
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 21 March 2022
Sec. Children and Health
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.842519
This article is part of the Research Topic Biomarkers to Predict, Prevent and Find the Appropriate Treatments of Disorders in Childhood View all 14 articles
Hypospadias is one of the most common congenital external genital malformations, which is characterized by abnormal urethral meatus. However, the etiology remains to be incompletely understood. HAAO is a gene that encodes a protein, which catalyzes the synthesis of quinolinic acid, and has been identified as a risk gene for hypospadias. Thus, this study was conducted to elaborate the association between HAAO gene polymorphism rs3816183 T>C and hypospadias in the largest hypospadias cohort from Asia, including 577 patients and 654 healthy controls in China. The strength of interrelation was evaluated using 95% confidence intervals (CIs) and odds ratios (ORs). Based on the stratified analysis of hypospadias subtypes, it was found that the HAAO risk allele rs386183[T] enhances the susceptibility for hypospadias among patients with anterior/middle hypospadias subtypes (adjusted OR = 1.31, 95% CI = 1.05–1.64, p = 0.017). Enhanced risk of hypospadias in the entirety could not be demonstrated (OR = 1.20, 95% CI = 1.00–1.47, p = 0.054). In summary, our study found that the rs3816183[T] polymorphism is associated with increased risk of anterior/middle hypospadias among Southern Han Chinese children. The mechanisms by which the variations in the HAAO gene require further research.
Hypospadias is one of the most common congenital external genital malformations, which is characterized by abnormal urethral meatus (1), and affects approximately 20.9 out of every 10,000 births and has shown significant increases worldwide (2). Over the past decade, an increasing trend in the prevalence of hypospadias has been observed in China (3, 4). The clinical characteristics of hypospadias include proximal urethral opening, ventrally deficient hooded prepuce, and chordee (5). Hypospadias can be classified into two subgroups based on the urethral meatus location: anterior/middle hypospadias and posterior hypospadias (6). The meatus localization is best evaluated during surgery when chordee is corrected.
Although the surgical approach to hypospadias treatment has a great progress over the past decades, its etiology remains incompletely understood (1, 7–9). Individual phenotypic differences, such as disease susceptibility, survival, and treatment response, were identified to be associated with different genetic variants (10). Genetic variants have been observed to be associated with hypospadias risk (11, 12). However, very few studies have focused on variants in potential genes, such as DGKK, MAMLD1, MID1, CYP1A1, GSTM1, and GSTT1, which are associated with susceptibility to hypospadias (9). Some single-nucleotide polymorphisms (SNPs) have been reported in association to hypospadias. Nevertheless, recent studies used small sample sizes and have not been consistently replicated (13, 14).
Geller et al. conducted a genome-wide association study (GWAS) and reported that 17 SNPs were independently associated with hypospadias (15). Yoshiyuki validated these 17 SNPs in a Japanese cohort. However, only HAAO rs3816183 T>C was significantly associated with an increased risk toward hypospadias (16). Considering that ethnic differences exist at some loci, it would prove meaningful to evaluate the effect of SNPs on hypospadias susceptibility in different ethnic groups. Thus, we conducted this study to validate the association of HAAO rs3816183 T>C polymorphism with hypospadias susceptibility.
We recruited 557 isolated hypospadias patients at the Guangzhou Women and Children's Medical Center from January 2016 to December 2019, all of whom were Han Chinese, and the diagnosis was confirmed by pediatric urologists before surgery repair. Hypospadias classification was performed by experienced pediatric urologists at our center. The meatus localization is best evaluated during surgery when chordee is corrected. Based on the urethral orifice, the patients were divided into two groups: patients with anterior/middle hypospadias were defined as having a urethral opening in glanular, subcoronal, distal penile, and midshaft penile areas, while patients with posterior hypospadias were identified as having the urethral opening in penoscrotal, scrotal, and perineal areas. The control group included 654 male children without a medical history of hypospadias, who were selected from the Guangzhou Women and Children's Medical Center. Since hypospadias can be inherited, all the patients and controls group with a first-degree relative who suffers from hypospadias were excluded.
Informed consent was obtained from all patients' parents or legal guardians. This study was approved by the Ethics Committee of Guangzhou Women and Children's Medical Center in China.
Genomic DNA was extracted from venous blood samples using TIANamp Blood DNA kits (Catalog No. DP335-02; TIANGEN Biotech Co. Ltd., Beijing, China) following the manufacturer's instructions (17). NanoPhotometer® N50 (Implen GmbH., Munich, Germany) was used to assess DNA purity and concentration. Genomic DNA was amplified using the ABI-7900 real-time quantitative PCR instrument (Applied Biosystems, Foster City, CA, USA) and was subjected to HAAO rs3816183 TaqMan genotyping (18). PCR reactions were run as described in the previous study (19) using TaqMan® SNP Genotyping Assays (Catalog No: 4351379,C_180222_20, Thermo Fisher, USA) and TIANexact genotyping qPCR PreMix (Probe) (Catalog No. FP211-02; TIANGEN Biotech Co. Ltd., Beijing, China). In addition, 10% of DNA samples were selected randomly for second genotyping. The accuracy of data was ensured by the replicated samples with 100% consistency (19).
SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) and GraphPad Prism version 8 (GraphPad Software, Inc., La Jolla, California, USA) were used to perform statistical analyses. Hardy–Weinberg equilibrium (HWE) test was performed in the control group using a goodness-of-fit chi-squared test. SNPs were analyzed for association with hypospadias susceptibility by comparing the risk of allele frequency (allelic test) in patients and controls, along with other tests using PLINK 1.9 (20). Association was stratified by subgroup through comparing controls with cases with a certain subgroup. A p-value of 0.05 was considered statistically significant (21).
In the present study, 534 of 557 patients and 634 of 654 controls could be successfully genotyped. The frequencies of controls and patients group genotypes are shown in Table 1. The frequency distribution of the rs3816183[T] genotype in the control groups was consistent with HWE (p = 0.64). The HAAO rs3816183 TT phenotype was associated with an increased risk of hypospadias (TT vs. CC: OR = 1.57, 95% CI = 1.12–2.19, p = 0.008). Nevertheless, the results showed that the HAAO rs3816183[T] polymorphism may not be associated with hypospadias susceptibility in dominant and recessive models (adjusted OR = 1.19, p = 0.15/adjusted OR = 1.59, p = 0.06).
Hypospadias can be divided into different subtypes based on the urethral meatus location after penile degloving. The HAAO risk allele rs3816183[T] was associated with an increased susceptibility toward anterior/middle hypospadias (OR = 1.35, 95% CI = 1.08–1.68, p < 0.01). Nevertheless, no significant association was found between the HAAO risk allele rs3816183 T and patients with posterior hypospadias (OR = 1.03, 95% CI = 0.80–1.32, p = 0.81).
Hypospadias is a complex, congenital, external genitalia malformation. Genetic factors are important causative reason in the development of hypospadias (11, 12). Kojima et al. replicated rs3816183 of HAAO polymorphism with hypospadias and found that rs3816183 [T] was significantly increased the hypospadias susceptibility toward both posterior and anterior/middle hypospadias (16). However, the HAAO rs3816183 polymorphism was only significantly associated with an increased susceptibility toward anterior/middle hypospadias susceptibility in the present study. Therefore, our study demonstrated that HAAO rs3816183 polymorphism is not equally associated with hypospadias risk in different populations.
The HAAO gene, which is widely distributed in various organs (22–24), encodes a protein that catalyzes the synthesis of quinolinic acid (QUIN) from 3-hydroxyanthranilic acid. Huang et al. showed that hypermethylation of the HAAO gene predicts disease-free survival in patients with endometrioid endometrial cancer (25). Martin et al. reported that hypercholesterolemia and atherosclerosis may be treated and prevented by targeting the HAAO gene (26). Previous studies have demonstrated that the HAAO gene is associated with cancer biomarkers and degenerative diseases. The relationship between the HAAO gene and developmental disorders has also been reported. HAAO has also been correlated with congenital malformations and miscarriage and, when combined with environmental factors, may impair embryo outcomes (27). Pathogenesis of hypospadias has been attributed to the incomplete fusion of the urethra in a portion of the penis and the expression of HAAO in male mouse genital tubercle. Moreover, genetic variants of HAAO may specifically impede the migration and proliferation of normal urethral cells. We hypothesized that the HAAO rs3816183 T>C polymorphism may disrupt the metabolism of its encoded protein leading to disorders of NAD synthesis, which contribute to the pathogenesis of hypospadias (Figure 1). Similar genetic studies have suggested that rs3816183[T] HAAO polymorphisms may result in increased hypospadias susceptibility (16). However, in our study, the association between rs3816183 T>C HAAO polymorphism and hypospadias susceptibility was observed in anterior/middle group but not in posterior hypospadias patients. This discrepancy could be attributed to the sample size and ethnic differences in patients. In addition, causes of hypospadias may be genetic, maternal, environmental, or a combination of all of these factors. Posterior hypospadias have been reported to be associated with maternal factors, such as oligohydramnios, premature birth, and hypertension, suggesting that the underlying placental insufficiency may be an important contributing factor (28). Environmental factors, such as phthalates, have been associated with a toxic effect on the male reproductive system and the development of hypospadias (29). The fact that there may be many complex causes for hypospadias and that the environmental and maternal factors were not accounted for in our study could be the reason that the HAAO rs3816183 variants was found to be associated only with anterior/middle hypospadias.
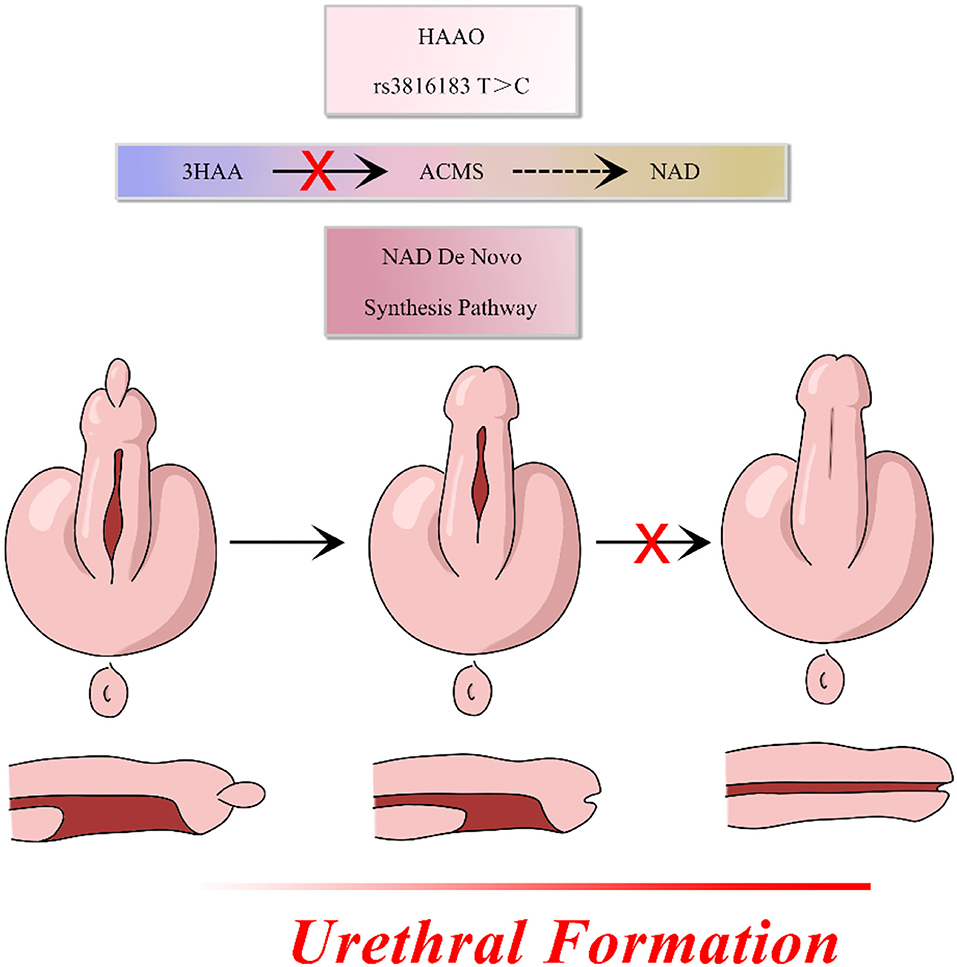
Figure 1. HAAO rs3816183 T>C polymorphism may disrupt the metabolism of its encoded protein leading to disorders of NAD synthesis, which contribute to the pathogenesis of hypospadias.
This is the largest Asian case–control study to investigate the association of HAAO polymorphism rs3816183 T>C with hypospadias susceptibility. Our results demonstrated that the SNPs rs3816183[T] in HAAO may be associated with increased anterior/middle hypospadias but not posterior hypospadias (Table 2), suggesting that HAAO may influence distal part of penile urethral formation.
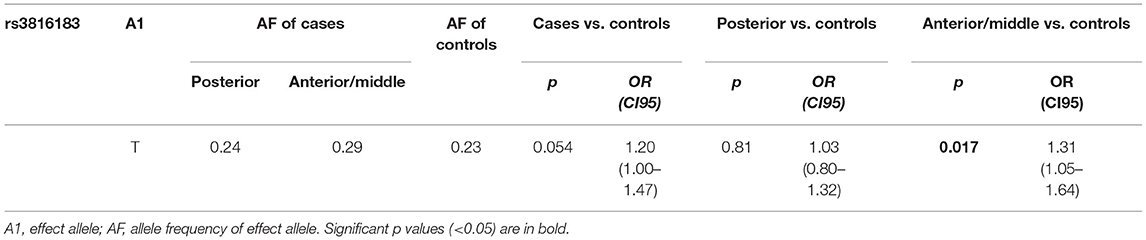
Table 2. Stratification analysis to evaluate the association between HAAO rs3816183 T>C polymorphism and hypospadias susceptibility (by subgroup).
However, there were some limitations to this study. First, environmental factors, such as difference in diet and geographic locations, were not analyzed. Second, in-depth exploration of HAAO rs3816183T>C and hypospadias sensitivity mechanisms is required. This may have potential implications for hypospadias prevention. Finally, multiple center studies are warranted to confirm our findings.
The HAAO rs3816183[T] is associated with increased risk to anterior/middle hypospadias in Southern Han Chinese population. Our findings support the hypothesis that the mechanism underlying the variations in the HAAO gene may contribute to the pathogenesis of hypospadias and thus requires in-depth research.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethical Standards of the Institutional Review Board of Guangzhou Women and Children's Medical Center (NO. 39401). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
FD designed experiment. YL, WF, KF, XZ, WJ, NW, GL, and FD collected samples and conducted the study. YZ and XZ analyzed the data. YL and FD wrote the paper. All authors have read and approved the manuscript.
FD thanks the fund from Guangzhou Institute of Pediatrics/Guangzhou Women and Children's Medical Center (Grant No. 0190026) and Science and Technology Project of Guangzhou (Grant No. 202102010238).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Carmichael SL, Shaw GM, Lammer EJ. Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence. Birth Defects Res A, Clin Molec Teratol. (2012) 94:499–510. doi: 10.1002/bdra.23021
2. Yu X, Nassar N, Mastroiacovo P, Canfield M, Groisman B, Bermejo-Sánchez E, et al. Hypospadias prevalence and trends in international birth defect surveillance systems, 1980-2010. Eur Urol. (2019) 76:482–90. doi: 10.1016/j.eururo.2019.06.027
3. Li Y, Mao M, Dai L, Li K, Li X, Zhou G, et al. Time trends and geographic variations in the prevalence of hypospadias in China. Birth Defects Res A, Clin Molec Teratol. (2012) 94:36–41. doi: 10.1002/bdra.22854
4. Sun G, Tang D, Liang J, Wu M. Increasing prevalence of hypospadias associated with various perinatal risk factors in Chinese newborns. Urology. (2009) 73:1241–5. doi: 10.1016/j.urology.2008.12.081
5. Fredell L, Kockum I, Hansson E, Holmner S, Lundquist L, Läckgren G, et al. Heredity of hypospadias and the significance of low birth weight. J Urol. (2002) 167:1423–7. doi: 10.1016/S0022-5347(05)65334-7
7. Cunha GR, Sinclair A, Risbridger G, Hutson J, Baskin LS. Current understanding of hypospadias: relevance of animal models. Nat Rev Urol. (2015) 12:271–80. doi: 10.1038/nrurol.2015.57
8. Leung AK, Robson WL. Hypospadias: an update. Asian J Androl. (2007) 9:16–22. doi: 10.1111/j.1745-7262.2007.00243.x
9. van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV, Roeleveld N. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. (2012) 18:260–83. doi: 10.1093/humupd/dms002
10. Lu YF, Goldstein DB, Angrist M, Cavalleri G. Personalized medicine and human genetic diversity. Cold Spring Harb Perspect Med. (2014) 4:a008581. doi: 10.1101/cshperspect.a008581
11. George M, Schneuer FJ, Jamieson SE, Holland AJ. Genetic and environmental factors in the aetiology of hypospadias. Pediatr Surg Int. (2015) 31:519–27. doi: 10.1007/s00383-015-3686-z
12. Söderhäll C, Körberg IB, Thai HT, Cao J, Chen Y, Zhang X, et al. Fine mapping analysis confirms and strengthens linkage of four chromosomal regions in familial hypospadias. Eur J Human Genet. (2015) 23:516–22. doi: 10.1038/ejhg.2014.129
13. Kojima Y, Kohri K, Hayashi Y. Genetic pathway of external genitalia formation and molecular etiology of hypospadias. J Pediatr Urol. (2010) 6:346–54. doi: 10.1016/j.jpurol.2009.11.007
14. van der Zanden LF, van Rooij IA, Feitz WF, Knight J, Donders AR, Renkema KY, et al. Common variants in dgkk are strongly associated with risk of hypospadias. Nat Genet. (2011) 43:48–50. doi: 10.1038/ng.721
15. Geller F, Feenstra B, Carstensen L, Pers TH, van Rooij IA, Körberg IB, et al. Genome-wide association analyses identify variants in developmental genes associated with hypospadias. Nat Genet. (2014) 46:957–63. doi: 10.1038/ng.3063
16. Kojima Y, Koguchi T, Mizuno K, Sato Y, Hoshi S, Hata J, et al. Single nucleotide polymorphisms of haao and irx6 genes as risk factors for hypospadias. J Urol. (2019) 201:386–92. doi: 10.1016/j.juro.2018.07.050
17. Lu T, Li L, Zhu J, Liu J, Lin A, Fu W, et al. Aurka Rs8173 G>C polymorphism decreases wilms tumor risk in chinese children. J Oncol. (2019) 2019:9074908. doi: 10.1155/2019/9074908
18. He J, Zhang X, Zhang J, Zhang R, Yang T, Zhu J, et al. Lmo1 Super-enhancer polymorphism Rs2168101 G>T correlates with decreased neuroblastoma risk in chinese children. J Cancer. (2018) 9:1592–7. doi: 10.7150/jca.24326
19. Deng F, Zhao J, Jia W, Fu K, Zuo X, Huang L, et al. Increased hypospadias risk by grem1 Rs3743104[G] in the southern han Chinese population. Aging. (2021) 13:13898–908. doi: 10.18632/aging.202983
20. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. Plink: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. (2007) 81:559–75. doi: 10.1086/519795
21. Liu J, Jia W, Hua RX, Zhu J, Zhang J, Yang T, et al. Apex1 polymorphisms and neuroblastoma risk in chinese children: a three-center case-control study. Oxid Med Cell Longev. (2019) 2019:5736175. doi: 10.1155/2019/5736175
22. Pawlak D, Tankiewicz A, Matys T, Buczko W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J Physiol Pharmacol. (2003) 54:175–89. doi: 10.1007/978-1-4615-0135-0_48
23. Schwarcz R, Okuno E, White RJ, Bird ED, Whetsell WO. 3-Hydroxyanthranilate oxygenase activity is increased in the brains of huntington disease victims. Proc Nation Acad Sci U S A. (1988) 85:4079–81. doi: 10.1073/pnas.85.11.4079
24. Köhler C, Eriksson LG, Okuno E, Schwarcz R. Localization of quinolinic acid metabolizing enzymes in the rat brain. Immunohistochemical studies using antibodies to 3-hydroxyanthranilic acid oxygenase and quinolinic acid phosphoribosyltransferase. Neuroscience. (1988) 27:49–76. doi: 10.1016/0306-4522(88)90219-9
25. Huang YW, Luo J, Weng YI, Mutch DG, Goodfellow PJ, Miller DS, et al. Promoter hypermethylation of cidea, haao and rxfp3 associated with microsatellite instability in endometrial carcinomas. Gynecol Oncol. (2010) 117:239–47. doi: 10.1016/j.ygyno.2010.02.006
26. Berg M, Polyzos KA, Agardh H, Baumgartner R, Forteza MJ, Kareinen I, et al. 3-Hydroxyanthralinic acid metabolism controls the hepatic srebp/lipoprotein axis, inhibits inflammasome activation in macrophages, and decreases atherosclerosis in ldlr-/- mice. Cardiovasc Res. (2019). doi: 10.1093/cvr/cvz258
27. Cuny H, Rapadas M, Gereis J, Martin E, Kirk RB, Shi H, et al. Nad deficiency due to environmental factors or gene-environment interactions causes congenital malformations and miscarriage in mice. Proc Natl Acad Sci U S A. (2020) 117:3738–47. doi: 10.1073/pnas.1916588117
28. Huisma F, Thomas M, Armstrong L. Severe hypospadias and its association with maternal-placental factors. Am J Med Genet A. (2013) 161a:2183–7. doi: 10.1002/ajmg.a.36050
Keywords: hypospadias, HAAO, single-nucleotide polymorphism (SNP), genetics, urethral abnormalities
Citation: Liu Y, Fu W, Fu K, Zuo X, Jia W, Wang N, Zhang Y, Liu G and Deng F (2022) HAAO rs3816183 Polymorphisms [T] Increase Anterior/Middle Hypospadias Risk in Southern Han Chinese Population. Front. Pediatr. 10:842519. doi: 10.3389/fped.2022.842519
Received: 23 December 2021; Accepted: 02 February 2022;
Published: 21 March 2022.
Edited by:
Ulrik Lausten-Thomsen, Copenhagen University Hospital Rigshospitalet, DenmarkReviewed by:
Lovro Lamot, University of Zagreb, CroatiaCopyright © 2022 Liu, Fu, Fu, Zuo, Jia, Wang, Zhang, Liu and Deng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuming Deng, Zm1fZGVuZ0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.