- 1Division of Allergy and Asthma, Department of Pediatrics, Hacettepe University Faculty of Medicine, Ankara, Turkey
- 2Department of Maternal and Child Health and Urological Sciences, Sapienza University of Rome, Rome, Italy
- 3Translational Research in Pediatric Specialities Area, Division of Allergy, Bambino Gesù Children's Hospital (IRCCS), Rome, Italy
There is evidence that in children with persistent IgE-mediated food allergy (FA) to cow's milk, hen's egg, and peanut, oral allergen-specific immunotherapy (OIT) may increase the reaction threshold to the culprit food allergen(s). OIT may protect patients from the occurrence of severe reactions in case of accidental ingestion of the culprit food during treatment. Notwithstanding, many gaps are still unsolved, including safety issues, identification of predictive biomarkers, and post-desensitization efficacy. In this perspective, the use of omalizumab (Anti-IgE monoclonal antibody) has been proposed as an adjunctive treatment to OIT in order to reduce the risk of allergic reactions related to OIT. This review aims to summarize the current evidence and unmet needs on OIT in children with FA to enhance the development of longitudinal, prospective, and well-designed studies able to fill the current gaps soon.
Introduction
Immunoglobulin E (IgE) mediated food allergies (FA) represent an adverse and potentially life-threatening condition caused by the exposure to a specific food allergen through an immediate IgE-mediated immunological mechanism (type 1 of Gell and Coombs) (1). Based on the underlying patho-mechanism, and specifically on the involvement of a hypersensitivity reaction, FA are generally classified into immunoglobulin (Ig)E-mediated FA, non-IgE mediated, and mixed ones (2). In the last few decades, reports show that FA prevalence has been increasing in industrialized countries (2–4). Globally the estimated incidence of FA ranges from 0.45 to 10% in infants and preschool-aged children, from 1 to 5% in school age, and about 4.5% in adult age (5–7). Although the majority of FA reactions are mild-moderate, they sometimes are severe and even fatal or near-fatal (8–12). Referring to retrospective case series, the fatality rate is estimated between 0.65 and 2% (13, 14).
The most allergenic foods are milk, egg, peanut, tree nut, wheat, soy, fish, and shellfish with a prevalence related to the age and local dietary habits (2, 4, 15–18). The natural history of FA is variable: the majority of children with allergies to egg, milk or soy allergy overcome their FAs, vice versa, FAs to peanuts, tree nuts, and seafood are more difficult to be resolved (18). Overall, for egg, tolerance is reached by 3 years of age in the 50% of cases and by school-age in 80%; for milk it is achieved by 5 years of age in about 50% of cases (19). Less than 10–20% of allergies to peanut or tree nuts achieves spontaneous clinical tolerance (20).
The negative impact of FAs on pediatric patients and their families' lives may be significant due to several reasons including: difficulties in practicing a strict allergen avoidance; possible nutritional impairment; fear of accidental exposure; feeling of being different from one's peers; absences from school and from work, respectively, for patients and their parents which generate a major detriment to a country's economy (21–27).
According to international guidelines, the current standard of care in the management of FA is the strict elimination diet and the use of adrenaline as rescue medication in case of severe allergic reactions, such as anaphylaxis (28). Alternative treatment strategies to the avoidance diet have been investigated; the most promising therapeutic strategies are currently oral immunotherapy (OIT) and biologicals (such as omalizumab) (29–31). Other routes of administrations other than the oral [such as epicutaneous (EPIT, epicutaneous immunotherapy) and sublingual ones (SLIT, sublingual immunotherapy)] have been investigated. Although their safety profile appears to be good, efficacy seems to be lower in both in magnitude and in number needed to treat than for OIT. Furthermore, there are no products for EPIT currently available on the market and none has been approved by a regulatory authority. This review aims to critically provide an overview on the current evidence and unmet needs related on OIT in children suffering from FA.
General Concepts on Allergen Immunotherapy
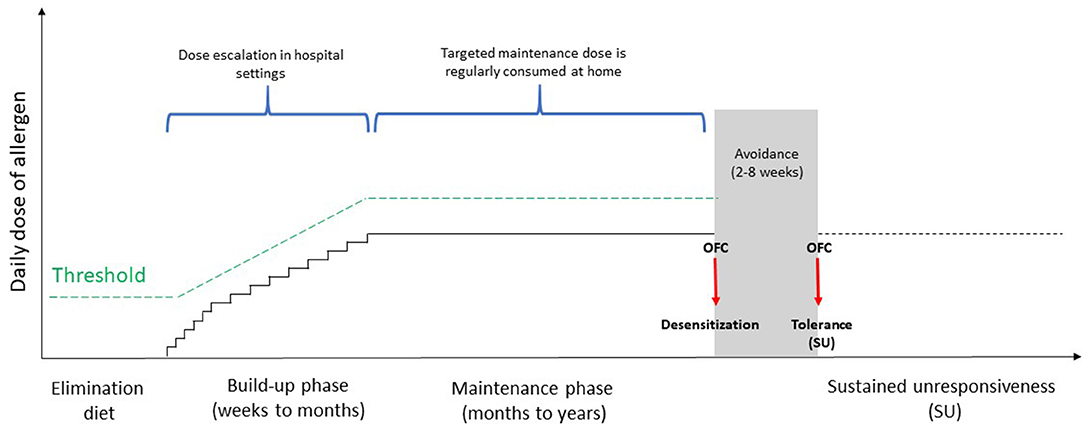
OIT consists of a titrated oral administration of the culprit food at regular intervals to induce tolerance [i.e., the possibility to take unlimited amounts of the culprit food without presenting reactions even after its intake is stopped indefinitely], starting with a build-up phase where increasing quantities of the food are administered in hospital. Usually, during the build-up phase the maximum tolerated dose is assumed daily at home in the interval during dose increases, usually on weekly or every other week basis. The build-up phase is followed by a maintenance phase with regular, daily intake of a maximum tolerated amount of food (32) (Figure 1). The protocols are heterogeneous and differ in relation to the type of food used (e.g., fresh or baked), the number of doses administered, the amount of allergenic protein per dose, the framework between the single doses and the maintenance one. OIT, as stated by the European Academy of Allergy and Clinical Immunology (EAACI), represents the only potentially curative treatment for FA so far, capable of modulating the immune system and modifying the natural history of disease (33). The primary aim of OIT is the increase of reactivity threshold in order to prevent patients from life-threatening events due to accidental ingestion of the culprit food (34, 35). The clinical effectiveness of OIT is commonly evaluated in terms of “desensitization” [i.e., an increase in the threshold of reactivity toward a specific food, allowing the patient to consume the culprit food without adverse reactions while continuing OIT (35–38)] and sustained unresponsiveness (SU) [i.e., the possibility to assume any amount of the incriminated food, even after a long period of its avoidance (34, 36, 39, 40)] (Figure 1). Factors associated with a greater chance of achieving SU include a longer duration of maintenance phase and younger age for commencing OIT (39). To date, there are no guidelines stating the perfect time when OIT should be discontinued prior to demonstrate SU but commonly it is a framework of 4–8 weeks. In addition, the length of time that SU must persist to confirm the achievement of tolerance, remains unknown, and it seems that this state may not be long-lasting after OIT (41).
Mechanisms of Allergen Immunotherapy
The mechanisms of action are not fully understood. It has been shown a reduction of specific IgE levels (after an initial raise) followed by an increase in specific immunoglobulin G4 (IgG4), reaching a balance in the achievement of tolerance. IgG4 compete with sIgE for allergen binding, suppressing the reactivity of mast cell and basophils, which are deprived of their preformed mediators through continuous degranulation (42, 43) (Figure 2).
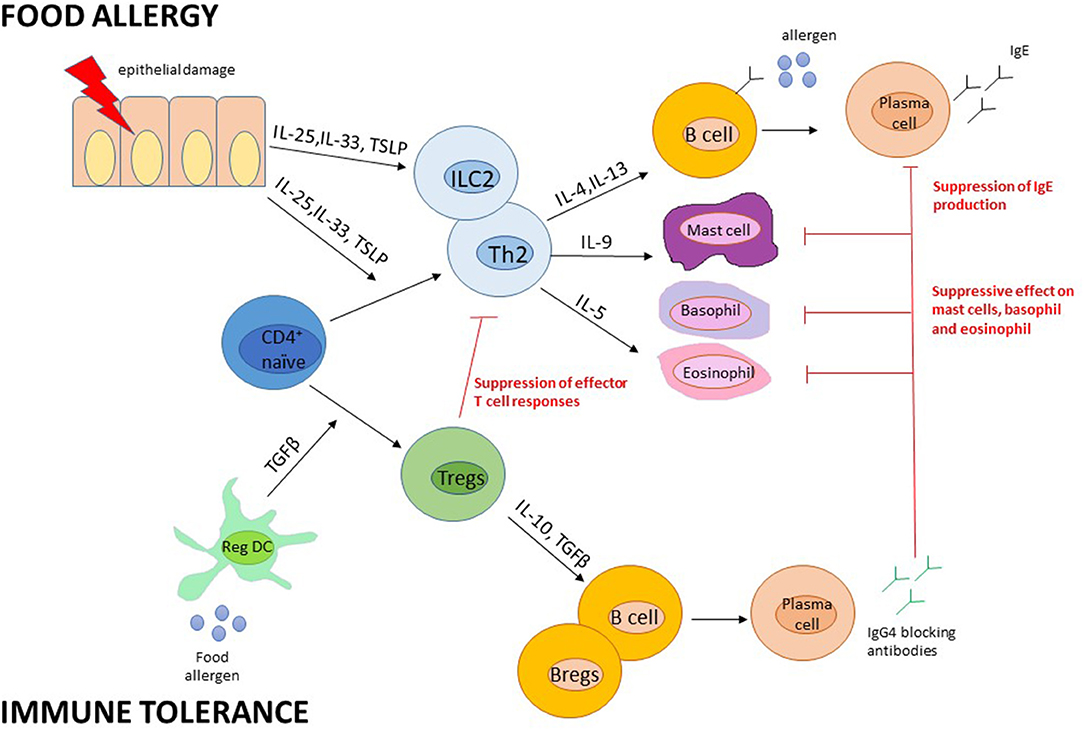
Figure 2. Presumed immune mechanisms in food allergy and immune tolerance. Adapted from Pajno et al. (43). B regs, B regulatory cells; DC, dendritic cells; Ig, Immunoglobulin; IL, interleukin; ILC, Innate Lymphoid cells; T regs, T regulatory cells; TSLP, thymic stromal lymphopoietin.
During OIT, thanks to the constant low dose allergen exposure, it is observed a reduced basophils activation with a global state of their hyporesponsiveness or anergy, not related to IgE levels. In fact, IgE generally increase after the onset of OIT, then remain elevated for months until they fall to baseline or lower levels. However, this OIT-induced basophils suppression is not definitive but seems to be necessary to maintain remission after OIT discontinuation (39).
Poor data are available on the role of mast cells in OIT, which has a long half-life that lasts from months to years. Recent studies on murine models, suggest that early degranulation of mast cells may have a pivotal role in desensitization and, similar to basophils, it provides a defense against allergens exposure (44, 45).
So it is reasonable to think that the suppression of mast cells and basophils activity due to continuous exposure to low-dose allergen is the basis of desensitization during OIT, and that the depletion of this suppression is related to the gradual reappearance of clinical reactivity in some patients after OIT suspension (39).
In OIT, the spotlight has been on T regulatory (Treg) cells with the conversion of allergen-specific T cells to anergic T cells, involving the epigenetic regulation of the FOXP3 (forkhead box P3) gene in allergen-specific Treg cells (46).
Potential Biomarkers
There is still a long way to find reliable biomarkers that can predict good responders to OIT and personalize the protocol schedule, including the duration of SU.
Some studies have shown that low skin prick tests' (SPTs) wheals diameters and serum sIgE at baseline and at the end of maintenance phase besides a reduced basophil reactivity may predict SU achievement; while IgG4 are not predictive of SU (39).
The regulatory markers, in particular FOXP3+ and latency associated peptide (LAP+) Tregs, seem to play a key-role in inducing long-term tolerance in patients successfully treated with OIT (47).
Contraindications to OIT
The major OIT contraindications include: non-IgE mediated allergy; uncontrolled asthma; treatments contraindicating adrenaline, low family compliance (33, 48). Further criticisms in OIT come from the lack of standardized protocols, the spontaneous development of tolerance especially for cow's milk and egg, the need for patients' compliance and the possibilities of side effects as well as the requirement of the availability of trained health care professionals, appropriate clinical facilities to provide OIT and deal with adverse effects (35, 49, 50). Decision aids might help individuals (and their parents) make decisions consistent with their values and preferences.
Cow'S Milk OIT
EAACI guidelines suggest to start OIT in children when they are about 4–5 years old because at this age 50–90% of them have already outgrown their allergy (33). Nevertheless, an early intervention, especially in children affected by a severe cow's milk allergy (CMA), is considered to be more effective (32).
Overall there is moderate-to-strong evidence on its benefit in terms of desensitization although with a higher risk of AEs, mainly mild to moderate (51–60) (Supplementary Table 1A). However, the risk of serious side effects should not be overlooked and a case of death due to this procedure has recently been reported in a non-scientific ambit.1 Results from an updated SR by the Diagnosis and Rationale for Action Against Cow's Milk Allergy (DRACMA) project are submitted.
Among the several studies reported, Longo et al. showed that rush a OIT protocol (i.e., an initial rush up-dosing phase with multiple increasing doses for 10 days in a hospital setting, followed by a slow increasing phase at home) in 97 children affected by severe CMA was effective and safe with data similar to a slower procedure (61). A weekly-up dosing OIT in 33 children with severe CMA over 4 months was reported to be an alternative method to achieve desensitization, being less time consuming than every other week up-dosing regimen and overall safe if performed in a well-equipped hospital setting (62). Factors that might predict adverse reactions during OIT are milk specific IgE levels, wheal size at SPTs, concomitant asthma / eczema or history of anaphylaxis (63–66).
The knowledge on SU is less robust. Factors that have been speculated to be linked to SU include the duration of maintenance phase (67). Biomarkers useful for the prediction of SU in CMA are the initial lower milk specific IgE levels, a small wheal diameter of the SPTs and a low basophil activity toward allergens (39, 68, 69). OIT in CMA induces also a reduction in the avidity of IgE and an increase of IgG4 binding to milk protein epitopes, resulting in a greater likelihood of obtaining SU (68, 70, 71). Nevertheless, the lack of acquisition regarding SU achievement in all treated subjects with CMA may underlie significant differences in individual immune systems, and further studies are desirable in the future to better understand these mechanisms.
Egg OIT
From the first egg-OIT reported in 1908 (72), several studies have shown its effectiveness and safety (73–89) (Supplementary Table 1B). The form of the egg ingested during OIT as well as the definition and rate of desensitization, the OIT protocol, the primary outcome were different between studies (36, 75, 79, 80, 82, 86) (Supplementary Table 1B). In the first double blind placebo-controlled food challenge (DBPCFC) egg-OIT study published (75), fifty-five egg-allergic children received OIT, consumed 2 g egg-white powder in maintenance phase and 55 and 75% of the OIT group passed oral food challenge (OFC) at 10 and 22 months of treatment, respectively. None of the patients in placebo group passed OFC. At 22 months, OIT was discontinued and children were instructed to avoid all egg consumption for 4–6 weeks. At 24 months, 28% of OIT group passed OFC, reaching SU. All children who had SU were consumed egg without any allergic reaction at 30 and 36 months. Long-term results of the study were reported by Jones et al. demonstrating that 50% of the OIT group had SU by 4 year (84). Kim et al. investigated the safety and efficacy of egg-OIT compared with baked egg (BE) consumption in children (aged 3–16 years) who were BE-tolerant but unbaked egg reactive (88). They concluded that OIT was more effective to achieve SU than ingesting BE alone.
Peanut OIT
The first open-label studies about peanut-OIT were published in 2009 (90, 91) followed by several studies in last decades (92–109) (Supplementary Table 1C). In STOP II study, after 6 months of OIT with 800 mg peanut protein (PP) maintenance dose, 24/39 children (62%) passed DBPCFC of 1,400 mg PP. No patients (n = 46) in control group (avoidance) passed DBPCFC. In the crossover phase of the study, 45 children from control group started OIT and 54% passed OFC of 1,400 mg at the end of the therapy (97). No serious adverse events were reported during treatment (97). In 2017 Vickery et al. compared the efficiency and safety of low-dose (300 mg PP, n = 20) and high dose (3,000 mg PP, n = 17) peanut-OIT in pre-school age children (100). They reported that the desensitization (85 vs. 75%) and SU rate (85 vs. 71%) did not differ significantly between low-dose and high-dose groups. Moreover, high-dose group experienced higher rate of moderate-to-severe adverse event than low-dose group. In 2018, PALISADE study investigated a standardized maintenance dose (phase 2 trial of AR101, 300 mg peanut PP) peanut OIT (103). They reported that 62% of OIT group passed OFC of 1,043 mg while no children in placebo group could pass it. After positive results of phase 2 trial, the outcome of phase 3 trial of AR101 was reported by Vickery et al. (105). In this multicenter study, 496 children underwent peanut-OIT using AR101 (standardized 300 mg PP). After 12 months maintenance period, 67% of OIT group passed OFC of 600 mg PP, whereas the rate was 4% in placebo group. Most patients (60%) had mild to moderate adverse events and 4.3% of the subjects reported severe adverse events. Besides, authors reported effectivity and safety of alternative dosing regimens from 2-year follow-on study of PALISADE participants (109). They observed the highest desensitization rate in the group who had longest daily dosing duration (300 mg/daily during 24–56 weeks) (Supplementary Table 1C). In addition, adverse events (AEs) rates were higher in non-daily dosing cohorts than daily cohorts and most children had mild/moderate AEs. Then, in January 2020, Palforzia (AR101), which is a standardized peanut OIT formulation approved by US Food and Drug Administration (FDA), became the first drug approved for OIT in FA treatment (110).
OIT for Other Foods
Few reports describe OIT to other foods, including tree nut, sesame, cashew and wheat (111–122) (Supplementary Table 1D). In the first reported walnut-OIT study (111), 73 patients were randomized in OIT (n = 55) group and control group (n = 18). At the end of the study, 89% of OIT group passed OFC of 4,000 mg walnut protein and all children co-allergic to pecan achieved desensitization to pecan. In addition, 60 and 93% of the patients co-allergic to hazelnut/cashew and hazelnut alone achieved desensitization, respectively. During the study, most of the patients (85%) experienced AEs mostly mild-moderate and intramuscular adrenaline was administered to 9 children.
Adjunctive Treatments to OIT
Omalizumab has been used as an adjuvant during OIT protocol to reduce risk of allergic reactions. In milk and egg OIT, Omalizumab reduced the number and severity of reactions during dose-escalation phase and allowed a rapid build-up phase (59, 123–125). However, no additional effect was found to achieve desensitization with omalizumab (59, 124). Similar effects have been described for peanut OIT, (95, 99, 126, 127) even in high-risk patients (95). In addition, the effectiveness of Omalizumab in multiple food allergens-OIT were comparable to the outcomes of a single food OIT (121, 128). However, the effects on efficacy and safety of dose adjustment, according to body weight and total IgE levels, or in fixed doses are uncertain. Hence, the duration, dosage, and effectiveness of Omalizumab treatment in OIT remains to be clarified. In particular, one of the main gaps in knowledge lies on the effectiveness after the discontinuation of omalizumab.
Discussion and Future Perspectives
FA is a major health problem with growing prevalence (129). The main treatment option of FA is dietary restriction, using rescue medications in case of severe allergic reactions (130). Moreover, novel therapies for FA treatment including microbiome, biologic agents, oral/sublingual/subcutaneous/epicutaneous immunotherapy (IT) were reported in the last decades (29, 50, 131, 132). While results of recent OIT studies are encouraging, the major issue of OIT is the heterogeneity of study protocols including the duration of maintenance doses, primary end points, definition of desensitization, OFC protocols to evaluate desensitization, SU and safety profiles (35). The natural raw form of food was usually used in OIT (51, 62, 71, 78). On the other hand, some studies revealed with processed food allergens, such as hydrolyzed, pasteurized, dry powdered, heated, undercooked, etc. (36, 75, 79, 80, 82, 86). In peanut OIT, the first standardized product protein (Palforzia, AR101, containing 300 mg PP) has been recently approved by FDA (110). Molecular IT for tree nut and peanut allergies might be another treatment option in the future, along with developments in the diagnosis of molecular allergy (133, 134).
Although, most of the reactions are mild to moderate and occur mostly during the build-up phase performed in clinical settings, allergic reactions may appear also during maintenance phase at home (33). A rare but important side effect of OIT is eosinophilic esophagitis (EoE) with a frequency rated of 0.3%, instead 8.3% of patients experiences gastrointestinal symptoms during OIT (135, 136). Further, two meta-analysis reported that OIT increases anaphylaxis risk and frequency of adrenaline use (35, 137). Of importance, two cases of lethal reaction to the intentional introduction of a food are reported1 (138). Although this can be considered an extremely rare eventuality, the clinician must inform the family of this possibility at the beginning of each treatment with OIT.
Several efforts are under investigation in order to improve the safety profile, including the slow introduction [i.e., slow progression schedule starting with baked food (e.g., milk/egg) and then less and less heated food over time (with a presumed lower risk of side-effects] (139) and low dose introduction (140). The main goal of OIT is an increase in the allergen reactivity threshold to achieve lower risk of severe allergic reactions after accidental ingestion (33). Accordingly, it is expected that patients have less fear of anaphylaxis after allergen exposure, less restriction in social life, and a consequent increase in their food allergy-related quality of life (FA-QoL) (27, 86, 97, 106, 141–147). However, the current data are not sufficient to make definitive conclusions about the impact of OIT on QoL and more studies are needed.
Another issue that should be highlighted is the cost-effectiveness of the treatment, recently investigated only for peanut OIT and EPIT (148–150). These studies reported that OIT and/or EPIT may be cost-effective in certain conditions: lower therapy cost, achieving SU, improvement in health state utility, and reduction of anaphylaxis risk (149, 150). However, further well-designed studies are needed to better explain health state improvement in OIT (150).
In conclusion, OIT is a promising treatment in FA and it will be important to define standardized protocols, considering also the possible use of Omalizumab as an adjuvant therapy. Understanding of the mechanism associated with remission or SU is fundamental, thus reaching the goal of therapy.
Author Contributions
SA designed the manuscript. AA and GB wrote the first draft. SA provided background, coordinated, and supervised the work. All authors critically revised the work and contributed to the article, approving the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.842196/full#supplementary-material
Abbreviations
AE, Adverse event; CMA, Cow's milk allergy; DBPCFC, Double blind placebo-controlled food challenge; DRACMA, Diagnosis and Rationale for Action Against Cow's Milk Allergy; EAACI, European Academy of Allergy and Clinical Immunology; EoE, Eosinophilic esophagitis; EPIT, Epicutaneous immunotherapy; FA, Food allergy; FA-QoL, Food-allergy-related quality of life; FDA, Food and Drug Administration (FDA); FOXP3, Forkhead Box P3; IgE, Immunoglobulin E; IgG4, Immunoglobulin G4; IT, immunotherapy; LAP, Latency associated peptide; OFC, Oral food challenge; OIT, Oral immunotherapy; PP, Peanut protein; SPTs, Skin prick tests; SLIT, Sublingual immunotherapy; SU, Sustained unresponsiveness; Treg cells, T regulatory cells.
Footnotes
1. ^Available online at: https://www.allergicliving.com/2021/12/20/girl-with-milk-allergy-dies-of-severe-reaction-related-to-desensitization/?s=09 (accessed December 22, 2021).
References
1. Halken S, Muraro A, de Silva D, Khaleva E, Angier E, Arasi S, et al. EAACI guideline: preventing the development of food allergy in infants and young children (2020 update). Pediatr Allergy Immunol. (2021) 32:843–58. doi: 10.1111/pai.13496
2. Gupta RS, Springston EE, Warrier MR, Smith B, Kumar R, Pongracic J, et al. The prevalence, severity, and distribution of childhood food allergy in the United States. Pediatrics. (2011) 128:e9–17. doi: 10.1542/peds.2011-0204
3. Chafen JJ, Newberry SJ, Riedl MA, Bravata DM, Maglione M, Suttorp MJ, et al. Diagnosing and managing common food allergies: a systematic review. JAMA. (2010) 303:1848–56. doi: 10.1001/jama.2010.582
4. Savage J, Johns CB. Food allergy: epidemiology and natural history. Immunol Allergy Clin North Am. (2015) 35:45–59. doi: 10.1016/j.iac.2014.09.004
5. Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn J, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. (2013) 6:21. doi: 10.1186/1939-4551-6-21
6. Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. (2014) 69:62–75. doi: 10.1111/all.12305
7. Koplin JJ, Tang ML, Martin PE, Osborne NJ, Lowe AJ, Ponsonby AL, et al. Predetermined challenge eligibility and cessation criteria for oral food challenges in the HealthNuts population-based study of infants. J Allergy Clin Immunol. (2012) 129:1145–7. doi: 10.1016/j.jaci.2011.09.044
8. Arasi S, Nurmatov U, Dunn-Galvin A, Daher S, Roberts G, Turner PJ, et al. Consensus on DEfinition of Food Allergy SEverity (DEFASE) an integrated mixed methods systematic review. World Allergy Organ J. (2021) 14:100503. doi: 10.1016/j.waojou.2020.100503
9. Arasi S, Nurmatov U, Turner PJ, Ansotegui IJ, Daher S, Dunn-Galvin A, et al. Consensus on DEfinition of Food Allergy SEverity (DEFASE): protocol for a systematic review. World Allergy Organ J. (2020) 13:100493. doi: 10.1016/j.waojou.2020.100493
10. Pouessel G, Turner PJ, Worm M, Cardona V, Deschildre A, Beaudouin E, et al. Food-induced fatal anaphylaxis: from epidemiological data to general prevention strategies. Clin Exp Allergy. (2018) 48:1584–93. doi: 10.1111/cea.13287
11. Michelson KA, Dribin TE, Vyles D, Neuman MI. Trends in emergency care for anaphylaxis. J Allergy Clin Immunol Pract. (2020) 8:767–8 e2. doi: 10.1016/j.jaip.2019.07.018
12. Kahveci M, Akarsu A, Koken G, Sahiner UM, Soyer O, Sekerel BE. Food-induced anaphylaxis in infants, as compared to toddlers and preschool children in Turkey. Pediatr Allergy Immunol. (2020) 31:954–61. doi: 10.1111/pai.13320
13. Tanno LK, Simons FE, Annesi-Maesano I, Calderon MA, Ayme S, Demoly P, et al. Fatal anaphylaxis registries data support changes in the who anaphylaxis mortality coding rules. Orphanet J Rare Dis. (2017) 12:8. doi: 10.1186/s13023-016-0554-4
14. Umasunthar T, Leonardi-Bee J, Hodes M, Turner PJ, Gore C, Habibi P, et al. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. (2013) 43:1333–41. doi: 10.1111/cea.12211
15. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, et al. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. (2014) 69:992–1007. doi: 10.1111/all.12423
16. Peters RL, Koplin JJ, Gurrin LC, Dharmage SC, Wake M, Ponsonby AL, et al. The prevalence of food allergy and other allergic diseases in early childhood in a population-based study: HealthNuts age 4-year follow-up. J Allergy Clin Immunol. (2017) 140:145–53.e8. doi: 10.1016/j.jaci.2017.02.019
17. Savage J, Sicherer S, Wood R. The natural history of food allergy. J Allergy Clin Immunol Pract. (2016) 4:196–203; quiz 4. doi: 10.1016/j.jaip.2015.11.024
18. Akarsu A, Ocak M, Koken G, Sahiner UM, Soyer O, Sekerel BE. IgE mediated food allergy in Turkey: different spectrum, similar outcome. Turk J Pediatr. (2021) 63:554–63. doi: 10.24953/turkjped.2021.04.002
19. Loh W, Tang MLK. Debates in allergy medicine: oral immunotherapy shortens the duration of milk and egg allergy - the con argument. World Allergy Organ J. (2018) 11:12. doi: 10.1186/s40413-018-0189-0
20. Ho MH, Wong WH, Heine RG, Hosking CS, Hill DJ, Allen KJ. Early clinical predictors of remission of peanut allergy in children. J Allergy Clin Immunol. (2008) 121:731–6. doi: 10.1016/j.jaci.2007.11.024
21. Wassenberg J, Cochard MM, Dunngalvin A, Ballabeni P, Flokstra-de Blok BM, Newman CJ, et al. Parent perceived quality of life is age-dependent in children with food allergy. Pediatr Allergy Immunol. (2012) 23:412–9. doi: 10.1111/j.1399-3038.2012.01310.x
22. Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy. (2010) 65:933–45. doi: 10.1111/j.1398-9995.2010.02342.x
23. Jones SM, Scurlock AM. The impact of food allergy: the real “fear factor”. Ann Allergy Asthma Immunol. (2006) 96:385–6. doi: 10.1016/S1081-1206(10)60903-9
24. Bollinger ME, Dahlquist LM, Mudd K, Sonntag C, Dillinger L, McKenna K. The impact of food allergy on the daily activities of children and their families. Ann Allergy Asthma Immunol. (2006) 96:415–21. doi: 10.1016/S1081-1206(10)60908-8
25. Mikkelsen A, Mehlig K, Borres MP, Oxelmark L, Bjorkelund C, Lissner L. Monitoring the impact of cow's milk allergy on children and their families with the FLIP questionnaire–a six-month follow-up study. Pediatr Allergy Immunol. (2015) 26:409–15. doi: 10.1111/pai.12406
26. Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D. The economic impact of childhood food allergy in the United States. JAMA Pediatr. (2013) 167:1026–31. doi: 10.1001/jamapediatrics.2013.2376
27. Arasi S, Otani IM, Klingbeil E, Begin P, Kearney C, Dominguez TL, et al. Two year effects of food allergen immunotherapy on quality of life in caregivers of children with food allergies. Allergy Asthma Clin Immunol. (2014) 10:57. doi: 10.1186/1710-1492-10-57
28. Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. (2014) 69:1008–25. doi: 10.1111/all.12429
29. Arasi S, Castagnoli R, Pajno GB. Oral immunotherapy in pediatrics. Pediatr Allergy Immunol. (2020) 31:51–3. doi: 10.1111/pai.13159
30. Arasi S, Mennini M, Cafarotti A, Fiocchi A. Omalizumab as monotherapy for food allergy. Curr Opin Allergy Clin Immunol. (2021) 21:286–91. doi: 10.1097/ACI.0000000000000744
31. Cafarotti A, Fiocchi A, Arasi S. Biologics as treatment options for anaphylaxis. Curr Opin Allergy Clin Immunol. (2021) 21:455–64. doi: 10.1097/ACI.0000000000000779
32. Ogata M, Kido J, Nakamura K. Oral immunotherapy for children with cow's milk allergy. Pathogens. (2021) 10:1328. doi: 10.3390/pathogens10101328
33. Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro-Lozano M, et al. EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy. (2018) 73:799–815. doi: 10.1111/all.13319
34. Leonard SA, Laubach S, Wang J. Integrating oral immunotherapy into clinical practice. J Allergy Clin Immunol. (2021) 147:1–13. doi: 10.1016/j.jaci.2020.11.011
35. Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta-analysis. Allergy. (2017) 72:1133–47. doi: 10.1111/all.13124
36. Tang ML, Hsiao K-C. An update on oral immunotherapy for the treatment of food allergy. Paediatr Child Health. (2016) 26:304–9. doi: 10.1016/j.paed.2016.03.004
37. Martorell A, Alonso E, Echeverria L, Escudero C, Garcia-Rodriguez R, Blasco C, et al. Oral immunotherapy for food allergy: a Spanish guideline. Egg and Milk Immunotherapy Spanish Guide (ITEMS GUIDE). Part II: Maintenance Phase of Cow Milk (CM) and Egg Oral Immunotherapy (OIT), special treatment dosing schedules. models of dosing schedules of OIT with CM and egg. J Investig Allergol Clin Immunol. (2017) 27:279–90. doi: 10.18176/jiaci.0178
38. Niggemann B, Staden U, Rolinck-Werninghaus C, Beyer K. Specific oral tolerance induction in food allergy. Allergy. (2006) 61:808–11. doi: 10.1111/j.1398-9995.2006.01066.x
39. Barshow SM, Kulis MD, Burks AW, Kim EH. Mechanisms of oral immunotherapy. Clin Exp Allergy. (2021) 51:527–35. doi: 10.1111/cea.13824
40. Vickery BP, Scurlock AM, Jones SM, Burks AW. Mechanisms of immune tolerance relevant to food allergy. J Allergy Clin Immunol. (2011) 127:576–84; quiz 85–6. doi: 10.1016/j.jaci.2010.12.1116
41. Wood RA. Food allergen immunotherapy: current status and prospects for the future. J Allergy Clin Immunol. (2016) 137:973–82. doi: 10.1016/j.jaci.2016.01.001
42. Pranger CL, Fazekas-Singer J, Kohler VK, Pali-Scholl I, Fiocchi A, Karagiannis SN, et al. PIPE-cloned human IgE and IgG4 antibodies: new tools for investigating cow's milk allergy and tolerance. Allergy. (2021) 76:1553–6. doi: 10.1111/all.14604
43. Pajno GB, Castagnoli R, Muraro A, Alvaro-Lozano M, Akdis CA, Akdis M, et al. Allergen immunotherapy for IgE-mediated food allergy: there is a measure in everything to a proper proportion of therapy. Pediatr Allergy Immunol. (2019) 30:415–22. doi: 10.1111/pai.13042
44. Ang WX, Church AM, Kulis M, Choi HW, Burks AW, Abraham SN. Mast cell desensitization inhibits calcium flux and aberrantly remodels actin. J Clin Invest. (2016) 126:4103–18. doi: 10.1172/JCI87492
45. Killoran KE, Kropp LE, Lindrose AR, Curtis HE, Cook D, Mitre E. Rush desensitization with a single antigen induces subclinical activation of mast cells and protects against bystander challenge in dually sensitized mice. Clin Exp Allergy. (2019) 49:484–94. doi: 10.1111/cea.13323
46. Ryan JF, Hovde R, Glanville J, Lyu SC, Ji X, Gupta S, et al. Successful immunotherapy induces previously unidentified allergen-specific CD4+ T-cell subsets. Proc Natl Acad Sci USA. (2016) 113:E1286–95. doi: 10.1073/pnas.1520180113
47. Schoos AM, Bullens D, Chawes BL, Costa J, De Vlieger L, DunnGalvin A, et al. Immunological outcomes of allergen-specific immunotherapy in food allergy. Front Immunol. (2020) 11:568598. doi: 10.3389/fimmu.2020.568598
48. Martorell A, Alonso E, Echeverria L, Escudero C, Garcia-Rodriguez R, Blasco C, et al. Oral immunotherapy for food allergy: a Spanish guideline. Immunotherapy Egg and Milk Spanish Guide (ITEMS Guide). Part I: cow milk and egg oral immunotherapy: introduction, methodology, rationale, current state, indications, contraindications, and oral immunotherapy build-up phase. J Investig Allergol Clin Immunol. (2017) 27:225–37. doi: 10.18176/jiaci.0177
49. Nurmatov U, Devereux G, Worth A, Healy L, Sheikh A. Effectiveness and safety of orally administered immunotherapy for food allergies: a systematic review and meta-analysis. Br J Nutr. (2014) 111:12–22. doi: 10.1017/S0007114513002353
50. Mori F, Giovannini M, Barni S, Jimenez-Saiz R, Munblit D, Biagioni B, et al. Oral immunotherapy for food-allergic children: a pro-con debate. Front Immunol. (2021) 12:636612. doi: 10.3389/fimmu.2021.636612
51. Morisset M, Moneret-Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. Eur Ann Allergy Clin Immunol. (2007) 39:12–9.
52. Pajno GB, Caminiti L, Ruggeri P, De Luca R, Vita D, La Rosa M, et al. Oral immunotherapy for cow's milk allergy with a weekly up-dosing regimen: a randomized single-blind controlled study. Ann Allergy Asthma Immunol. (2010) 105:376–81. doi: 10.1016/j.anai.2010.03.015
53. Salmivesi S, Korppi M, Makela MJ, Paassilta M. Milk oral immunotherapy is effective in school-aged children. Acta Paediatr. (2013) 102:172–6. doi: 10.1111/j.1651-2227.2012.02815.x
54. Skripak JM, Nash SD, Rowley H, Brereton NH, Oh S, Hamilton RG, et al. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow's milk allergy. J Allergy Clin Immunol. (2008) 122:1154–60. doi: 10.1016/j.jaci.2008.09.030
55. Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. (2012) 129:448–55:55.e1–5. doi: 10.1016/j.jaci.2011.10.023
56. Yeung JP, Kloda LA, McDevitt J, Ben-Shoshan M, Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. (2012) 11:CD009542. doi: 10.1002/14651858.CD009542
57. Martorell Calatayud C, Muriel Garcia A, Martorell Aragones A, De La Hoz Caballer B. Safety and efficacy profile and immunological changes associated with oral immunotherapy for IgE-mediated cow's milk allergy in children: systematic review and meta-analysis. J Investig Allergol Clin Immunol. (2014) 24:298–307.
58. Martorell A, De la Hoz B, Ibanez MD, Bone J, Terrados MS, Michavila A, et al. Oral desensitization as a useful treatment in 2-year-old children with cow's milk allergy. Clin Exp Allergy. (2011) 41:1297–304. doi: 10.1111/j.1365-2222.2011.03749.x
59. Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. (2016) 137:1103–10.e11. doi: 10.1016/j.jaci.2015.10.005
60. Nagakura KI, Sato S, Miura Y, Nishino M, Takahashi K, Asaumi T, et al. A randomized trial of oral immunotherapy for pediatric cow's milk-induced anaphylaxis: heated vs. unheated milk. Pediatr Allergy Immunol. (2021) 32:161–9. doi: 10.1111/pai.13352
61. Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow's milk-induced reactions. J Allergy Clin Immunol. (2008) 121:343–7. doi: 10.1016/j.jaci.2007.10.029
62. Caminiti L, Passalacqua G, Barberi S, Vita D, Barberio G, De Luca R, et al. A new protocol for specific oral tolerance induction in children with IgE-mediated cow's milk allergy. Allergy Asthma Proc. (2009) 30:443–8. doi: 10.2500/aap.2009.30.3221
63. Petersen TH, Mortz CG, Bindslev-Jensen C, Eller E. Cow's milk allergic children-Can component-resolved diagnostics predict duration and severity? Pediatr Allergy Immunol. (2018) 29:194–9. doi: 10.1111/pai.12854
64. Sampson HA, Berin MC, Plaut M, Sicherer SH, Jones S, Burks AW, et al. The Consortium for Food Allergy Research (CoFAR): the first generation. J Allergy Clin Immunol. (2019) 143:486–93. doi: 10.1016/j.jaci.2018.12.989
65. Koike Y, Sato S, Yanagida N, Asaumi T, Ogura K, Ohtani K, et al. Predictors of persistent milk allergy in children: a retrospective cohort study. Int Arch Allergy Immunol. (2018) 175:177–80. doi: 10.1159/000486311
66. Kauppila TK, Paassilta M, Kukkonen AK, Kuitunen M, Pelkonen AS, Makela MJ. Outcome of oral immunotherapy for persistent cow's milk allergy from 11 years of experience in Finland. Pediatr Allergy Immunol. (2019) 30:356–62. doi: 10.1111/pai.13025
67. Takahashi M, Taniuchi S, Soejima K, Hatano Y, Yamanouchi S, Kaneko K. Two-weeks-sustained unresponsiveness by oral immunotherapy using microwave heated cow's milk for children with cow's milk allergy. Allergy Asthma Clin Immunol. (2016) 12:44. doi: 10.1186/s13223-016-0150-0
68. Suarez-Farinas M, Suprun M, Chang HL, Gimenez G, Grishina G, Getts R, et al. Predicting development of sustained unresponsiveness to milk oral immunotherapy using epitope-specific antibody binding profiles. J Allergy Clin Immunol. (2019) 143:1038–46. doi: 10.1016/j.jaci.2018.10.028
69. Kido J, Hirata M, Ueno H, Nishi N, Mochinaga M, Ueno Y, et al. Evaluation of the skin-prick test for predicting the outgrowth of cow's milk allergy. Allergy Rhinol. (2016) 7:139–43. doi: 10.2500/ar.2016.7.0175
70. Savilahti EM, Kuitunen M, Valori M, Rantanen V, Bardina L, Gimenez G, et al. Use of IgE and IgG4 epitope binding to predict the outcome of oral immunotherapy in cow's milk allergy. Pediatr Allergy Immunol. (2014) 25:227–35. doi: 10.1111/pai.12186
71. Lee JH, Kim WS, Kim H, Hahn YS. Increased cow's milk protein-specific IgG4 levels after oral desensitization in 7- to 12-month-old infants. Ann Allergy Asthma Immunol. (2013) 111:523–8. doi: 10.1016/j.anai.2013.09.001
72. Graham F, Tardio N, Paradis L, Des Roches A, Begin P. Update on oral immunotherapy for egg allergy. Hum Vaccin Immunother. (2017) 13:2452–61. doi: 10.1080/21645515.2017.1339844
73. Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. (2007) 119:199–205. doi: 10.1016/j.jaci.2006.09.016
74. Vickery BP, Pons L, Kulis M, Steele P, Jones SM, Burks AW. Individualized IgE-based dosing of egg oral immunotherapy and the development of tolerance. Ann Allergy Asthma Immunol. (2010) 105:444–50. doi: 10.1016/j.anai.2010.09.030
75. Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. (2012) 367:233–43. doi: 10.1056/NEJMoa1200435
76. Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen's egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol. (2013) 24:66–74. doi: 10.1111/j.1399-3038.2012.01349.x
77. Fuentes-Aparicio V, Alvarez-Perea A, Infante S, Zapatero L, D'Oleo A, Alonso-Lebrero E. Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergol Immunopathol. (2013) 41:143–50. doi: 10.1016/j.aller.2012.02.007
78. Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen's egg allergy: a new protocol with raw hen's egg. Pediatr Allergy Immunol. (2013) 24:75–83. doi: 10.1111/j.1399-3038.2012.01341.x
79. Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, et al. Oral immunotherapy for egg allergy: a double-blind placebo-controlled study, with postdesensitization follow-up. J Allergy Clin Immunol Pract. (2015) 3:532–9. doi: 10.1016/j.jaip.2015.01.017
80. Escudero C, Rodriguez Del Rio P, Sanchez-Garcia S, Perez-Rangel I, Perez-Farinos N, Garcia-Fernandez C, et al. Early sustained unresponsiveness after short-course egg oral immunotherapy: a randomized controlled study in egg-allergic children. Clin Exp Allergy. (2015) 45:1833–43. doi: 10.1111/cea.12604
81. Yanagida N, Sato S, Asaumi T, Nagakura K, Ogura K, Ebisawa M. Safety and efficacy of low-dose oral immunotherapy for hen's egg allergy in children. Int Arch Allergy Immunol. (2016) 171:265–8. doi: 10.1159/000454807
82. Giavi S, Vissers YM, Muraro A, Lauener R, Konstantinopoulos AP, Mercenier A, et al. Oral immunotherapy with low allergenic hydrolysed egg in egg allergic children. Allergy. (2016) 71:1575–84. doi: 10.1111/all.12905
83. Akashi M, Yasudo H, Narita M, Nomura I, Akasawa A, Ebisawa M, et al. Randomized controlled trial of oral immunotherapy for egg allergy in Japanese patients. Pediatr Int. (2017) 59:534–9. doi: 10.1111/ped.13210
84. Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA, et al. Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol. (2016) 137:1117–27.e10. doi: 10.1016/j.jaci.2015.12.1316
85. Perez-Rangel I, Rodriguez Del Rio P, Escudero C, Sanchez-Garcia S, Sanchez-Hernandez JJ, Ibanez MD. Efficacy and safety of high-dose rush oral immunotherapy in persistent egg allergic children: a randomized clinical trial. Ann Allergy Asthma Immunol. (2017) 118:356–64.e3. doi: 10.1016/j.anai.2016.11.023
86. Itoh-Nagato N, Inoue Y, Nagao M, Fujisawa T, Shimojo N, Iwata T, et al. Desensitization to a whole egg by rush oral immunotherapy improves the quality of life of guardians: a multicenter, randomized, parallel-group, delayed-start design study. Allergol Int. (2018) 67:209–16. doi: 10.1016/j.alit.2017.07.007
87. Martin-Munoz MF, Belver MT, Alonso Lebrero E, Zapatero Remon L, Fuentes Aparicio V, Piquer Gibert M, et al. Egg oral immunotherapy in children (SEICAP I): daily or weekly desensitization pattern. Pediatr Allergy Immunol. (2019) 30:81–92. doi: 10.1111/pai.12974
88. Kim EH, Perry TT, Wood RA, Leung DYM, Berin MC, Burks AW, et al. Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. J Allergy Clin Immunol. (2020) 146:851–62.e10. doi: 10.1016/j.jaci.2020.05.040
89. Palosuo K, Karisola P, Savinko T, Fyhrquist N, Alenius H, Makela MJ. A randomized, open-label trial of hen's egg oral immunotherapy: efficacy and humoral immune responses in 50 children. J Allergy Clin Immunol Pract. (2021) 9:1892–901.e1. doi: 10.1016/j.jaip.2021.01.020
90. Hofmann AM, Scurlock AM, Jones SM, Palmer KP, Lokhnygina Y, Steele PH, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. (2009) 124:286–91:91.e1–6. doi: 10.1016/j.jaci.2009.03.045
91. Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. (2009) 124:292–300.e1–97. doi: 10.1016/j.jaci.2009.05.022
92. Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LC, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. (2010) 126:83–91.e1. doi: 10.1016/j.jaci.2010.04.030
93. Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. (2011) 127:654–60. doi: 10.1016/j.jaci.2010.12.1111
94. Anagnostou K, Clark A, King Y, Islam S, Deighton J, Ewan P. Efficacy and safety of high-dose peanut oral immunotherapy with factors predicting outcome. Clin Exp Allergy. (2011) 41:1273–81. doi: 10.1111/j.1365-2222.2011.03699.x
95. Schneider LC, Rachid R, LeBovidge J, Blood E, Mittal M, Umetsu DT. A pilot study of omalizumab to facilitate rapid oral desensitization in high-risk peanut-allergic patients. J Allergy Clin Immunol. (2013) 132:1368–74. doi: 10.1016/j.jaci.2013.09.046
96. Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. (2014) 133:468–75. doi: 10.1016/j.jaci.2013.11.007
97. Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. (2014) 383:1297–304. doi: 10.1016/S0140-6736(13)62301-6
98. Tang ML, Ponsonby AL, Orsini F, Tey D, Robinson M, Su EL, et al. Administration of a probiotic with peanut oral immunotherapy: a randomized trial. J Allergy Clin Immunol. (2015) 135:737–44.e8. doi: 10.1016/j.jaci.2014.11.034
99. MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A, et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol. (2017) 139:873–81.e8. doi: 10.1016/j.jaci.2016.08.010
100. Vickery BP, Berglund JP, Burk CM, Fine JP, Kim EH, Kim JI, et al. Early oral immunotherapy in peanut-allergic preschool children is safe and highly effective. J Allergy Clin Immunol. (2017) 139:173–81.e8. doi: 10.1016/j.jaci.2016.05.027
101. Kukkonen AK, Uotila R, Malmberg LP, Pelkonen AS, Makela MJ. Double-blind placebo-controlled challenge showed that peanut oral immunotherapy was effective for severe allergy without negative effects on airway inflammation. Acta Paediatr. (2017) 106:274–81. doi: 10.1111/apa.13668
102. Hsiao KC, Ponsonby AL, Axelrad C, Pitkin S, Tang MLK, Team PS. Long-term clinical and immunological effects of probiotic and peanut oral immunotherapy after treatment cessation: 4-year follow-up of a randomised, double-blind, placebo-controlled trial. Lancet Child Adolesc Health. (2017) 1:97–105. doi: 10.1016/S2352-4642(17)30041-X
103. Bird JA, Spergel JM, Jones SM, Rachid R, Assa'ad AH, Wang J, et al. Efficacy and safety of AR101 in oral immunotherapy for peanut allergy: results of ARC001, a randomized, double-blind, placebo-controlled phase 2 clinical trial. J Allergy Clin Immunol Pract. (2018) 6:476–85.e3. doi: 10.1016/j.jaip.2017.09.016
104. Nagakura KI, Sato S, Yanagida N, Nishino M, Asaumi T, Ogura K, et al. Oral immunotherapy in Japanese children with anaphylactic peanut allergy. Int Arch Allergy Immunol. (2018) 175:181–8. doi: 10.1159/000486310
105. Investigators PGoC, Vickery BP, Vereda A, Casale TB, Beyer K, du Toit G, et al. AR101 oral immunotherapy for peanut allergy. N Engl J Med. (2018) 379:1991–2001. doi: 10.1056/NEJMoa1812856
106. Blumchen K, Trendelenburg V, Ahrens F, Gruebl A, Hamelmann E, Hansen G, et al. Efficacy, safety, and quality of life in a multicenter, randomized, placebo-controlled trial of low-dose peanut oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol Pract. (2019) 7:479–91.e10. doi: 10.1016/j.jaip.2018.10.048
107. Chinthrajah RS, Purington N, Andorf S, Long A, O'Laughlin KL, Lyu SC, et al. Sustained outcomes in oral immunotherapy for peanut allergy (POISED study): a large, randomised, double-blind, placebo-controlled, phase 2 study. Lancet. (2019) 394:1437–49. doi: 10.1016/S0140-6736(19)31793-3
108. Hourihane JOB, Beyer K, Abbas A, Fernandez-Rivas M, Turner PJ, Blumchen K, et al. Efficacy and safety of oral immunotherapy with AR101 in European children with a peanut allergy (ARTEMIS): a multicentre, double-blind, randomised, placebo-controlled phase 3 trial. Lancet Child Adolesc Health. (2020) 4:728–39. doi: 10.1016/S2352-4642(20)30234-0
109. Vickery BP, Vereda A, Nilsson C, du Toit G, Shreffler WG, Burks AW, et al. Continuous and daily oral immunotherapy for peanut allergy: results from a 2-year open-label follow-on study. J Allergy Clin Immunol Pract. (2021) 9:1879–89.e14. doi: 10.1016/j.jaip.2020.12.029
110. U.S. Food & Drug Administration. Palforzia. (2020). Available online at: https://www.fda.gov/vaccines-blood-biologics/allergenics/palforzia (accessed November 10, 2021).
111. Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Pontoppidan B, et al. Walnut oral immunotherapy for desensitisation of walnut and additional tree nut allergies (Nut CRACKER): a single-centre, prospective cohort study. Lancet Child Adolesc Health. (2019) 3:312–21. doi: 10.1016/S2352-4642(19)30029-X
112. Moraly T, Pelletier de Chambure D, Verdun S, Preda C, Seynave M, Vilain AC, et al. Oral immunotherapy for hazelnut allergy: a single-center retrospective study on 100 patients. J Allergy Clin Immunol Pract. (2020) 8:704–9 e4. doi: 10.1016/j.jaip.2019.10.045
113. Nachshon L, Goldberg MR, Levy MB, Appel MY, Epstein-Rigbi N, Lidholm J, et al. Efficacy and safety of sesame oral immunotherapy-a real-world, single-center study. J Allergy Clin Immunol Pract. (2019) 7:2775–81.e2. doi: 10.1016/j.jaip.2019.05.031
114. Nowak-Wegrzyn A, Wood RA, Nadeau KC, Pongracic JA, Henning AK, Lindblad RW, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. (2019) 143:651–61.e9. doi: 10.1016/j.jaci.2018.08.041
115. Sato S, Utsunomiya T, Imai T, Yanagida N, Asaumi T, Ogura K, et al. Wheat oral immunotherapy for wheat-induced anaphylaxis. J Allergy Clin Immunol. (2015) 136:1131–e7. doi: 10.1016/j.jaci.2015.07.019
116. Pacharn P, Vichyanond P. Immunotherapy for IgE-mediated wheat allergy. Hum Vaccin Immunother. (2017) 13:2462–6. doi: 10.1080/21645515.2017.1356499
117. Nagakura KI, Yanagida N, Sato S, Nishino M, Takahashi K, Asaumi T, et al. Low-dose-oral immunotherapy for children with wheat-induced anaphylaxis. Pediatr Allergy Immunol. (2020) 31:371–9. doi: 10.1111/pai.13220
118. Furuta T, Tanaka K, Tagami K, Matsui T, Sugiura S, Kando N, et al. Exercise-induced allergic reactions on desensitization to wheat after rush oral immunotherapy. Allergy. (2020) 75:1414–22. doi: 10.1111/all.14182
119. Wongteerayanee C, Tanticharoenwiwat P, Rutrakool N, Senavonge A, Jeekungwal N, Pacharn P, et al. Feasibility of a 3-step protocol of wheat oral immunotherapy in children with severe wheat allergy. Asia Pac Allergy. (2020) 10:e38. doi: 10.5415/apallergy.2020.10.e38
120. Sasamoto K, Nagakura KI, Sato S, Yanagida N, Ebisawa M. Low-dose oral immunotherapy for walnut allergy with anaphylaxis: three case reports. Allergol Int. (2021) 70:392–4. doi: 10.1016/j.alit.2021.01.007
121. Andorf S, Purington N, Kumar D, Long A, O'Laughlin KL, Sicherer S, et al. A Phase 2 randomized controlled multisite study using omalizumab-facilitated rapid desensitization to test continued vs discontinued dosing in multifood allergic individuals. EClinicalMedicine. (2019) 7:27–38. doi: 10.1016/j.eclinm.2018.12.006
122. He Z, Dongre P, Lyu SC, Manohar M, Chinthrajah RS, Galli SJ, et al. Identification of cross-reactive allergens in cashew- and pistachio-allergic children during oral immunotherapy. Pediatr Allergy Immunol. (2020) 31:709–14. doi: 10.1111/pai.13258
123. Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. (2011) 127:1622–4. doi: 10.1016/j.jaci.2011.04.009
124. Martorell-Calatayud C, Michavila-Gomez A, Martorell-Aragones A, Molini-Menchon N, Cerda-Mir JC, Felix-Toledo R, et al. Anti-IgE-assisted desensitization to egg and cow's milk in patients refractory to conventional oral immunotherapy. Pediatr Allergy Immunol. (2016) 27:544–6. doi: 10.1111/pai.12567
125. Takahashi M, Soejima K, Taniuchi S, Hatano Y, Yamanouchi S, Ishikawa H, et al. Oral immunotherapy combined with omalizumab for high-risk cow's milk allergy: a randomized controlled trial. Sci Rep. (2017) 7:17453. doi: 10.1038/s41598-017-16730-6
126. Yee CSK, Albuhairi S, Noh E, El-Khoury K, Rezaei S, Abdel-Gadir A, et al. Long-term outcome of peanut oral immunotherapy facilitated initially by omalizumab. J Allergy Clin Immunol Pract. (2019) 7:451–61. doi: 10.1016/j.jaip.2018.09.015
127. Brandstrom J, Vetander M, Sundqvist AC, Lilja G, Johansson SGO, Melen E, et al. Individually dosed omalizumab facilitates peanut oral immunotherapy in peanut allergic adolescents. Clin Exp Allergy. (2019) 49:1328–41. doi: 10.1111/cea.13469
128. Andorf S, Purington N, Block WM, Long AJ, Tupa D, Brittain E, et al. Anti-IgE treatment with oral immunotherapy in multifood allergic participants: a double-blind, randomised, controlled trial. Lancet Gastroenterol Hepatol. (2018) 3:85–94. doi: 10.1016/S2468-1253(17)30392-8
129. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-sponsored expert panel report. J Allergy Clin Immunol. (2010) 126:1105–18. doi: 10.1016/j.jaci.2010.10.008
130. Sampson HA. Food allergy. Part 2: diagnosis and management. J Allergy Clin Immunol. (1999) 103:981–9. doi: 10.1016/S0091-6749(99)70167-3
131. Albuhairi S, Rachid R. Novel therapies for treatment of food allergy. Immunol Allergy Clin North Am. (2020) 40:175–86. doi: 10.1016/j.iac.2019.09.007
132. Fiocchi A, Vickery BP, Wood RA. The use of biologics in food allergy. Clin Exp Allergy. (2021) 51:1006–18. doi: 10.1111/cea.13897
133. Akarsu A, Ocak M, Sahiner UM, Soyer O, Sekerel BE. Multiplex component-based allergen macroarray test is useful to predict clinical reactivity to tree nuts in children. Allergol Int. (2021) S1323-8930(21)00127-1. doi: 10.1016/j.alit.2021.10.001
134. Fuhrmann V, Huang HJ, Akarsu A, Shilovskiy I, Elisyutina O, Khaitov M, et al. From allergen molecules to molecular immunotherapy of nut allergy: a hard nut to crack. Front Immunol. (2021) 12:742732. doi: 10.3389/fimmu.2021.742732
135. Jin H, Trogen B, Nowak-Wegrzyn A. Eosinophilic esophagitis as a complication of food oral immunotherapy. Curr Opin Allergy Clin Immunol. (2020) 20:616–23. doi: 10.1097/ACI.0000000000000688
136. Lucendo AJ, Arias A, Tenias JM. Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. (2014) 113:624–9. doi: 10.1016/j.anai.2014.08.004
137. Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S, et al. Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet. (2019) 393:2222–32. doi: 10.1016/S0140-6736(19)30420-9
138. Statement Statement by the American College of Allergy Asthma & Immunology American American Academy of Allergy Asthma & Immunology and the Canadian Society of Allergy Clinical Immunology. Allergists Respond to Death of 3 Year-Old Boy During Oral Food Challenge. Available online at: https://acaai.org/allergists-respond-death-3-year-old-boy-during-oral-food-challenge (accessed December 22, 2018).
139. Amat F, Kouche C, Gaspard W, Lemoine A, Guiddir T, Lambert N, et al. Is a slow-progression baked milk protocol of oral immunotherapy always a safe option for children with cow's milk allergy? A randomized controlled trial. Clin Exp Allergy. (2017) 47:1491–6. doi: 10.1111/cea.13022
140. Sugiura S, Kitamura K, Makino A, Matsui T, Furuta T, Takasato Y, et al. Slow low-dose oral immunotherapy: threshold and immunological change. Allergol Int. (2020) 69:601–9. doi: 10.1016/j.alit.2020.03.008
141. DunnGalvin A, Blumchen K, Timmermans F, Regent L, Schnadt S, Podesta M, et al. APPEAL-1: a multiple-country European survey assessing the psychosocial impact of peanut allergy. Allergy. (2020) 75:2899–908. doi: 10.1111/all.14363
142. Factor JM, Mendelson L, Lee J, Nouman G, Lester MR. Effect of oral immunotherapy to peanut on food-specific quality of life. Ann Allergy Asthma Immunol. (2012) 109:348–52.e2. doi: 10.1016/j.anai.2012.08.015
143. Reier-Nilsen T, Carlsen KCL, Michelsen MM, Drottning S, Carlsen KH, Zhang C, et al. Parent and child perception of quality of life in a randomized controlled peanut oral immunotherapy trial. Pediatr Allergy Immunol. (2019) 30:638–45. doi: 10.1111/pai.13066
144. Carraro S, Frigo AC, Perin M, Stefani S, Cardarelli C, Bozzetto S, et al. Impact of oral immunotherapy on quality of life in children with cow milk allergy: a pilot study. Int J Immunopathol Pharmacol. (2012) 25:793–8. doi: 10.1177/039463201202500329
145. Epstein-Rigbi N, Goldberg MR, Levy MB, Nachshon L, Elizur A. Quality of life of food-allergic patients before, during, and after oral immunotherapy. J Allergy Clin Immunol Pract. (2019) 7:429–36.e2. doi: 10.1016/j.jaip.2018.06.016
146. Otani IM, Begin P, Kearney C, Dominguez TL, Mehrotra A, Bacal LR, et al. Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol. (2014) 10:25. doi: 10.1186/1710-1492-10-25
147. Rigbi NE, Goldberg MR, Levy MB, Nachshon L, Golobov K, Elizur A. Changes in patient quality of life during oral immunotherapy for food allergy. Allergy. (2017) 72:1883–90. doi: 10.1111/all.13211
148. Fanning L, Woods E, Hornung CJ, Perrett KP, Tang MLK, Dalziel K. Cost-effectiveness of food allergy interventions in children: a systematic review of economic evaluations. Value Health. (2021) 24:1360–76. doi: 10.1016/j.jval.2021.02.010
149. Shaker M, Greenhawt M. Providing cost-effective care for food allergy. Ann Allergy Asthma Immunol. (2019) 123:240–8 e1. doi: 10.1016/j.anai.2019.05.015
Keywords: children, cow's milk allergy, desensitization, food allergy, egg allergy, oral immunotherapy, peanut allergy, sustained responsiveness
Citation: Akarsu A, Brindisi G, Fiocchi A, Zicari AM and Arasi S (2022) Oral Immunotherapy in Food Allergy: A Critical Pediatric Perspective. Front. Pediatr. 10:842196. doi: 10.3389/fped.2022.842196
Received: 23 December 2021; Accepted: 17 January 2022;
Published: 22 February 2022.
Edited by:
Betul Buyuktiryaki, Koç University Hospital, TurkeyReviewed by:
Noriyuki Yanagida, National Sagamihara Hospital, JapanCopyright © 2022 Akarsu, Brindisi, Fiocchi, Zicari and Arasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefania Arasi, c3RlZmFuaWEuYXJhc2lAb3BiZy5uZXQ=
 Aysegul Akarsu
Aysegul Akarsu Giulia Brindisi
Giulia Brindisi Alessandro Fiocchi
Alessandro Fiocchi Anna Maria Zicari
Anna Maria Zicari Stefania Arasi
Stefania Arasi