
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 17 March 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.839775
 Ilaria Fotzi1†
Ilaria Fotzi1† Francesco Pegoraro1,2†
Francesco Pegoraro1,2† Elena Chiocca1
Elena Chiocca1 Tommaso Casini1
Tommaso Casini1 Massimo Mogni3
Massimo Mogni3 Marinella Veltroni1
Marinella Veltroni1 Claudio Favre1*
Claudio Favre1*Introduction: εγδβ thalassemia is a rare form of β-thalassemia mostly described in children originating from Northern Europe. Only anecdotic cases from the Mediterranean area are reported. The diagnosis is challenging, considering the rarity of the disease and its heterogeneous clinical presentation. Most patients have neonatal microcytic anemia, sometimes requiring in utero and/or neonatal transfusions, and typically improving with age.
Case Description: We report on an Italian newborn presenting with severe neonatal anemia that required red blood cell transfusion. After the first months of life, hemoglobin levels improved with residual very low mean corpuscular volume. β and α thalassemia, IRIDA syndrome, and sideroblastic anemia were excluded. Finally, a diagnosis of εγδβ thalassemia was made after microarray analysis of single nucleotide polymorphisms revealed a 26 kb single copy loss of chromosome 11p15.4, including the HBD, HBBP1, HBG1, and HBB genes.
Conclusions: Despite its rarity, the diagnosis of εγδβ thalassemia should be considered in newborns with severe neonatal anemia requiring in utero and/or neonatal transfusions, but also in older infants with microcytic anemia, after excluding more prevalent red blood cell disorders.
The εγδβ thalassemia is an extremely rare heterozygous form of β-thalassemia, with around 40 reported cases in 2019 (1). In most cases, patients originated from ethnic backgrounds where β-thalassemia was not prevalent (Table 1). Despite the extreme heterogeneity of the molecular bases of β-thalassemia in Italy, the first εγδβ thalassemia deletion has been only identified in 2016 (23).
εγδβ thalassemias are caused by long deletions in the β-globin cluster and exist only in heterozygous form. Except for one case (8, 27), the reported deletions are almost exclusively unique and in most cases de novo, explaining the phenotypic heterogeneity of the disease. Indeed, multiple clinical phenotypes of εγδβ thalassemia have been reported, ranging from normal blood cell count to severe anemia requiring in utero and/or neonatal transfusions (Table 1) (20). The underlying reasons for such a spectrum of clinical characteristics are unknown, but the type and length of the deletion are not responsible, as contrasting phenotypes have been reported in heterozygotes with identical deletions within the same family (8). At the molecular level εγδβ thalassemias fall into two distinct categories: in group I all, or a greater part of the β-globin cluster, are removed, including the β-globin gene, whereas in group II extensive upstream regions are removed, leaving the β-globin gene itself intact although its expression is silenced because of inactivation of the upstream β-locus control region (23). Furthermore, co-existent α-globin gene triplication has been suggested to exacerbate the phenotype of εγδβ thalassemia increasing the imbalance between the α and non-α globin chain ratio during fetal life (16).
Most patients with εγδβ-thalassemia had neonatal erythroblastosis, reticulocytosis, hypochromia, and microcytosis (Table 1), that later improved with age. Anemia usually remitted spontaneously during the first months of life, and the adult phenotype is similar to that of the β-thalassemia trait, but with more severe microcytosis (13).
Herein, we describe the clinical phenotype of a novel Italian εγδβ deletion, the second patient from Italy described in the literature and the third from the Mediterranean Area, presenting with severe microcytic anemia in the neonatal period.
A male, full-term infant of Tuscanian origin was born by induced vaginal delivery due to meconium-stained amniotic fluid. He presented with clinical and laboratory signs of sepsis (increased white blood cell count, C-reactive protein, and indirect bilirubin) and received wide spectrum antibiotics. Laboratory evaluations revealed microcytic anemia (hemoglobin, Hb, 10.8 g/dL, mean corpuscular volume, MCV, 65.4 fL). The clinical condition rapidly improved and hemoglobin rose to 12 g/dL, with persistent microcytemia. At the first follow-up visit at 1 month of age, hemoglobin had dropped to 6.4 g/dL, with a MCV of 53.8 fL, a mean cell hemoglobin concentration of 18.3 g/dL, a hematocrit of 19.9%, and an increased reticulocyte count (0.2 × 106/L) (Figure 1). No other signs of hemolysis were detected (normal bilirubin and lactate dehydrogenase levels). The peripheral blood smear revealed microcytic hypochromic erythrocytes with anisopoikilocytosis (Figure 2). Hemoglobin electrophoresis showed a normal pattern with an unusually high proportion of HbA (HbF 47%, HbA2 0.8%, HbA 52.2%), no abnormal hemoglobin variants, nor evidence of β-thalassemia; abdominal ultrasound showed splenomegaly. The patient received a red blood cell transfusion and supplementation of iron and folic acid, which proved ineffective. Therefore, bone marrow aspiration was performed to exclude the presence of ring sideroblasts with Prussian blue staining (Figure 2); normal plasmatic hepcidin values ruled out an Iron-Refractory Iron Deficiency Anemia (IRIDA) syndrome.
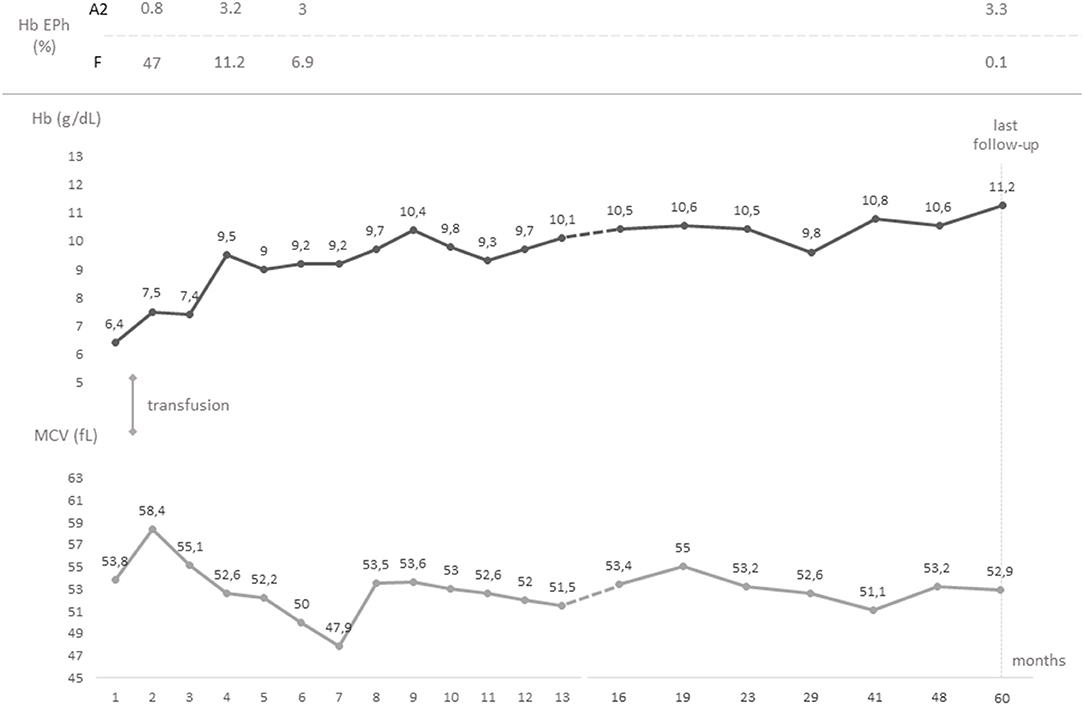
Figure 1. Timeline graph showing the chronological evolution of the values of hemoglobin and mean corpuscular volume from birth to last follow-up. Hemoglobin electrophorese studies and transfusions are also reported. Hb, hemoglobin; EPh, electrophoresis; MCV, mean corpuscular volume.
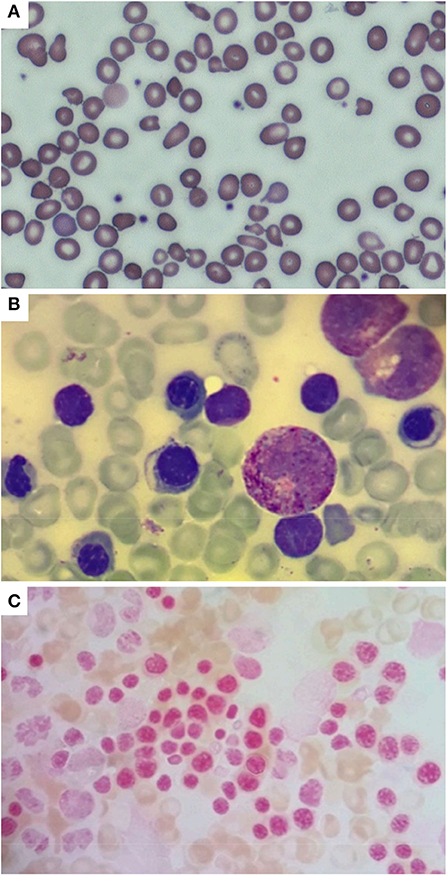
Figure 2. Peripheral blood smear (A) performed in the neonatal period, showing hypochromic erythrocytes with anisopoikilocytosis; isolated target cells, ovalocytes, ellissocytes, and dacrocytes are also visible (600x magnification, MGG). Bone marrow aspirate (B) performed at 2 months of age showing mild dyserythropoiesis (1000x magnification, MGG); no ring sideroblasts were found in the smear [(C) 1000x magnification, Pearls coloration].
At 6 months of age, the blood cell count of the patient was consistent with a thalassemia trait (hemoglobin 9 g/dL, red blood cell 6.19 × 1012/L, MCV 52 fL, mean cell hemoglobin, MCH, 6.3 pg). The hemoglobin electrophoresis showed HbA2 value of 3.3%, and α gene deletions were excluded using Multiplex Ligation Probe Amplification (MLPA). Conversely, MLPA showed a heterozygous deletion in the short arm of chromosome 11 (Figure 3). This was confirmed by microarray analysis of single nucleotide polymorphisms that revealed a 26 kb single-copy loss of a genomic region localized at 11p15.4. The lost genetic material included the HBD, HBBP1, HBG1- and partially HBB- genes, a finding consistent with εγδβ thalassemia. The family history was negative for similarly affected individuals and targeted parental testing via quantitative polymerase chain reaction confirmed the presence of a de novo deletion.
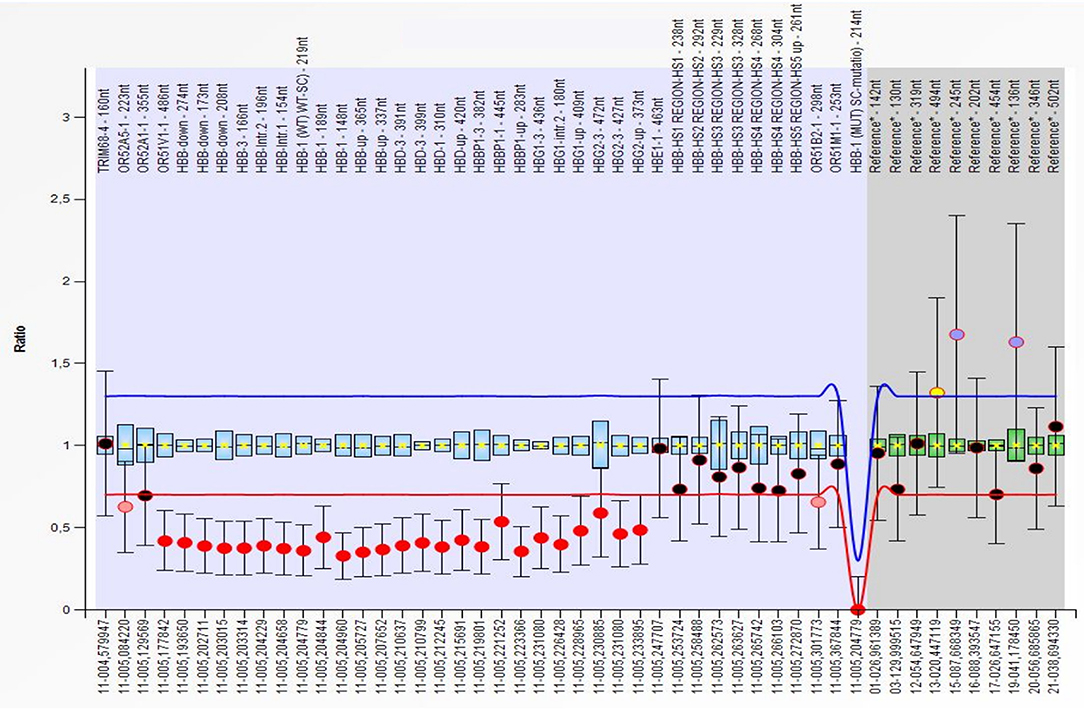
Figure 3. Multiplex Ligation Probe Amplification (MLPA) showing a deletion of the OR51V1-1, HBB, HBD, HBBP1, HBG1 and HBG2 genes (red dots) on the short arm of chromosome 11. All deletion were detected in the heterozygous form. The first deleted probe was the 486 on the OR51V1-1 gene (hg18 loc.11–005,177842), while the last was the 373, after the end of the HBG2 gene (hg18 loc.11–005,233895).
At last follow-up (5 years of age), the patient had a hemoglobin of 11.2 g/dL, a MCV of 52.9 fL, and a MCH of 17 pg; hemoglobin electrophoresis revealed 0.1% of HbF and 3.3% of HbA2 (Figure 1). The patient was in good clinical condition, with normal growth (72nd centile of height and 80th centile of weight, WHO curves) and cognitive development. No splenomegaly was found at the abdominal ultrasound, nor signs of iron overload/deficiency. Therefore, no specific follow-up plan nor specific interventions in case of minor ailments were deemed necessary, as for β-trait carriers.
Anemia during the neonatal period represents a challenge for the pediatrician, mainly for the multiplicity of conditions that are responsible for the condition during the first weeks of life. The etiology of neonatal anemia usually falls into three major categories: blood loss, decreased production, and increased destruction of erythrocytes (28). The differential diagnosis for hemolytic anemia in the newborn period includes alloimmunity, erythrocyte membrane defects, enzyme deficiencies, and hemoglobinopathies. The most frequent hemoglobinopathy associated with critically ill infants and hemolytic anemia is α thalassemia with deletion of three α globin genes (28, 29).
εγδβ thalassemia usually presents as severe neonatal hemolytic anemia that requires in utero and/or neonatal transfusions but this condition is rarely considered among the causes of neonatal anemia and therefore misdiagnosed, as in our case. A reduced MCV without abnormalities on hemoglobin electrophoresis in a newborn is not always detected in εγδβ thalassemia (Table 1), but when it is found, it can orient toward the diagnosis. Despite the high incidence of thalassemias in Italy, the significant microcytosis in our patient was initially deemed secondary to iron deficiency, as the intercurrent sepsis misdirected high indirect bilirubin values as a sign of hemolysis.
Although uncommon during the neonatal period, microcytosis can occur secondary to iron deficiency following feto-maternal hemorrhage. However, in most cases, it is associated with thalassemia, also depending on the α thalassemia allele frequency, which varies in different populations (30). After the neonatal period, the hematologic phenotype of microcytosis associated with normal hemoglobin electrophoresis, which is typical of εγδβ thalassemia, can be associated to or confused with α thalassemia, but also, in presence of normal ferritin levels, with IRIDA. Unlike previously suggested, the severe phenotype of our patient was not justified by the presence of α triplication, which was excluded by MLPA analysis.
There is no established explanation for the phenotypic heterogeneity of the disease, but it is not dependent on the type and length of deletion (8). Although at the molecular level εγδβ thalassemias fall into two distinct categories, the associated phenotypes of the two groups are similar. Therefore, the variable severity is likely to be influenced by other genetic and environmental factors.
The remission of anemia after the first months of life is a consequence of the increasing production of β-globin that reduces the imbalance between α/non-α globin chain synthesis. The residual adult phenotype is similar to that of the β-thalassemia trait but with normal, rather than increased, levels of hemoglobin A2 due to the loss of one δ locus, while the fetal hemoglobin is normal or minimally increased (13). The normal HbA2 levels make the hematologic phenotype also similar to that of carriers of α-thalassemia (23). However, data collected by Rooks et al. suggest that adult heterozygotes for εγδβ-thalassemias tend to have more severe microcytosis and hypochromia even than β0-thalassemia carriers (13).
In conclusion, this case remarks the importance of considering the εγδβ thalassemia in the differential diagnosis of hypochromic microcytic hemolytic anemias in the newborn period. In the post-natal period, microcytosis with normal ferritin values and without abnormalities on hemoglobin electrophoresis should also raise the suspicion for εγδβ thalassemia.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
IF and FP wrote the manuscript and MV and CF critically reviewed it. IF, EC, and TC followed the patient. MM performed the genetic analysis. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Repnikova E, Roberts J, Mc Dermott S, Farooqi MS, Iqbal NT, Silvey M, et al. Clinical and molecular characterization of novel deletions causing epsilon gamma delta beta thalassemia: report of two cases. Pathol Res Pract. (2019) 215:152578. doi: 10.1016/j.prp.2019.152578
2. Kan YW, Forget BG, Nathan DG. Gamma-beta thalassemia: a cause of hemolytic disease of the newborn. N Engl J Med. (1972) 286:129–34. doi: 10.1056/NEJM197201202860304
3. Oort M, Heerspink W, Roos D, Flavell RA, Bernini LF. Hemolytic disease of the newborn and chronic hypochromic microcytic anemia in one family: gamma-delta-beta thalassemia. Tijdschr Kindergeneeskd. (1981) 49:199–207.
4. Fearon ER, Kazazian HH, Waber PG, Lee JI, Antonarakis SE, Orkin SH, et al. The entire beta-globin gene cluster is deleted in a form of gamma delta beta-thalassemia. Blood. (1983) 61:1269–74. doi: 10.1182/blood.V61.6.1269.1269
5. Curtin P, Pirastu M, Kan YW, Gobert-Jones JA, Stephens AD, Lehmann H, et al. distant gene deletion affects beta-globin gene function in an atypical gamma delta beta-thalassemia. J Clin Invest. (1985) 76:1554–8. doi: 10.1172/JCI112136
6. Diaz-Chico JC, Huang HJ, Juricić D, Efremov GD, Wadsworth LD, Huisman TH. Two new large deletions resulting in epsilon gamma delta beta-thalassemia. Acta Haematol. (1988) 80:79–84. doi: 10.1159/000205607
7. Driscoll MC, Dobkin CS, Alter BP. Gamma delta beta-thalassemia due to a de novo mutation deleting the 5' beta-globin gene activation-region hypersensitive sites. Proc Natl Acad Sci U S A. (1989) 86:7470–4. doi: 10.1073/pnas.86.19.7470
8. Trent RJ, Williams BG, Kearney A, Wilkinson T, Harris PC. Molecular and hematologic characterization of Scottish-Irish type (epsilon gamma delta beta)zero thalassemia. Blood. (1990) 76:2132–8. doi: 10.1182/blood.V76.10.2132.2132
9. Fortina P, Delgrosso K, Werner E, Haines K, Rappaport E, Schwartz E, et al. A greater than 200 kb deletion removing the entire beta-like globin gene cluster in a family of Irish descent. Hemoglobin. (1991) 15:23–41. doi: 10.3109/03630269109072482
10. Abels J, Michiels JJ, Giordano PC, Bernini LF, Baysal E, Smetanina NS, et al. A de novo deletion causing epsilon gamma delta beta-thalassemia in a Dutch patient. Acta Haematol. (1996) 96:108–9. doi: 10.1159/000203726
11. Game L, Bergounioux J, Close JP, Marzouka BE, Thein SL. A novel deletion causing (epsilon gamma delta beta) degrees thalassaemia in a Chilean family. Br J Haematol. (2003) 123:154–9. doi: 10.1046/j.1365-2141.2003.04564.x
12. Harteveld CL, Osborne CS, Peters M, van der Werf S, Plug R, Fraser P, et al. Novel 112 kb (epsilonGgammaAgamma) deltabeta-thalassaemia deletion in a Dutch family. Br J Haematol. (2003) 122:855–8. doi: 10.1046/j.1365-2141.2003.04505.x
13. Rooks H, Bergounioux J, Game L, Close JP, Osborne C, Best S, et al. Heterogeneity of the epsilon gamma delta beta-thalassaemias: characterization of three novel English deletions. Br J Haematol. (2005) 128:722–9. doi: 10.1111/j.1365-2141.2005.05368.x
14. Furuya C, Yamashiro Y, Hattori Y, Hino M, Nishioka H, Shimizu Y, et al. A novel epsilon gamma delta beta thalassemia of 1. 4 Mb deletion found in a Japanese patient. Am J Hematol. (2008) 83:84–6. doi: 10.1002/ajh.21040
15. Brantberg A, Eik-Nes SH, Roberts N, Fisher C, Wood WG. Severe intrauterine anemia: a new form of epsilongammagammadeltabeta thalassemia presenting in utero in a Norwegian family. Haematologica. (2009) 94:1157–9. doi: 10.3324/haematol.2009.007534
16. Rose C, Rossignol J, Lambilliotte A, Depret S, Le Metayer N, Pissard S, et al. novel (epsilongammadeltabeta)(o)-thalassemia deletion associated with an alpha globin gene triplication leading to a severe transfusion dependent fetal thalassemic syndrome. Haematologica. (2009) 94:593–4. doi: 10.3324/haematol.2008.002675
17. Verhovsek M, Shah NR, Wilcox I, Koenig SC, Barros T, Thornburg CD, et al. Severe fetal and neonatal hemolytic anemia due to a 198 kb deletion removing the complete β-globin gene cluster. Pediatr Blood Cancer. (2012) 59:941–4. doi: 10.1002/pbc.24094
18. Rooks H, Clark B, Best S, Rushton P, Oakley M, Thein OS, et al. A novel 506kb deletion causing εγδβ thalassemia. Blood Cells Mol Dis. (2012) 49:121–7. doi: 10.1016/j.bcmd.2012.05.010
19. Von Kanel T, Röthlisberger B, Schanz U, Dutly F, Huber AR, Saller E, et al. Swiss (εγδβ)°-thalassemia patient with a novel 3-Mb deletion associated with mild mental impairment. Am J Hematol. (2013) 88:158–9. doi: 10.1002/ajh.23364
20. Shalev H, Landau D, Pissard S, Krasnov T, Kapelushnik J, Gilad O, et al. A novel epsilon gamma delta beta thalassemia presenting with pregnancy complications and severe neonatal anemia. Eur J Haematol. (2013) 90:127–33. doi: 10.1111/ejh.12047
21. Zebisch A, Schulz E, Grosso M, Lombardo B, Acierno G, Sill H, et al. Identification of a novel variant of epsilon-gamma-delta-beta thalassemia highlights limitations of next generation sequencing. Am J Hematol. (2015) 90:E52–4. doi: 10.1002/ajh.23913
22. Shooter C, Rooks H, Thein SL. Barnaby Clark. Next generation sequencing identifies a novel rearrangement in the HBB cluster permitting to-the-base characterization. Hum Mutat. (2015) 36:142–50. doi: 10.1002/humu.22707
23. Cardiero G, Prezioso R, Dembech S, Del Vecchio Blanco F, Scarano C, Lacerra G. Identification and molecular characterization of a novel 163 kb deletion: The Italian (ϵγδβ)(0)-thalassemia. Hematology. (2016) 21:317–24. doi: 10.1080/10245332.2015.1133007
24. Goel R, Snow J, Pri-Paz SM, Cushing M, Vasovic LV. Intrauterine transfusions for severe fetal anemia and hydrops due to de novo εγδβ-thalassemia. Transfusion. (2017) 57:876. doi: 10.1111/trf.13926
25. Hui ASY, Au PKC, Ting YH, Kan ASY, Cheng YKY, Leung AWK, et al. First Report of a Novel Deletion Due to εγδβ-Thalassemia in a Chinese Family. Hemoglobin. (2017) 41:175–9. doi: 10.1080/03630269.2017.1366918
26. Muñoz Tormo-Figueres Á, Sanchis Calvo A, Guibert Zafra B. Epsilon gamma delta beta thalassemia: A rare cause of fetal and neonatal anemia. Med Clin (Barc). (2018) 150:368–9. doi: 10.1016/j.medcli.2017.10.011
27. Pirastu M, Kan YW, Lin CC, Baine RM, Holbrook CT. Hemolytic disease of the newborn caused by a new deletion of the entire beta-globin cluster. J Clin Invest. (1983) 72:602–9. doi: 10.1172/JCI111008
28. Allali S, Brousse V, Sacri AS, Chalumeau M, de Montalembert M. Anemia in children: prevalence, causes, diagnostic work-up, and long-term consequences. Expert Rev Hematol. (2017) 10:1023–8. doi: 10.1080/17474086.2017.1354696
29. Cappellini MD, Russo R, Andolfo I, Iolascon A. Inherited microcytic anemias. Hematology Am Soc Hematol Educ Program. (2020) 2020:465–70. doi: 10.1182/hematology.2020000158
Keywords: thalassemia, εγδβ, children, newborn, anemia
Citation: Fotzi I, Pegoraro F, Chiocca E, Casini T, Mogni M, Veltroni M and Favre C (2022) Case Report: Clinical and Hematological Characteristics of εγδβ Thalassemia in an Italian Patient. Front. Pediatr. 10:839775. doi: 10.3389/fped.2022.839775
Received: 20 December 2021; Accepted: 07 February 2022;
Published: 17 March 2022.
Edited by:
Monica Hulbert, Washington University in St. Louis, United StatesReviewed by:
Mariane De Montalembert, Assistance Publique Hopitaux de Paris, FranceCopyright © 2022 Fotzi, Pegoraro, Chiocca, Casini, Mogni, Veltroni and Favre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Favre, Y2xhdWRpby5mYXZyZUBtZXllci5pdA==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.