
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 14 April 2022
Sec. Obstetric and Pediatric Pharmacology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.832363
 Fang Zhang1†
Fang Zhang1† Gulbanur Amat2†
Gulbanur Amat2† Yanjing Tang1†
Yanjing Tang1† Ru Chen3
Ru Chen3 Xin Tian4
Xin Tian4 Wenting Hu1
Wenting Hu1 Changcheng Chen1
Changcheng Chen1 Shuhong Shen1*
Shuhong Shen1* Yangyang Xie1*
Yangyang Xie1*
Background: Thiopurines are widely used as anti-cancer and immunosuppressant agents, but have a narrow therapeutic index owing to frequent toxicity and life-threatening bone marrow suppression. The nudix hydrolase 15 (NUDT15) genetic polymorphism is strongly associated with the tolerance and myelosuppressive effect of mercaptopurine administration, but the frequency of NUDT15 variants is known to vary among different ethnic groups or nationalities. At present, the NUDT15 gene polymorphism in ethnic minorities such as the Uighur, Kirghiz, and Dai nationalities in China is unclear.
Procedure: DNA samples were isolated from 1,071 Chinese children, including 675 Han children with acute lymphoblastic leukemia and 396 healthy minority children, including 118 Uighur, 126 Kirghiz, and 152 Dai participants. The coding regions of NUDT15 exons 1 to 3 were amplified by polymerase chain reaction. NUDT15 genotypes were identified by Sanger sequencing.
Results: Five NUDT15 genetic variants of coding regions including rs746071566 (c.55_56insGAGTCG), rs186364861 (c.52G > A), c.137C > G, and c.138T > G in exon 1, and the variant rs116855232 (c.415C > T) in exon 3 were found among the participants. The frequency of NUDT15 rs746071566 variants was lower in the Uighur and Kirghiz populations than in the Han population and in other East Asian nationalities, while the frequency of c.415C > T variants was lower in the Dai population. The c.52G > A variant was relatively uncommon in children of the Han, Uighur, Kirghiz, and Dai ethnic groups. Notably, the rare variants c.137C > G and c.138T > G in a Uighur child were predicted to be disruptive sites.
Conclusion: In summary, our results illustrate the NUDT15 polymorphisms in Chinese children of Han, Uighur, Kirghiz, and Dai nationalities, and provide the most effective detection recommendations for different ethnic groups to predict thiopurine-related toxicity, which could be used to guide future clinical thiopurine dose adjustment.
Mercaptopurine is widely used as a chemotherapeutic medication for acute lymphoblastic leukemia (ALL) and an immunosuppressive agent for the treatment of inflammatory bowel disease (IBD) (1–4). In the maintenance phase of childhood ALL, mercaptopurine is used as a first-line drug for 2–3 years in modern treatment regimens (5). However, mercaptopurine can cause severe toxicities, especially leukopenia, leading to frequent treatment interruptions, increasing the risk of relapse and fatal infections (6–8). In IBD, thiopurines are also commonly used to preserve the potential of steroids and maintain remission, but up to 40% of patients have to stop treatment due to adverse effects such as hematopoietic toxicity, which leads to subsequent disease recurrence (9, 10). Therefore, there is an urgent need to accurately guide the use of such drugs to reduce adverse reactions and ensure therapeutic effects.
Mercaptopurine is a prodrug that is converted into the active intermediate metabolite thioguanosine triphosphate (TGTP) through a variety of enzymatic reactions. TGTP is further reduced to deoxythioguanosine triphosphate (TdGTP), which binds to DNA during replication to produce DNA-TG, leading to DNA strand breaks, chromosome damage, and ultimately apoptosis. A variety of metabolic enzymes affect the production of mercaptopurine-active products and are closely related to the toxic response (11).
Thiopurine methyltransferase (TPMT) is a key enzyme in the metabolism of mercaptopurine, and has been established to be associated with mercaptopurine toxicity. TPMT genotype-guided mercaptopurine therapy has been incorporated into treatment regimens in some European countries (12). However, the frequency of TPMT variants is only 2% or less in Asians, which cannot fully explain the high incidence of mercaptopurine-related toxicity in these populations (13, 14).
Recent studies have shown that nudix hydrolase 15 (NUDT15) polymorphisms have a higher mutation frequency in Asian populations compared to European and African populations (15, 16) and is closely associated with thiopurine-related myelosuppression in children with ALL and IBD (17–19). Defective TGTP degradation mediated by the low enzymatic activity of NUDT15 results in more TGTP available for incorporation into DNA to generate DNA-TG, leading to DNA strand breaks and apoptosis, and thus mitigating toxicity (20). The most common NUDT15 variants are c.415C > T, c.416G > A, c.52G > A, and c.55_56insGAGTCG, which have been reported to be associated with mercaptopurine-induced myelotoxicity (8, 21, 22). Of these, the NUDT15 c.415C > T variant has been strongly associated with thiopurine-induced leukopenia, and individuals carrying this homozygous variant are exceptionally sensitive to mercaptopurine and tolerate only 8% of the standard dose (17, 19). Therefore, NUDT15 genetic variation is the main genetic cause of thiopurine toxicity in Asian populations, while the frequency of NUDT15 mutations differs among ethnic populations. In Asia, China has 56 ethnic groups, and the total population of the Uighur, Kirghiz, and Dai groups is almost 10 million.
In this study, we investigated the polymorphisms of NUDT15 in healthy children from three ethnic minorities, including the Uighur, Kirghiz, and Dai ethnic groups and Han Chinese children with ALL, and analyzed their allele frequencies to provide a reference for the individualized dosing of clinical mercaptopurine drugs.
A total of 1,071 Chinese children were enrolled in this study, of which 675 were Han children with ALL, and 396 were healthy children from three ethnic minorities. The Han children with ALL were treated with the ALL-SCMC-2005 modified protocol at Shanghai Children’s Medical Center from May 2009 to February 2014. In order to compare with ethnic minorities, 118 Uighur (male: 61, female: 57), 126 Kirghiz (male: 51, female: 75), and 152 Dai (male: 88, female: 64) healthy participants younger than 18 years of age were included in the study. Samples of the remaining peripheral blood from healthy ethnic minority children were provided by Kunming Children’s Hospital. We determined the ethnicity of children and their parents by using their registered household registration information, and the ethnicity of previous generations by asking their parents to confirm the three generations of children involved are from the same ethnic group. We collected information on the participants’ name, nationality, sex, and age, and this study was approved by the Ethics Committee of the Shanghai Children’s Medical Center (SCMCIRB – K2020078-1).
Bone marrow karyocytes from Han ALL children in the remission phase and peripheral blood samples from ethnic minority participants were collected. Genomic DNA was extracted from the frozen samples of participants using the TIANamp DNA Kit (Tiangen, Beijing, China), following the manufacturer’s instructions. The quantity and quality of the extracted DNA were assessed using a Nanodrop 8000 (Thermo Fisher Scientific, United States). The three coding sequences (exon 1 to exon 3) of NUDT15 were amplified by polymerase chain reaction (PCR) and sequenced by Sanger sequencing. For PCR, the forward primer for exon 1 was 5′-CAAAGCACAACTGTAAGCGACT-3′ and the reverse primer was 5′-GAAAGACCCAGCTAGCAAAGAC-3′. The forward and reverse primers for exon 2 were 5′-CGGCCTTCC AAAAGATTACA-3′ and 5′-TGATCTAATCACCTCCCAAGG-3′. The primer sequences for exon 3 were 5′-GCATAGCCTTTGTAAACTGGGC-3′ in the forward direction and 5′-CTCACTGGAAAAAGATTCCTTAGC-3′ in the reverse direction. Each 50 μL PCR mixture contained 25 μL 2X Taq PCR Master Mix (with dye, Sangon Biotech, Shanghai, China), 20 μM of each primer, and 200 ng of genomic DNA; and ddH2O was added to 50 μL. PCR for exon 1 and exon 2 was performed by heating to 94°C for 1 min, followed by 30 cycles at 94°C for 30 s, maintenance at 53°C for 30 s, and then 72°C for 1 min; with the final extension performed at 72°C for 5 min. PCR for exon 3 was performed with a starting temperature of 94°C for 1 min, followed by 35 cycles of 30 s at 94°C, 1 min at 55°C, and 3 min at 72°C, with a final extension at 72°C for 5 min. The PCR products were separated by 2% agarose gel electrophoresis, and the target bands were extracted using a Gel Extraction Kit D2500 (Omega, Guangzhou, China). The variants shown in this article correspond to version NM_018283.4.
The NUDT15 coding regions of exons 1 to 3 were determined by direct sequencing, and the sequencing data were analyzed using SnapGene software (GSL Biotech, Chicago, Illinois, United States). The Pearson chi-square test was used to verify that the NUDT15 c.52G > A, c.415C > T distributions in the three ethnic minority populations were consistent with the Hardy-Weinberg equilibrium law. Allele frequency was calculated as:
The chi-square test and Fisher exact test with an R × C column was used to analyze differences in distribution between ethnic minorities and other populations.
NUDT15 genotyping was conducted for a total of 1071 Chinese children with 675 Han Chinese children diagnosed with ALL and 396 healthy children from ethnic minorities, including 118 children of Uighur nationality (male: female = 1.07: 1), 126 Kirghiz children (male: female = 0.68: 1), and 152 Dai children (male: female = 1.38: 1) (Table 1). They all belonged to families of the same ethnicity, with at least three generations. The median ages of the Han, Uighur, Kirghiz, and Dai children were 5, 5, 7, and 5 years, respectively.
In the present study, the previously reported variants rs746071566 (c.55_56insGAGTCG) and rs186364861 (c.52G > A) in exon 1 and the variant rs116855232 (c.415C > T) in exon 3 were confirmed (Figures 1A,C). Genotyping results are presented in Table 2. In all three minority populations, NUDT15 c.415C > T was the most common variant type, followed by c.55_56insGAGTCG and c.52G > A. Our results showed that 29.9% (n = 202) of Han children with ALL carried the NUDT15 gene with one or more variants. The proportion of healthy children carrying variants in the NUDT15 gene was 16.1% (n = 19) (P = 0.003), 19.0% (n = 24) (P = 0.017), and 16.4% (n = 25) (P = 0.001) for the Uighur, Kirghiz, and Dai ethnic groups compared to Han children, respectively.
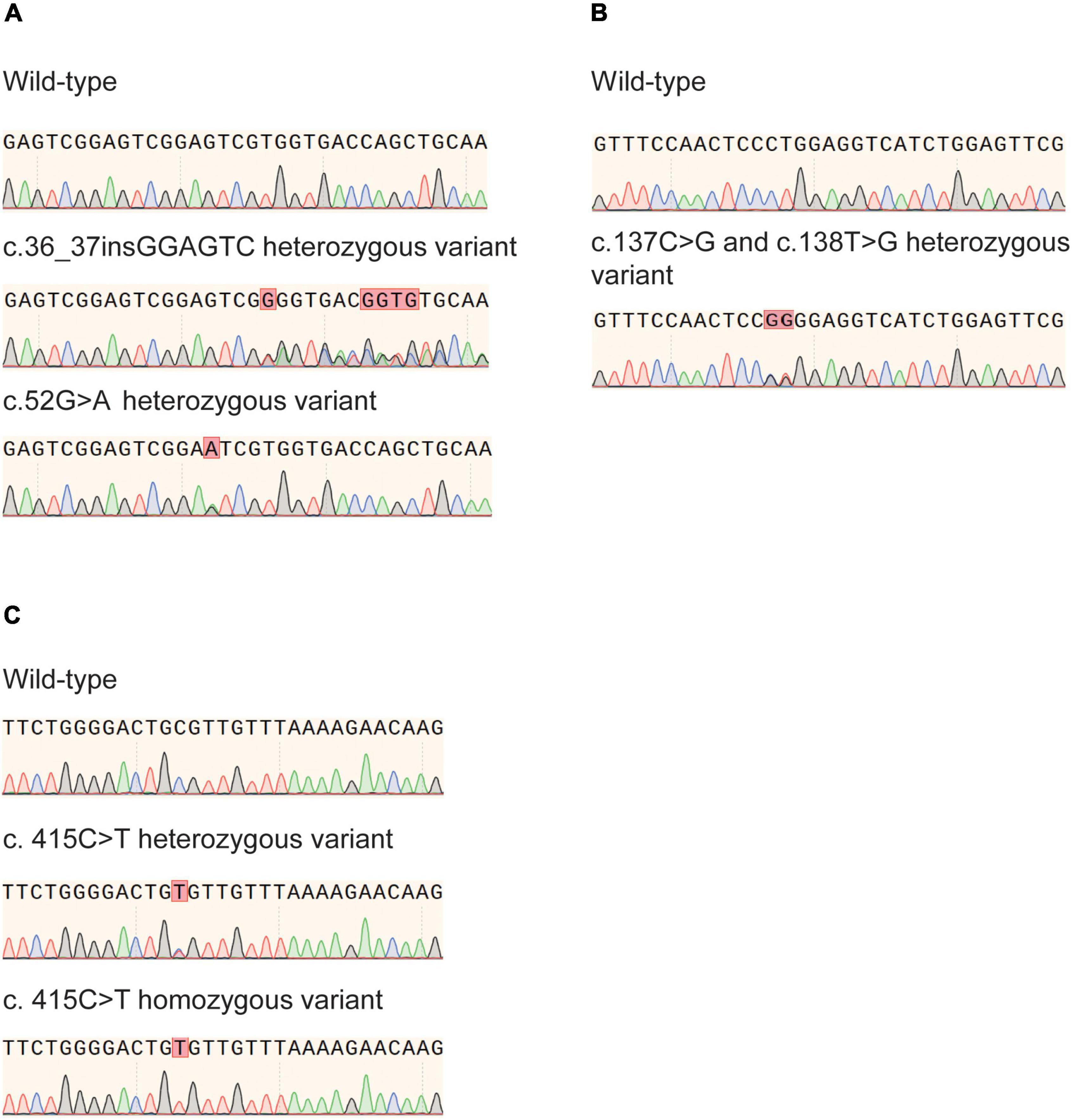
Figure 1. Nudix hydrolase 15 (NUDT15) gene variants identified by Sanger sequencing. (A) c.55_56insGAGTCG and c.52G>A heterozygous variant in exon 1. (B) Rare heterozygous variants c.137C>G and c.138T > G in exon 1 were confirmed. (C) c. 415C > T heterozygous and homozygous variant in exon 3.
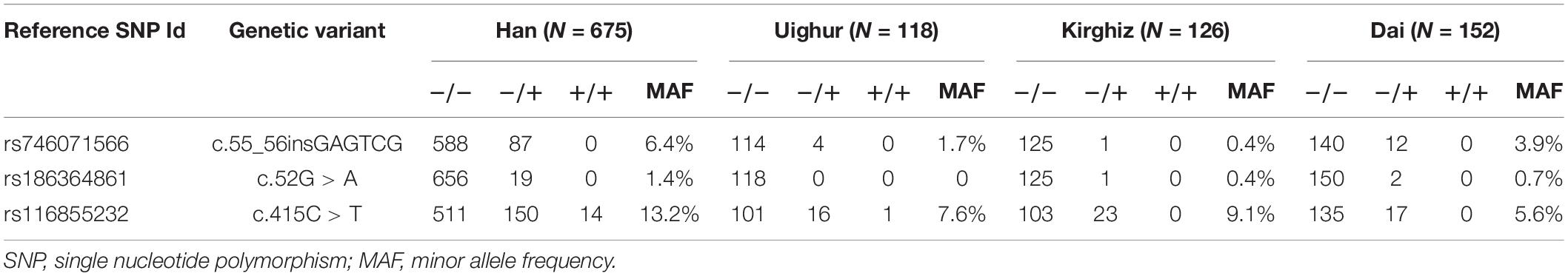
Table 2. Distribution of nudix hydrolase 15 (NUDT15) variants among Han and ethnic minority populations.
We described the results of NUDT15 gene polymorphism in Han Chinese children with ALL, showing the minor allele frequencies (MAFs) of 6.4%, 1.4%, and 13.2% for the NUDT15 c.55_56insGAGTCG, c.52G > A and c.415C > T variants, respectively. According to NUDT15 genotyping in 118 children of Uighur nationality, 99 children were wild-type (83.9%), and MAFs of NUDT15 c.55_56insGAGTCG and c.415C > T were 1.7% and 7.6%, respectively (Table 2). In addition, 13.6% Uighur children were heterozygous for NUDT15 c.415C > T, and 0.8% were homozygous variants. However, the NUDT15 c.52G > A variant was not found in the Uighur participants. In the current study of 126 Kirghiz children, the MAF of c.55_56insGAGTCG was 0.4%, while 9.1% for NUDT15 c.415C > T. Only one Kirghiz child with a variant of c.52G > A was observed. Among 152 Dai children, the highest variation frequency was c.55_56insGAGTCG, with an MAF of 3.9%; however, the c.415C > T locus had the lowest MAF of 5.6% compared to the other two minorities. Moreover, two Dai children were identified carrying heterozygous c.52G > A variants.
The star allele nomenclature in Figure 2 is derived from PharmVar.1 We calculated the frequencies of each star allele and compared them with those published by PharmVar (Table 3). Among the four ethnic groups in this study and the Central/South Asian, East Asian, European and Latin populations in PharmVar, the most common star allele was *1 (wild-type), whose frequencies were 83.8%, 91.5%, 90.5% and 91.8% in Han, Uighur, Kirghiz and Dai populations, respectively, and higher than 85% in the Central/South Asian, East Asian, European and Latin populations. Regarding the star alleles carrying variants, *3 was the most common, and its frequency was highest in the Kirghiz population (8.7%), followed by Han (8.1%), Central/South Asian (6.7%), Uighur (6.4%), East Asian (6.1%) and Dai (3.6%). The frequency of *2 was highest for Han Chinese at 5%, with East Asian and Latino populations at close to 3.5% and under 2% for Uighur, Kirghiz and Dai. The frequencies of all other star alleles were lower. In addition, we also compared diplotype frequencies in different populations (Table 4) and the most common variant diplotype was *1/*3, with the frequency of *1/*3 in the four ethnic groups in this study being closer to that of Central/South and East Asian and much higher than that of European and Latino populations.
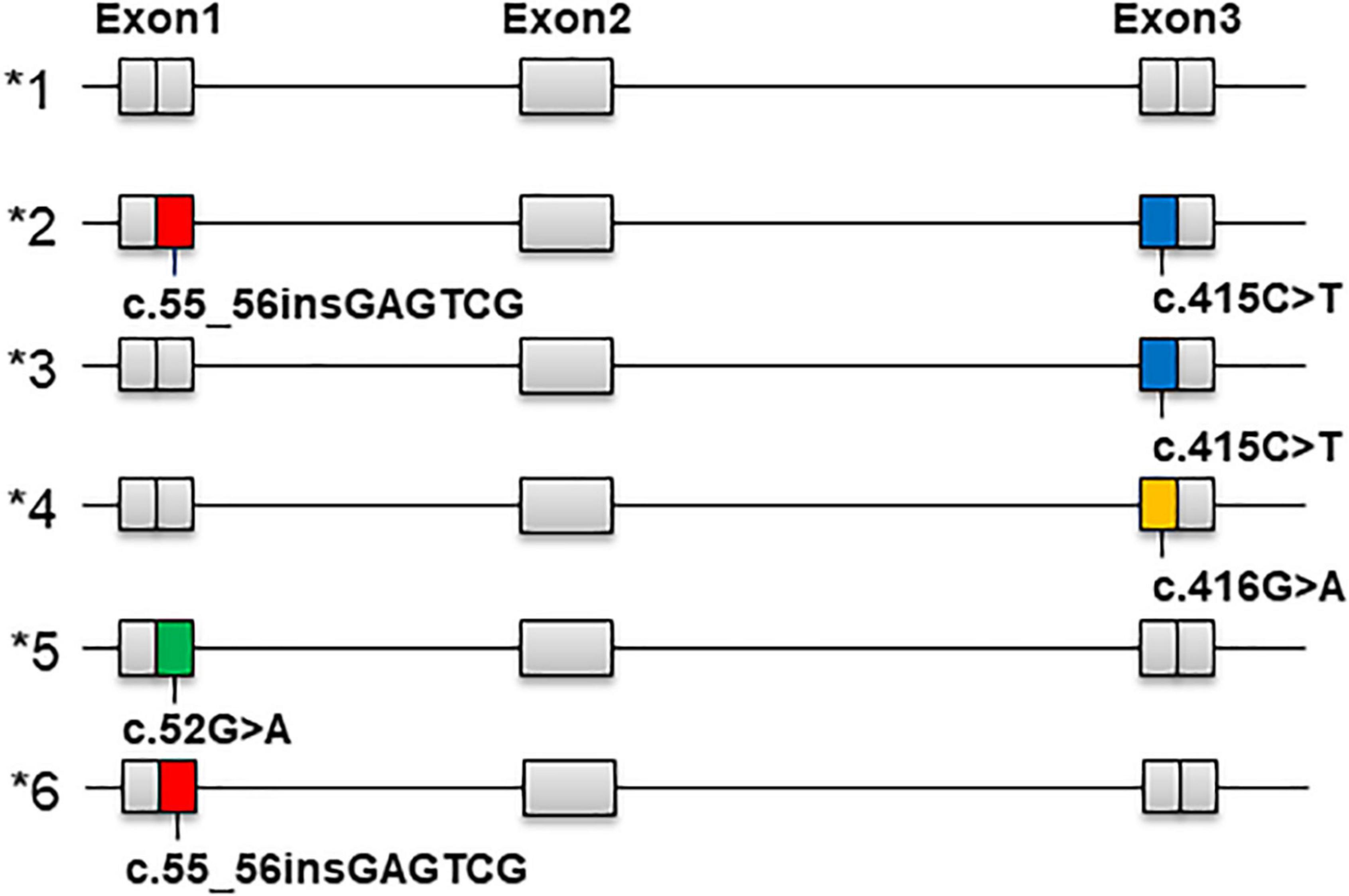
Figure 2. Nomenclature of NUDT15 star alleles (*1 to *6), corresponding to the variant version NM_018283.4.
The Hardy Weinberg genetic equilibrium test for genotypes at the c.415C > T and c.52G > A sites in the Han, Uighur, Kirghiz, and Dai ethnic groups, showed that the distribution of both loci was consistent with Hardy Weinberg genetic equilibrium (P > 0.05), indicating that the selected samples were from the same Mendelian population and could reflect the distribution of c.415C > T and c.52G > A in the target population.
To clarify the distribution of NUDT15 variants in Chinese ethnic minority populations, we conducted a two-by-two comparison of the c.55_56insGAGTCG, c.52G > A, and c.415C > T variant frequencies in seven ethnic groups: Korean (23), Japanese (24), Chinese Han, Bai (25), Uighur, Kirghiz, and Dai, of which Bai is also an ethnic minority in China. The allele frequency of NUDT15 c.55_56insGAGTCG was 5.6% in Korea (23), 5.4% in Japan (24), 6.4% in Chinese Han, and 5.6% in Chinese Bai (25). We found that the c.55_56insGAGTCG variant was significantly less frequent in the Uighur and Kirghiz ethnic groups than in the other ethnic groups, and the frequency of variation at this site in the Dai ethnic group was not significantly different from that of the other ethnic groups. The NUDT15 c.52G > A allele frequency was below 2% for all ethnic groups except Japan (2.9%) (24) (Figure 3A). The MAFs of c.52G > A variant were lower in children of Han, Uighur, Kirghiz and Dai ethnic groups.
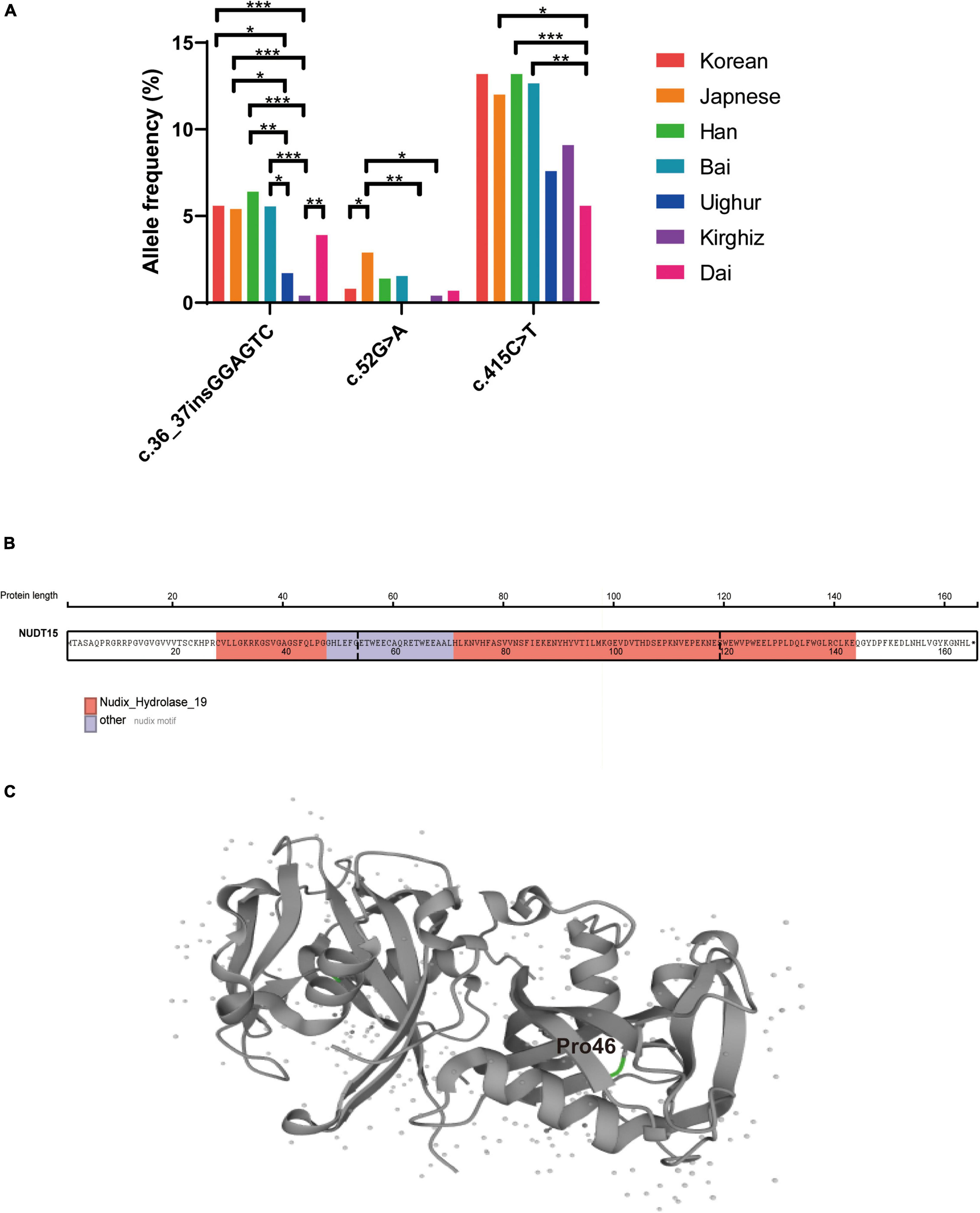
Figure 3. Comparison of the frequencies of variation at multiple sites of NUDT15 in different ethnic groups and the NUDT15 structural domain and protein structure. (A) *P < 0.05; **P < 0.01; ***P < 0.001. (B) The NUDT15 structural domain obtained from St. Jude Cloud is marked in color, and p.P46R (c.137C > G and c.138T > G) was located on the Nudix-Hydrolase-19 functional domain. (C) The position of p.P46R in the NUDT15 protein derived from Uniprot is highlighted in green.
The single nucleotide polymorphism (SNP) c.415C > T is the most important variant of NUDT15 related to thiopurine toxicity, which leads to almost complete loss of enzyme activity and protein stability in vitro and induces severe bone marrow suppression. It has been reported that the allele frequency of the NUDT15 c.415C > T variant is 13.2% in South Korea (23), 12.0% in Japan (24), and 12.7% in the Bai nationality in China (25). The distribution of NUDT15 c.415C > T variants in our results for the Han, Uighur and Kirghiz ethnic groups was similar to that for other ethnic groups, except for the Dai nationality group. The frequency of NUDT15 c.415C > T variants was significantly lower in the Dai population than in the Japanese (P = 0.02), Han populations (P = 0.0007), and Bai populations (P = 0.004).
We identified eight rare heterozygous variants in Han and minority children and showed the protein abundance score, drug sensitivity score and final classification referred to a previous article by Jun J Yang (26). There were four variants (c.37G > A, c.137C > G, c.138T > G, c.154T > C) in exon 1, three variants (c.202G > A, c.254T > C and c.272A > C) in exon 2, and one variant (c.422T > G) in exon 3 (Table 5). Remarkably, c.137C > G and c.138T > G heterozygous mutations at adjacent loci were found in one Uighur child (Figure 1B). This missense mutation resulted in a change of amino acid 46 from proline to arginine (P46R). To explore the impact of this variation, we revealed that this variant site was located in the Nudix-Hydrolase-19 functional domain (Figure 3B), which is a structural part of the beta fold in the protein (Figure 3C).
Mercaptopurine is a cornerstone drug for ALL and IBD, but is limited by the adverse effects such as myelosuppression, alopecia, hepatotoxicity, and pancreatitis (27). Leukopenia is the most common myelosuppressive manifestation of thiopurines, occurring in 15.36% of IBD patients (28). Severe myelosuppression can be life-threatening when treating ALL with 6-MP (6-mercaptopurine), and interruption of mercaptopurine therapy due to induced toxicity can increase the risk of relapse. Therefore special caution must be exercised toward mercaptopurine effects on myelotoxicity (29, 30).
NUDT15 germline variants are closely associated with mercaptopurine-induced hematotoxicity and vary considerably in their distribution across ethnic groups (31, 32), with the risk allele of NUDT15 c.415C > T being the most common among East Asians (9.8%), very rare in Europe (0.2%), and not observed in Africa (19). Among the 56 ethnic groups in China, only the Bai ethnic groups of the Yunnan and Han nationality have been studied for the distribution of the NUDT15 polymorphism (25, 33). This study investigated the distribution of NUDT15 gene polymorphisms among healthy Uighur and Kirghiz children in Xinjiang and Dai children in Yunnan, China. Each of these minorities has a population of over one million. We also conducted a large sample study on the distribution of NUDT15 genotypes in Han Chinese children with ALL. The NUDT15 gene encodes an enzyme that negatively regulates the activation and toxicity of thiopurine and has no effect on the pathogenesis of leukemia. Therefore, this cohort of children with ALL can be considered as a reference for the normal Han population. The frequencies of children carrying the NUDT15 variants were 29.9%, 16.1%, 19.0%, and 16.4% in the Han, Uighur, Kirghiz, and Dai ethnic groups in China, respectively. The distribution of both the c.415C > T and c.52G > A variants in the four ethnic groups is consistent with Hardy-Weinberg genetic equilibrium, indicating that the population surveyed is in genetic equilibrium and that the data from this population survey are reliable.
Numerous studies have reported increased susceptibility to mercaptopurine toxicity with the NUDT15 variant c.415C > T in patients with ALL (19, 34, 35) and IBD (36–38). Compared to ALL patients with the CC genotype who tolerated 83.5% of the standard dose, patients with the TC and TT genotypes tolerated 63% and 8.3%, respectively (19). The allele frequency of NUDT15 c.415C > T was 13.2% in Han, 7.6% in Uighur, 9.1% in Kirghiz, and 5.6% in Dai. Previous reports have shown that the allele frequency for NUDT15 c.415C > T was 16.3% and 11.62% in Japan (18, 35); 11.6% and 15.7% in China (14, 33); and 13.2% in Korea (23). In terms of c.415C > T variant frequency, our report of allele frequency of the Uighurs and Kirghiz is similar to other regional findings, while Dai has significantly lower variant frequencies than the majority East Asian population.
In addition to c.415C > T, a Chinese study of 732 patients with IBD concluded that NUDT15 c.55_56insGAGTCG and c.52G > A variants are also risk factors for thiopurine-induced leukopenia (21). However, a Japanese study in IBD patients concluded that c.55_56insGAGTCG and c.52G > A variants were not significantly associated with leukopenia. In addition, Moriyama et al. identified that NUDT15 coding variants (c.415C > T, c.55_56insGAGTCG and c.52G > A) resulted in 74.4–100% loss of nucleotide diphosphatase activity. Therefore, the effect of c.55_56insGAGTCG and c.52G > A variants on mercaptopurine induced toxicity is not yet determined. In our study, the c.55_56insGAGTCG variant was significantly lower in Uighur and Kirghiz children than in Han and other Asian nationalities, and the c.52G > A variant in Uighur, Kirghiz, Dai, and Han nationality was also lower. Since the frequency of NUDT15 genetic variants varies among different races, our results support that ethnic differences should be considered when using NUDT15 genotyping. We recommend that it is not necessary to detect these two variants in Uighur and Kirghiz patients and the c.52G > A variant in Dai and Han patients, but the c.415C > T variant in exon 3 must be detected. This helps to use the least economic burden to predict toxicity and guide thiopurine dosing adjustment.
The rare NUDT15 c.137C > G and c.138T > G heterozygous mutation in this study were detected in a healthy child from Uighur, and these variants resulted in a change in amino acid 46 encoded by NUDT15 from proline to arginine (p.P46R). This site is located in the functional structural domain of the protein encoded by NUDT15. Yang et al. determined the effect of variation in protein abundance and thiopurine cytotoxicity using a massively parallel NUDT15 functional assay, showing that the p.P46R variant of NUDT15 had protein abundance and drug sensitivity scores of 0.14 and 0.04, respectively (26). The protein abundance of this variant is lower than that of the known toxicity risk variant Arg139Cys, suggesting that this missense mutation exhibits a significant decrease in the thermostability of the NUDT15 protein, and the drug sensitivity score was also low, indicating low enzymatic activity of the p.P46R variant. Therefore, the p.P46R missense mutation is a disruptive mutation site that affects the structural stability of the enzyme and the activity of NUDT15. We need to further investigate the relationship between patients carrying this disruptive mutant and mercaptopurine dose-related side effects and drug tolerance in Uighur patients with ALL or IBD.
Our study has some limitations. The sample size of this study on three ethnic minorities was relatively small, and it is expected that the sample size will be further expanded in future studies to confirm our results. Subsequent studies should include clinical tolerance and treatment response to mercaptopurine in ethnic minority children with ALL carrying different NUDT15 genotypes.
In conclusion, this study revealed polymorphisms in the NUDT15 gene in Han, Uighur, Kirghiz, and Dai children, providing a basis for the clinical use of mercaptopurine in Han Chinese and ethnic minority patients. In order for the clinical test to be most meaningful for Uighur or Kirghiz patients, the test needs to include the c.415C > T variant, and for Dai and Han Chinese the test requires the inclusion of the c.55_56insGAGTCG and c.415C > T variants. The NUDT15 genetic variants in various ethnic groups may guide future studies in the individualized administration of mercaptopurine drugs in a clinical setting for patients from Han and ethnic minority populations (such as Uighur, Kirghiz, and Dai) in China.
The original contributions presented in the study are included in the article/supplementary material, and further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Shanghai Children’s Medical Center. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YX and SS conceived the idea of the study. FZ, GA, YT, and RC performed the experiments and analyzed the data. FZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by grants from the Natural Science Foundation of China (No. 81900114), the People’s Livelihood Scientific Research Project of Shanghai Pudong New Area Science and Technology Development Fund (No.PKJ2020-Y03), and the National Research Center for Translational Medicine at Shanghai [No. NRCTM(SH)-2019-04].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the timely help given by Yu Liu for assistance with the experiments and Han Wang for valuable advice.
1. Koren G, Ferrazini G, Sulh H, Langevin AM, Kapelushnik J, Klein J, et al. Systemic exposure to mercaptopurine as a prognostic factor in acute lymphocytic leukemia in children. N Engl J Med. (1990) 323:17–21. doi: 10.1056/NEJM199007053230104
2. Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. (2011) 29:551–65. doi: 10.1200/JCO.2010.30.7405
3. Timmer A, Patton PH, Chande N, Mcdonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. (2016) 2016:Cd000478.
4. Kakuta Y, Kawai Y, Okamoto D, Takagawa T, Ikeya K, Sakuraba H, et al. NUDT15 codon 139 is the best pharmacogenetic marker for predicting thiopurine-induced severe adverse events in Japanese patients with inflammatory bowel disease: a multicenter study. J Gastroenterol. (2018) 53:1065–78. doi: 10.1007/s00535-018-1486-7
5. Childhood All Collaborative Group. Duration and intensity of maintenance chemotherapy in acute lymphoblastic leukaemia: overview of 42 trials involving 12 000 randomised children. Lancet. (1996) 347:1783–8. doi: 10.1016/s0140-6736(96)91615-3
6. Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. (1993) 34:1081–5. doi: 10.1136/gut.34.8.1081
7. Goldberg R, Irving PM. Toxicity and response to thiopurines in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. (2015) 9:891–900. doi: 10.1586/17474124.2015.1039987
8. Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. (2016) 48:367–73. doi: 10.1038/ng.3508
9. Jharap B, Seinen ML, De Boer NK, Van Ginkel JR, Linskens RK, Kneppelhout JC, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. (2010) 16:1541–9. doi: 10.1002/ibd.21221
10. Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M, et al. Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis. (2013) 19:1404–10. doi: 10.1097/MIB.0b013e318281f28f
11. Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. (2008) 8:24–36. doi: 10.1038/nrc2292
12. Ford LT, Berg JD. Thiopurine S-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. (2010) 63:288–95. doi: 10.1136/jcp.2009.069252
13. Collie-Duguid ES, Pritchard SC, Powrie RH, Sludden J, Collier DA, Li T, et al. The frequency and distribution of thiopurine methyltransferase alleles in caucasian and Asian populations. Pharmacogenetics. (1999) 9:37–42. doi: 10.1097/00008571-199902000-00006
14. Liang DC, Yang CP, Liu HC, Jaing TH, Chen SH, Hung IJ, et al. NUDT15 gene polymorphism related to mercaptopurine intolerance in Taiwan Chinese children with acute lymphoblastic leukemia. Pharmacogenomics J. (2016) 16:536–9. doi: 10.1038/tpj.2015.75
15. Zhang AL, Yang J, Wang H, Lu JL, Tang S, Zhang XJ. Association of NUDT15 c.415C>T allele and thiopurine-induced leukocytopenia in Asians: a systematic review and meta-analysis. Ir J Med Sci. (2018) 187:145–53. doi: 10.1007/s11845-017-1608-x
16. Relling MV, Schwab M, Whirl-Carrillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. Clinical Pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. (2019) 105:1095–105. doi: 10.1002/cpt.1304
17. Yang S-K, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet. (2014) 46:1017–20. doi: 10.1038/ng.3060
18. Tanaka Y, Kato M, Hasegawa D, Urayama KY, Nakadate H, Kondoh K, et al. Susceptibility to 6-MP toxicity conferred by a NUDT15 variant in Japanese children with acute lymphoblastic leukaemia. Br J Haematol. (2015) 171:109–15. doi: 10.1111/bjh.13518
19. Yang JJ, Landier W, Yang W, Liu C, Hageman L, Cheng C, et al. Inherited NUDT15 variant is a genetic determinant of mercaptopurine intolerance in children with acute lymphoblastic leukemia. J Clin Oncol. (2015) 33:1235–42. doi: 10.1200/JCO.2014.59.4671
20. Valerie NC, Hagenkort A, Page BD, Masuyer G, Rehling D, Carter M, et al. NUDT15 hydrolyzes 6-thio-deoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. (2016) 76:5501–11. doi: 10.1158/0008-5472.CAN-16-0584
21. Chao K, Wang X, Cao Q, Qian J, Wu K, Zhu X, et al. Combined Detection of NUDT15 variants could highly predict thiopurine-induced leukopenia in Chinese patients with inflammatory bowel disease: a multicenter analysis. Inflamm Bowel Dis. (2017) 23:1592–9. doi: 10.1097/MIB.0000000000001148
22. Walker GJ, Harrison JW, Heap GA, Voskuil MD, Andersen V, Anderson CA, et al. Association of genetic variants in NUDT15 With thiopurine-induced myelosuppression in patients with inflammatory bowel disease. Jama. (2019) 321:773–85. doi: 10.1001/jama.2019.0709
23. Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, et al. NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels in children with acute lymphoblastic leukemia. Cancer Res Treat. (2018) 50:872–82. doi: 10.4143/crt.2017.283
24. Tsujimoto S, Osumi T, Uchiyama M, Shirai R, Moriyama T, Nishii R, et al. Diplotype analysis of NUDT15 variants and 6-mercaptopurine sensitivity in pediatric lymphoid neoplasms. Leukemia. (2018) 32:2710–4. doi: 10.1038/s41375-018-0190-1
25. Pu G, Wang Y, Duan S, Chen J, Yang C, Cui T, et al. NUDT15 polymorphism in healthy children with bai nationality in Yunnan of China. Pediatr Int. (2021) 63:790–6. doi: 10.1111/ped.14480
26. Suiter CC, Moriyama T, Matreyek KA, Yang W, Scaletti ER, Nishii R, et al. Massively parallel variant characterization identifies NUDT15 alleles associated with thiopurine toxicity. Proc Natl Acad Sci USA. (2020) 117:5394–401. doi: 10.1073/pnas.1915680117
27. Schmiegelow K, Nielsen SN, Frandsen TL, Nersting J. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia: clinical facts and fiction. J Pediatr Hematol Oncol. (2014) 36:503–17. doi: 10.1097/MPH.0000000000000206
28. Qiu Y, Mao R, Zhang S-H, Li M-Y, Guo J, Chen B-L, et al. Safety Profile of thiopurines in crohn disease: analysis of 893 patient-years follow-up in a southern China cohort. Medicine. (2015) 94:e1513. doi: 10.1097/MD.0000000000001513
29. Relling MV, Hancock ML, Boyett JM, Pui CH, Evans WE. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. (1999) 93:2817–23. doi: 10.1182/blood.v93.9.2817.409k04_2817_2823
30. Bhatia S, Landier W, Shangguan M, Hageman L, Schaible AN, Carter AR, et al. Nonadherence to oral mercaptopurine and risk of relapse in hispanic and non-hispanic white children with acute lymphoblastic leukemia: a report from the children’s oncology group. J Clin Oncol. (2012) 30:2094–101. doi: 10.1200/JCO.2011.38.9924
31. Kim HT, Choi R, Won HH, Choe YH, Kang B, Lee K, et al. NUDT15 genotype distributions in the Korean population. Pharmacogenet Genomics. (2017) 27:197–200. doi: 10.1097/FPC.0000000000000274
32. Kakuta Y, Kinouchi Y, Shimosegawa T. Pharmacogenetics of thiopurines for inflammatory bowel disease in East Asia: prospects for clinical application of NUDT15 genotyping. J Gastroenterol (2018) 53:172–80. doi: 10.1007/s00535-017-1416-0
33. Zhou H, Li L, Yang P, Yang L, Zheng JE, Zhou Y, et al. Optimal predictor for 6-mercaptopurine intolerance in Chinese children with acute lymphoblastic leukemia: NUDT15, TPMT, or ITPA genetic variants? BMC Cancer. (2018) 18:516. doi: 10.1186/s12885-018-4398-2
34. Chiengthong K, Ittiwut C, Muensri S, Sophonphan J, Sosothikul D, Seksan P, et al. NUDT15 c.415C>T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica. (2016) 101:e24–6. doi: 10.3324/haematol.2015.134775
35. Suzuki H, Fukushima H, Suzuki R, Hosaka S, Yamaki Y, Kobayashi C, et al. Genotyping NUDT15 can predict the dose reduction of 6-MP for children with acute lymphoblastic leukemia especially at a preschool age. J Hum Genet. (2016) 61:797–801. doi: 10.1038/jhg.2016.55
36. Asada A, Nishida A, Shioya M, Imaeda H, Inatomi O, Bamba S, et al. NUDT15 R139C-related thiopurine leukocytopenia is mediated by 6-thioguanine nucleotide-independent mechanism in Japanese patients with inflammatory bowel disease. J Gastroenterol. (2016) 51:22–9. doi: 10.1007/s00535-015-1142-4
37. Kakuta Y, Naito T, Onodera M, Kuroha M, Kimura T, Shiga H, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. (2016) 16:280–5. doi: 10.1038/tpj.2015.43
Keywords: NUDT15 polymorphism, Uighur, Kirghiz, Dai, mercaptopurine, hematotoxicity
Citation: Zhang F, Amat G, Tang Y, Chen R, Tian X, Hu W, Chen C, Shen S and Xie Y (2022) NUDT15 Genetic Variants in Chinese Han, Uighur, Kirghiz, and Dai Nationalities. Front. Pediatr. 10:832363. doi: 10.3389/fped.2022.832363
Received: 09 December 2021; Accepted: 17 March 2022;
Published: 14 April 2022.
Edited by:
Catherine M. T. Sherwin, Wright State University, United StatesReviewed by:
Laura B. Ramsey, Cincinnati Children’s Hospital Medical Center, United StatesCopyright © 2022 Zhang, Amat, Tang, Chen, Tian, Hu, Chen, Shen and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuhong Shen, c2hlbnNodWhvbmdAc2NtYy5jb20uY24=; Yangyang Xie, eGlleWFuZ3lhbmdAc2NtYy5jb20uY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.