF, Daniels SJ, Leeming DJ, Karsdal MA, Nielsen MJ. the signalling and functional role of the extracellular matrix in the development of liver fibrosis. Aliment Pharmacol Therap. (2020) 52:85–97. doi: 10.1111/apt.15773
12. Di Sessa A, Umano GR, Miraglia del Giudice E. The association between non-alcoholic fatty liver disease and cardiovascular risk in children. Children. (2017) 4:57. doi: 10.3390/children4070057
13. Wong CR, Lim JK. The association between nonalcoholic fatty liver disease and cardiovascular disease outcomes. Clin Liver Dis. (2018) 12:39. doi: 10.1002/cld.721
14. Eslam M, Alkhouri N, Vajro P, Baumann U, Weiss R, Socha P, et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: an international expert consensus statement. Lancet Gastroenterol Hepatol. (2021) 6:864–73. doi: 10.1016/S2468-1253(21)00183-7
15. Di Sessa A, Guarino S, Umano GR, Arenella M, Alfiero S, Quaranta G, et al. MAFLD in obese children: a challenging definition. Children. (2021) 8:247. doi: 10.3390/children8030247
16. Madsen JO, Jørgensen NR, Pociot F, Johannesen J. Bone turnover markers in children and adolescents with type 1 diabetes—a systematic review. Pediatric Diabetes. (2019) 20:510–22. doi: 10.1111/pedi.12853
17. Choi JS, Park I, Lee SJ, Ju HJ, Lee H, Kim J. Serum procollagen type I N-terminal propeptide and osteocalcin levels in Korean children and adolescents. Yonsei Med J. (2019) 60:1174–80. doi: 10.3349/ymj.2019.60.12.1174
18. Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De Francisco A, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocrine Dis. (2013) 13:1–10. doi: 10.1186/1472-6823-13-47
19. Qu H-Q, Li Q, Rentfro AR, Fisher-Hoch SP, McCormick JB. The definition of insulin resistance using HOMA-IR for Americans of Mexican descent using machine learning. PLoS ONE. (2011) 6:e21041. doi: 10.1371/journal.pone.0021041
20. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan W-K, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. (2020) 5:362–73. doi: 10.1016/S2468-1253(19)30383-8
21. Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. (2007) 46:32–6. doi: 10.1002/hep.21669
22. Kang B-K, Kim M, Shin S-J, Kim YJ. Correlation of clinical and histopathologic parameters with ultrasonographic grades in pediatric non-alcoholic fatty liver disease. J Korean Med Sci. (2019) 34:e298. doi: 10.3346/jkms.2019.34.e298
23. Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children with nonalcoholic fatty liver disease (NAFLD). J Pediatric Gastroenterol Nutr. (2011) 53:190. doi: 10.1097/MPG.0b013e31821b4b61
24. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1717–30. doi: 10.1053/j.gastro.2019.01.042
25. Anderson LN, Carsley S, Lebovic G, Borkhoff CM, Maguire JL, Parkin PC, et al. Misclassification of child body mass index from cut-points defined by rounded percentiles instead of Z-scores. BMC Res Notes. (2017) 10:1–4. doi: 10.1186/s13104-017-2983-0
26. Luger M, Kruschitz R, Kienbacher C, Traussnigg S, Langer FB, Schindler K, et al. Prevalence of liver fibrosis and its association with non-invasive fibrosis and metabolic markers in morbidly obese patients with vitamin D deficiency. Obesity Surgery. (2016) 26:2425–32. doi: 10.1007/s11695-016-2123-2
27. Wang N, Wang Y, Chen X, Zhang W, Chen Y, Xia F, et al. Bone turnover markers and probable advanced nonalcoholic fatty liver disease in middle-aged and elderly men and postmenopausal women with type 2 diabetes. Front Endocrinol. (2020) 10:926. doi: 10.3389/fendo.2019.00926
28. Deng H, Dai Y, Lu H, Li S, Gao L, Zhu D. Analysis of the correlation between non-alcoholic fatty liver disease and bone metabolism indicators in healthy middle-aged men. Eur Rev Med Pharmacol Sci. (2018) 22:1457–62. doi: 10.26355/eurrev_201803_14493
29. Veidal SS, Vassiliadis E, Bay-Jensen A-C, Tougas G, Vainer B, Karsdal MA. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrog Tissue Repair. (2010) 3:1–7. doi: 10.1186/1755-1536-3-5
30. Van Snick J. Interleukin-6: an overview. Ann Rev Immunol. (1990) 8:253–78. doi: 10.1146/annurev.iy.08.040190.001345
31. Bansal MB, Kovalovich K, Gupta R, Li W, Agarwal A, Radbill B, et al. Interleukin-6 protects hepatocytes from CCl4-mediated necrosis and apoptosis in mice by reducing MMP-2 expression. J Hepatol. (2005) 42:548–56. doi: 10.1016/j.jhep.2004.11.043
32. Jin X, Zimmers TA, Perez EA, Pierce RH, Zhang Z, Koniaris LG. Paradoxical effects of short-and long-term interleukin-6 exposure on liver injury and repair. Hepatology. (2006) 43:474–84. doi: 10.1002/hep.21087
33. Streetz K, Luedde T, Manns M, Trautwein C. Interleukin 6 and liver regeneration. Gut. (2000) 47:309–12. doi: 10.1136/gut.47.2.309
34. Sattar N, Forrest E, Preiss D. Non-alcoholic fatty liver disease. BMJ. (2014) 349:g4596. doi: 10.1136/bmj.g4596
35. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. (2010) 59:1265–9. doi: 10.1136/gut.2010.216077
36. Jang SY, Tak WY, Park SY, Kweon Y-O, Lee YR, Kim G, et al. Diagnostic efficacy of serum Mac-2 binding protein glycosylation isomer and other markers for liver fibrosis in non-alcoholic fatty liver diseases. Ann Lab Med. (2021) 41:302–9. doi: 10.3343/alm.2021.41.3.302
37. Cheng Y-M, Wang C-C. Serum Mac-2 binding protein glycosylation isomer (M2BPGi) can predict mild or significant liver fibrosis in non-alcoholic fatty liver disease. Hepat Mon. (2021) 21:e115400. doi: 10.5812/hepatmon.115400
38. Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. (2015) 50:776–84. doi: 10.1007/s00535-014-1007-2
39. Ogawa Y, Honda Y, Kessoku T, Tomeno W, Imajo K, Yoneda M, et al. Wisteria floribunda agglutinin-positive Mac-2-binding protein and type 4 collagen 7S: useful markers for the diagnosis of significant fibrosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. (2018) 33:1795–803. doi: 10.1111/jgh.14156
40. Behairy OG, El-Gendy SA, Ibrahim DY, Mansour AI, El-Shimi OS. Mac-2 binding protein glycan isomer as noninvasive tool to assess liver fibrosis in children with chronic liver disease. Hepatol Res. (2021) 51:277–83. doi: 10.1111/hepr.13608
41. El-Gendy S, Behairy O, Mansour A, El-Shimi O, Ibrahim D. Rule of Mac-2 binding protein glycan isomer in assessment of liver fibrosis in children with chronic liver disease. Benha J Appl Sci. (2020) 5:1–9. doi: 10.21608/bjas.2020.135971
42. Ueno T, Kodama T, Noguchi Y, Nomura M, Saka R, Takama Y, et al. Serum Mac-2-binding protein (M2BPGi) as a marker of chronological liver fibrosis in biliary atresia patients with cirrhosis. Pediatric Surgery Int. (2019) 35:1065–70. doi: 10.1007/s00383-019-04535-9
Keywords: non-alcoholic fatty liver disease (NAFLD), liver steatosis, liver steatohepatitis, liver fibrosis, children
Citation: Kwon Y, Kim ES, Choe YH and Kim MJ (2022) Stratification by Non-invasive Biomarkers of Non-alcoholic Fatty Liver Disease in Children. Front. Pediatr. 10:846273. doi: 10.3389/fped.2022.846273
Received: 31 December 2021; Accepted: 21 February 2022;
Published: 04 April 2022.
Edited by:
Claudia Mandato, AORN Santobono-Pausilipon, ItalyReviewed by:
Anna Di Sessa, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Kwon, Kim, Choe and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mi Jin Kim, bWlqaW4xMjE3LmtpbUBzYW1zdW5nLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.