- Department of Pediatric Intensive Care Unit (PICU), Maternal and Child Health Hospital of Hubei Province, Wuhan, China
Objective: Fungal infections are common in pediatric intensive care units (PICUs), but the monitoring methods are limited. This study analyzed the differences in clinical features, diagnosis, and treatment between PICU patients with and without fungal infection.
Methods: This retrospective study analyzed PICU patients at the Maternal and Child Health Hospital of Hubei Province diagnosed with severe pneumonia between January 2015 and January 2020. The patients were divided into the fungal (F) and non-fungal (NF) infection groups. Levels of 1,3-beta-D-glucan (BDG) and galactomannan (GM) in serum and bronchoalveolar lavage fluid (BALF) were analyzed. Chest computed tomography (CT) images were reviewed.
Results: A total of 357 patients were included. In the F group, fever, moist rales, coarse rales, shortness of breath, and sepsis were more common (all P < 0.05); PICU time, hospitalization duration, and BDG- and GM-positive rates in serum and BALF were all significantly higher than in the NF group (all P < 0.05). The BDG- and GM-positive rates in serum and BALF were higher in the F than in the NF group (all P < 0.05). The abnormal lymphocyte ratios in serum were higher in the F group (all P < 0.05). Wedge-shaped, patchy, streaky shadows and subpleural reticulation were higher in CT images of the F group (all P < 0.05). Tracheobronchial stenosis was more common in pulmonary fibroscopy results of the F group (P = 0.04).
Conclusion: PICU pneumonia patients with fungal infection have specific clinical and laboratory features compared with those without fungal infection, including higher rates of BALF, serum BDG, GM positivity and tracheobronchial stenosis.
Introduction
Fungal infections are common in pediatric intensive care units (PICUs) and neonatal intensive care units (NICUs). Invasive fungal disease is among the main causes of morbidity and mortality of hospitalized pediatric patients, especially premature infants (1–4). Yeast and mold are the most common clinical fungal pathogens. According to a report by the Centers for Disease Control and Prevention, fungal infection is the sixth largest cause of nosocomial infections, and Candida spp. is the fourth-most common pathogen responsible for hospital-acquired infections (5). In the NICU, fungal infection is the third most common cause of mortality, with a rate as high as 20–40% (6). In 2000, nosocomial fungal infections in China increased significantly compared with 1993–1996 (24.4 vs. 13.9%) (7). Candidemia ranked fourth in the United States and seventh in Europe among blood infections responsible for the high mortality rate among children (8).
With improvements in diagnostic technologies and treatment methods, the incidence of fungal infections has shown a downward trend in recent years, but its mortality remains high (1, 4). Unfortunately, the methods available to monitor fungal infections are limited and definitive diagnosis in many cases is still difficult. Despite their low sensitivity and long delays before providing results, the fungal culture of blood, body fluids, and respiratory secretions is still considered the gold standard.
Novel methods have shown promise, including serum marker tests such as the G test and galactomannan (GM) test, polymerase chain reaction (PCR), matrix-assisted laser desorption/ionization-time of flight, high-throughput pathogen sequencing, and other molecular approaches (9–12). Because the diagnostic accuracy of culture and imaging is low for patients with fungal infections (12, 13), they are often misdiagnosed as tumors, tuberculosis, or inflammatory lesions (14–16), resulting in delayed treatment. Bronchoscopic manifestations and testing of the bronchoalveolar lavage fluid (BALF) in children with pulmonary fungal infections could have a high diagnostic accuracy (17, 18).
Accurate identification and timely diagnosis of fungal infections are crucial to the early control of the disease, as well as reducing medical costs and the economic burden on society and families. Therefore, this paper summarizes the clinical diagnosis and treatment of patients in the PICU of the Maternal and Child Health Hospital from January 1, 2015 to January 1, 2020 and analyzes the general clinical manifestations, chest computed tomography (CT), laboratory examination, fiberoptic bronchoscopy examination, and BALF. The results could provide a reference for medical practitioners.
Patients and Methods
Study Design and Patients
This retrospective study examined the data of 357 patients with proven or probable pulmonary fungal infection admitted to the PICU of the Maternal and Child Health Hospital, Hubei Province, between January 1, 2015, to January 1, 2020. All data were prospectively collected in a database. The study was approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province (approval number: 2021 IECLW008). Informed consent was waived due to the retrospective nature of the study.
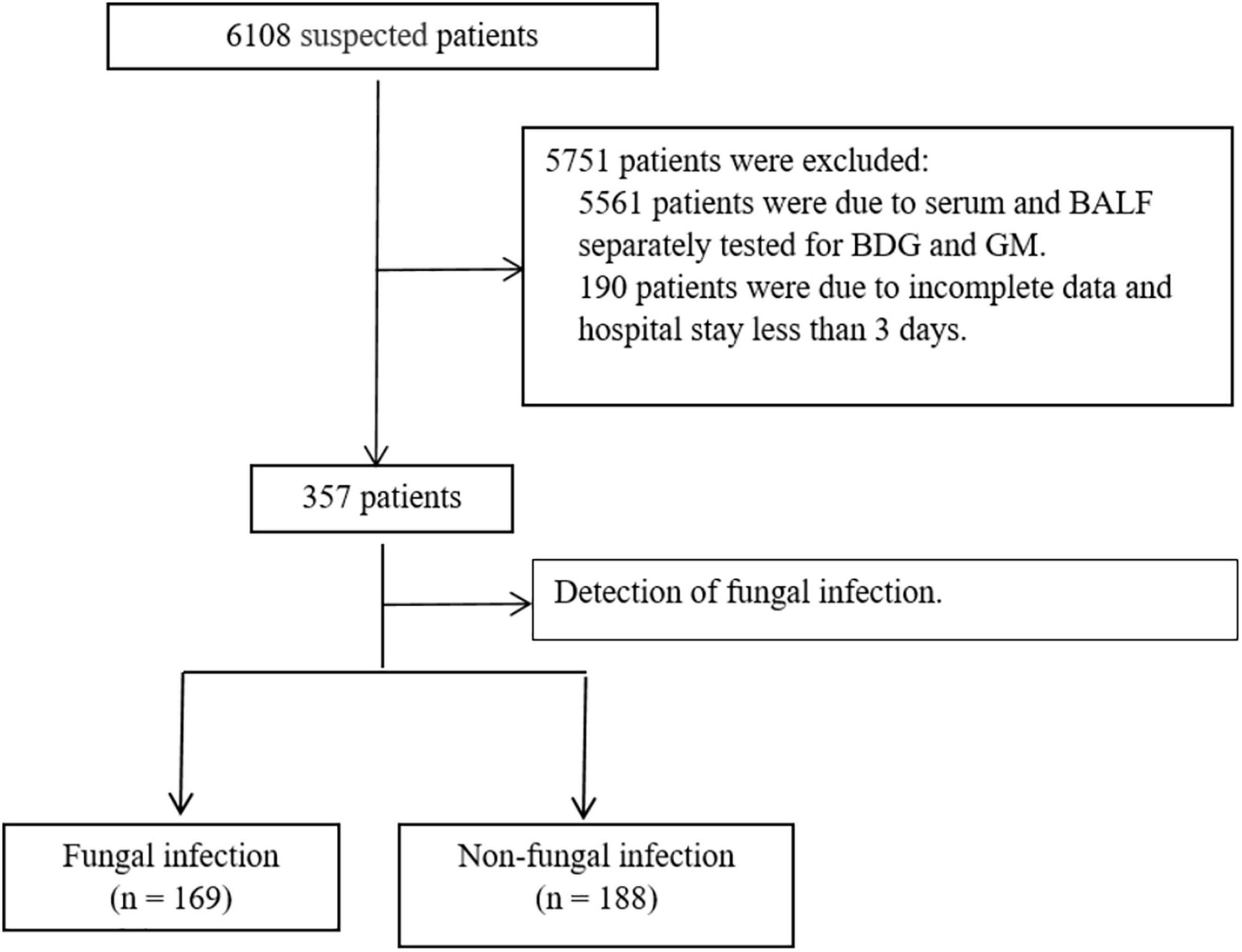
According to the European Organization for Research and Treatment of Cancer/Mycoses Study Group, fungal diagnostic criteria include clinically diagnosed and suspected patients (12, 19). Therefore, the inclusion criteria were (1) patients younger than 18 years and (2) patients who met the diagnostic criteria of severe pneumonia in community-acquired pneumonia (20). Patients with incomplete data, a hospital stay of < 3 days and serum and BALF were not simultaneously tested for 1,3-beta-D-glucan (BDG), and GM were excluded.
Data Collection and Grouping
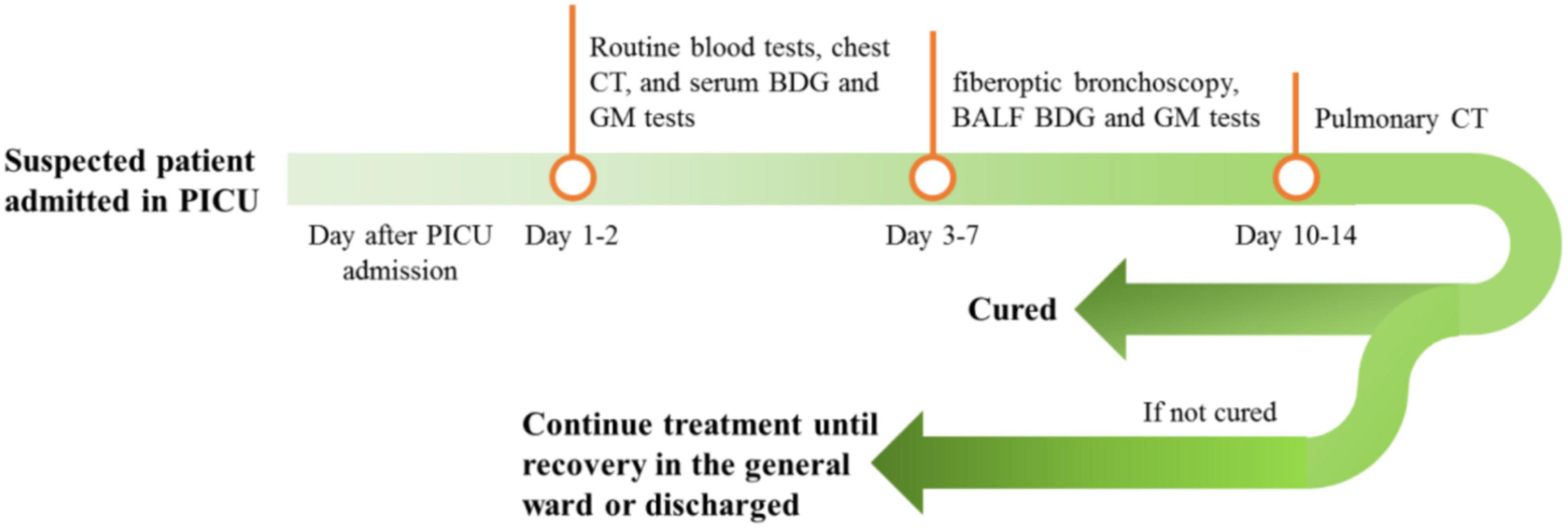
Demographic data and clinical characteristics such as clinical manifestation and acute physiology and chronic health evaluation (APACHE) score were obtained from medical records. Routine blood biochemical tests, chest CT, and serum 1,3-beta-D-glucan (BDG) and GM tests were performed on days 1 and 2 of PICU admission. For suspected patients and those with unfavorable outcomes after routine anti-infection treatment, fiberoptic bronchoscopy and alveolar lavage were performed from days 3 to 7; BALF was tested using BDG and GM tests. Chest CT was performed for all patients after 10–14 days of antifungal infection treatment (see Figure 1). All test results and clinical data were recorded and retrospectively analyzed. Patients were divided into fungal (F) and non-fungal (NF) groups, depending on the presence or absence of fungal infection. The diagnostic criteria for fungal infection were (1) the child had a history of cough, wheezing, and fever, (2) pulmonary rales and sounds, (3) no obvious improvement with antibiotic treatment, (4) pulmonary CT showed signs of fungal infection, (5) fungi were cultivated in blood or BALF, (6) the G and GM tests were positive in blood or BALF, and (7) the condition was significantly improved with antifungal treatment (21–23).
GM and BDG Testing of BALF and Serum
BALF was collected according to the routine bronchoscope operating procedure: 20 mL of normal sterile saline, warmed to body temperature (37°C), was injected for bronchoalveolar lavage. BALF volume of 5–10 mL was recovered and sent for microbial inspection in a disposable sterile silicon plastic bottle. Microbial inspection was performed using an ELx808IU microplate reader (BIoTek, Winooski, VT, United States). The recovered BALF was transferred to a 10-mL centrifuge tube using a disposable sterile pipette. After centrifugation at 1,000 rpm for 10 min using a microfuge 20R centrifuge (Beckman Coulter, Brea, CA, United States), 600–800 μL of supernatant was collected and stored at –40°C for further tests. Venous blood (3 mL) was collected and centrifuged at 3,500 rpm for 10 min; serum was collected and stored at 4°C.
The GM test was performed to detect GM levels in serum and BALF samples using the one-step enzyme immunoassay sandwich method (Aspergillus antigen detection kit). The BDG test was mainly performed to detect BDG in serum and BALF samples using the dynamic turbidimetric method (fungal dextran detection kit). All tests were conducted following the manufacturer’s instructions.
Serum GM > 0.5, BALF GM > 0.7, serum BDG > 100 pg/mL, and BALF BDG > 200 pg/mL were defined as positive values for fungal infection (9, 20).
Chest CT Scan and Interpretation
All patients were placed in the supine position and scanned using a 64-row multi-slice spiral CT. Chest scans were obtained at 1.25-mm intervals using 1.5-mm collimation and were reconstructed with a high-spatial-frequency algorithm. The images were photographed at window settings appropriate for assessing the lung parenchyma (window level, –600 Hounsfield units; window width, 1,600 Hounsfield units). The CT images were retrospectively reviewed by two experienced deputy chief radiologists. The pulmonary nodules or masses, wedge shadow, and consolidation shadow were recorded.
Fiberoptic Bronchoscopy Examination and Interpretation
Fiberoptic bronchoscopy was performed by a deputy director or a senior doctor using an Olympus fiberoptic bronchoscope. All patients who required bronchoscopy provided informed consent obtained from their parents prior to the treatment (excluding those with interfering factors, such as those receiving antibiotics and antifungal treatment). Routine bronchoscopic alveolar lavage was then performed, and their heart rate, oxygen saturation, and blood pressure were monitored. Under general anesthesia with propofol, 2% lidocaine was locally infused into the larynx via the fiberoptic bronchoscope. The top of the fiberoptic bronchoscope was in close contact with the bronchial opening of the lung lobe with abnormal imaging; sterile sodium chloride (0.5–1 mL/kg each time, 15–30 mL in total, at 37°C) was then rapidly injected through the biopsy port. The recovery rate was 40–60%. Furthermore, 50–60 mmHg (1 mmHg = 0.133 kPa) negative pressure suction was applied, and the total amount of lavage fluid was recovered and recorded. The recovered lavage fluid was placed in a uniform sterile container without a heat source and sent for timely inspection.
Disease Outcome
Pneumonia recovery was defined as the resolution or less cough, wheezing, or fever; lung imaging showed that the infection changes disappeared; the BDG and GM levels became normal. Improved pneumonia was defined as decreased cough and wheezing; lung CT still showed lung shadow (<50%); the GM and BDG levels were partially or completely normal. Uncured was defined as no change or improvement was less than 50%, i.e., lung shadow > 50%.
Statistical Methods
IBM SPSS statistics 24 (IBM, Armonk, NY, United States) was used for analysis. The categorical variables are presented as frequencies and percentages [n (%)] and were compared between groups using the chi-square test or continuous correction method if the effective value was < 5. The continuous variables in accordance with a normal distribution were displayed as mean ± standard deviation (SD) and were analyzed using an independent sample t-test; those not in accordance with a normal distribution are displayed as medians (ranges) and were analyzed using the Mann–Whitney U-test. P < 0.05 was considered statistically significant.
Results
General Data and Clinical Features
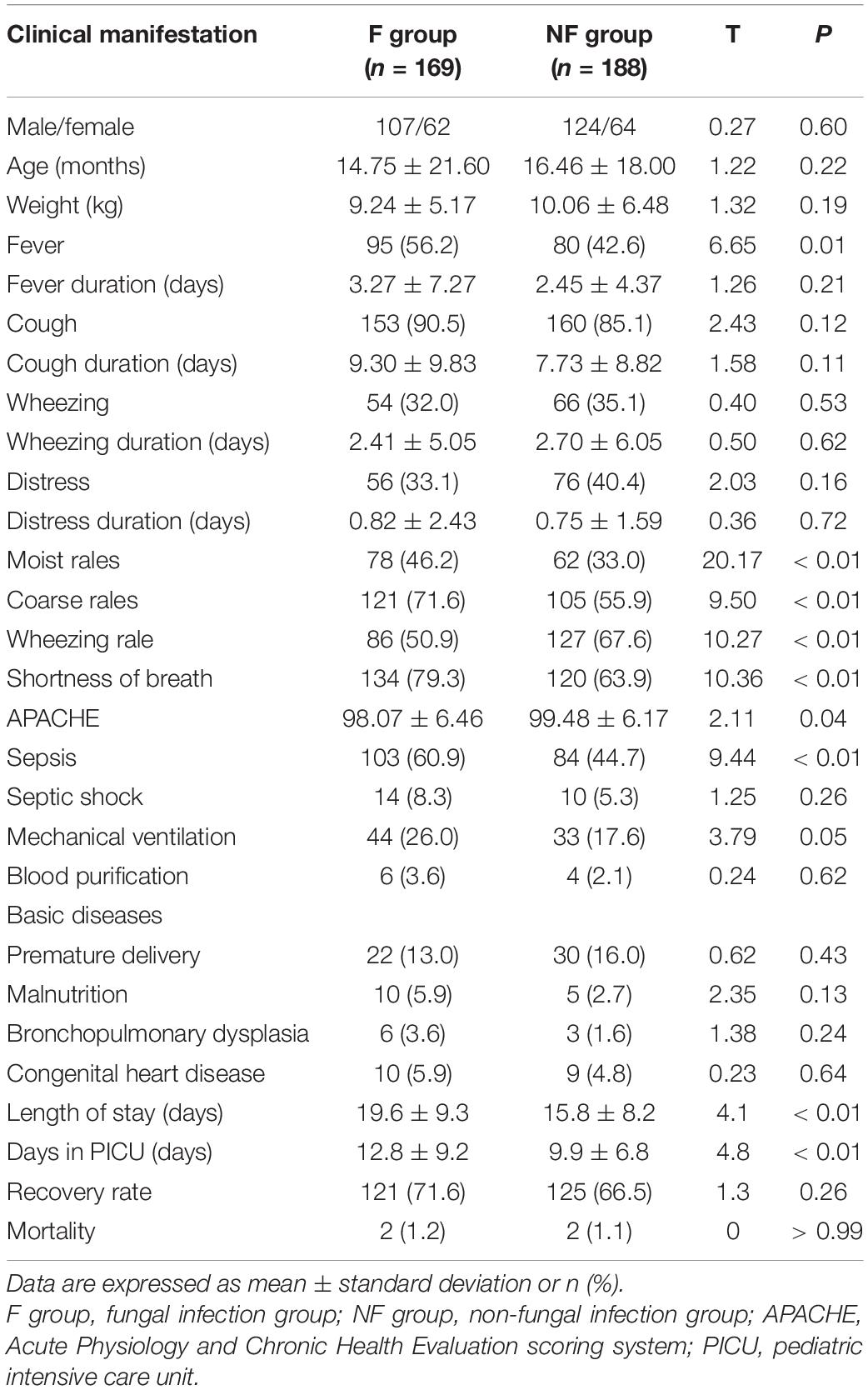
Of the total 357 patients in the PICU, 169 were in the F group, and 188 were in the NF group (Figure 2). The incidence of fever (56.2 vs. 42.6%, P = 0.01), moist rales (46.2 vs. 33.0%, P < 0.01), coarse rales (71.6 vs. 55.9%, P < 0.01), shortness of breath (79.3 vs. 63.9%, P < 0.01), and sepsis (60.9 vs. 44.7%, P < 0.01) were higher in the F group than in the NF group, while wheezing rale (50.9 vs. 67.6%, P < 0.01) was lower. Furthermore, the days in the hospital and PICU were significantly increased (days in PICU: 12.80 ± 9.20 vs. 9.91 ± 6.84 days, P < 0.01; duration of hospital stay: 19.55 ± 9.29 vs. 15.93 ± 9.23 days, P < 0.01; Table 1).
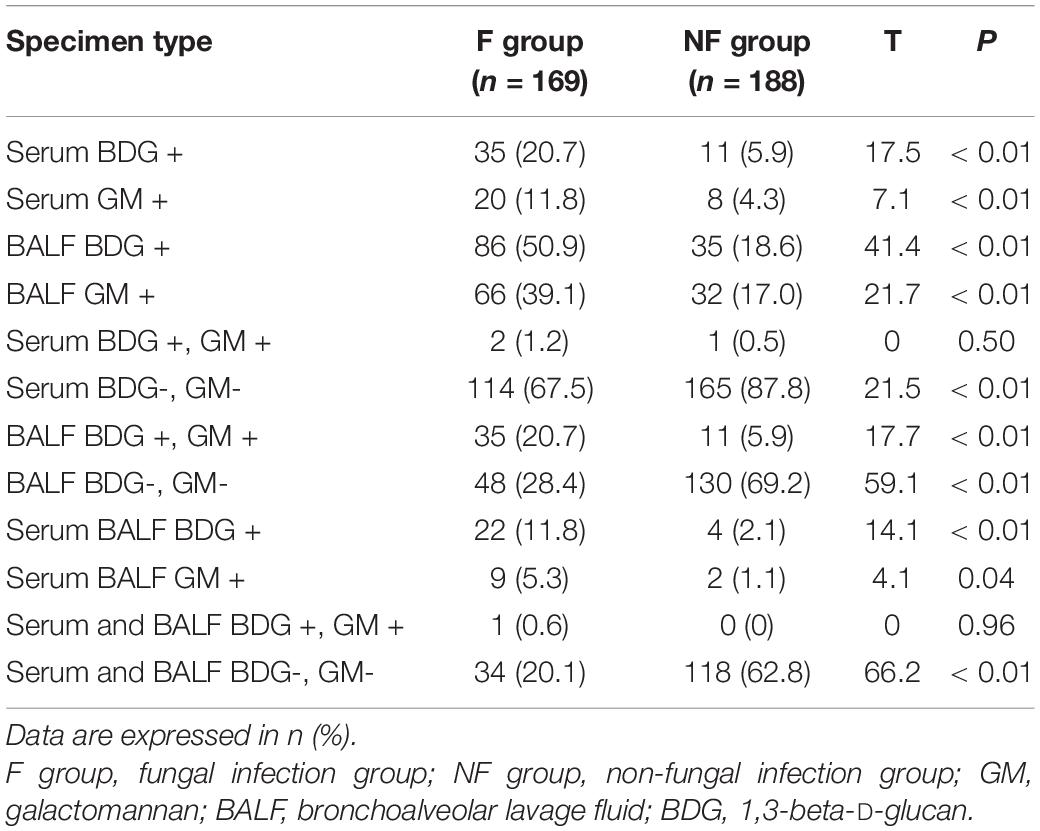
Results of the BDG and GM Tests
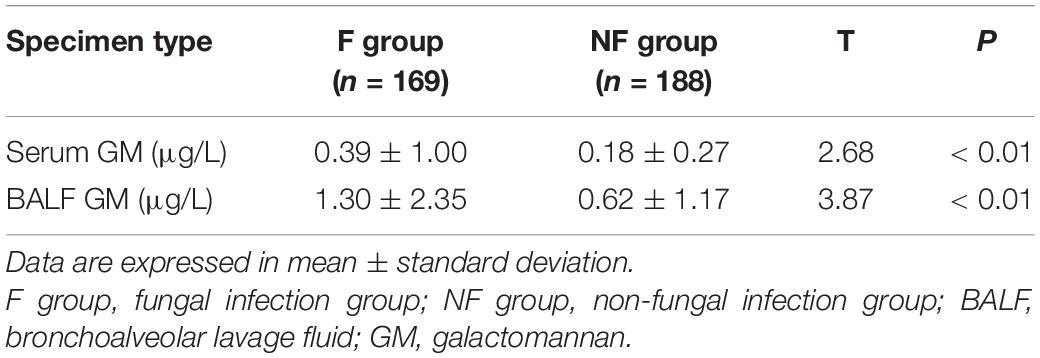
The BDG and GM values of serum and BALF in the F group were significantly higher than those in the NF group (Tables 2, 3). More patients in the F group had positive serum BDG and GM than in the NF group (BDG: 20.7 vs. 5.9%; GM: 11.8 vs. 4.3%; both P < 0.01). Similarly, more patients in the F group had positive BALF BDG and GM than in the NF group (BDG: 50.9 vs. 18.6%; GM: 39.1 vs. 17.0%; both P < 0.01). Fewer patients in the F group had negative serum BDG and GM than the NF group (67.5 vs. 87.8%, P < 0.01). In addition, fewer patients in the F group had negative serum and BALF BDG and GM than the NF group (20.1 vs. 62.8%, P < 0.01; Table 4).
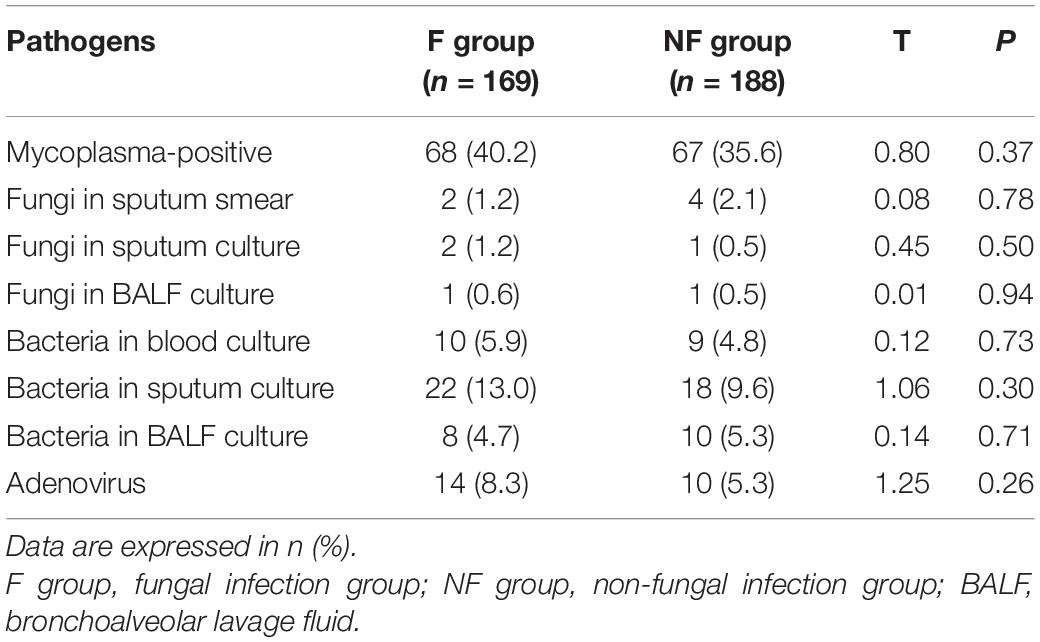
Pathogens
Results of the mycoplasma, adenovirus, blood culture, sputum culture, and lavage fluid culture showed no significant specificity in the F group compared with the NF group (Table 5).
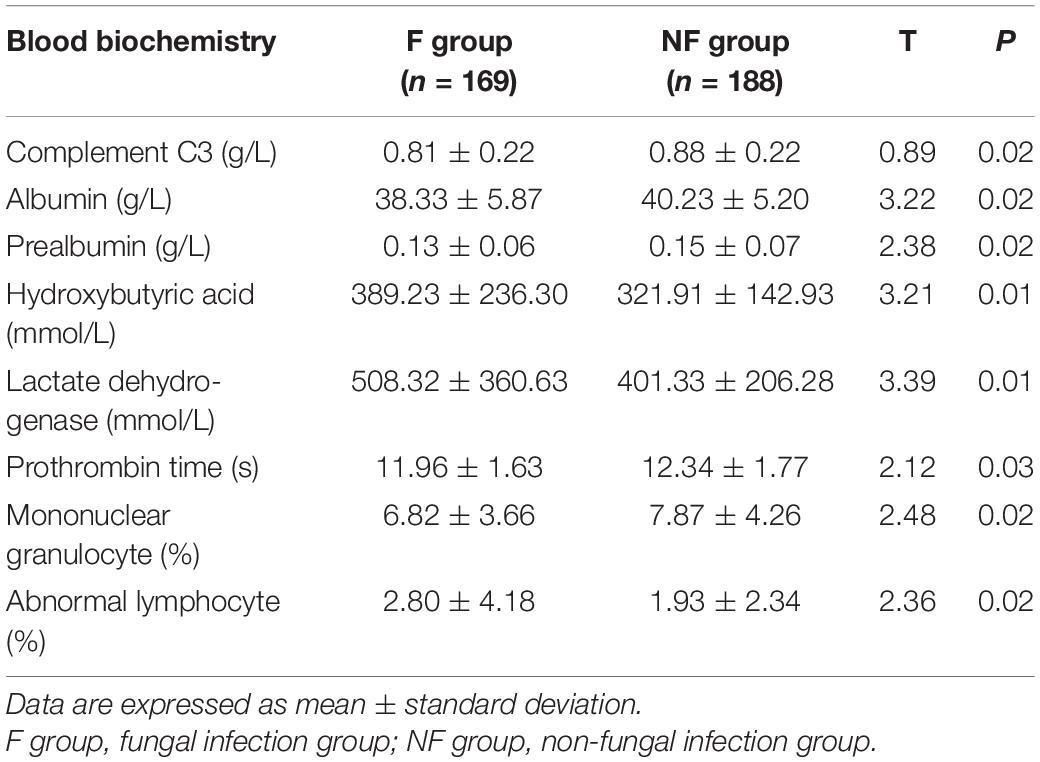
Routine Blood Tests
The routine blood test results were compared between the F and NF groups (Table 6). The proportion of C3, serum albumin, prealbumin, prothrombin, and blood monocytes was significantly lower in the F group, whereas the proportion of hydroxybutyric acid, lactate dehydrogenase, and abnormal lymphocytes in blood smear was significantly higher in the F group than that in the NF group (all P < 0.05).
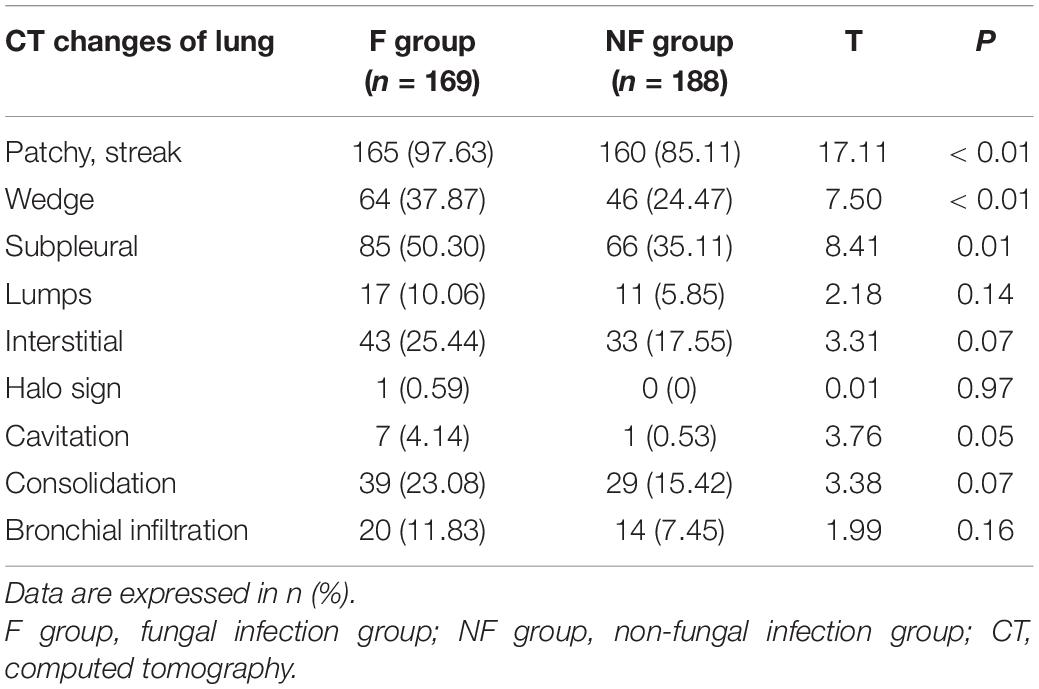
Chest CT Imaging and Fibroscopy of Both Groups
Chest CT imagining revealed that the F group showed a significant increase in wedge-shaped, patchy, streaky shadow and subpleural reticulation (all P < 0.05); however, halo sign, cavity, and consolidation did not significantly increase in the F group (Table 7).
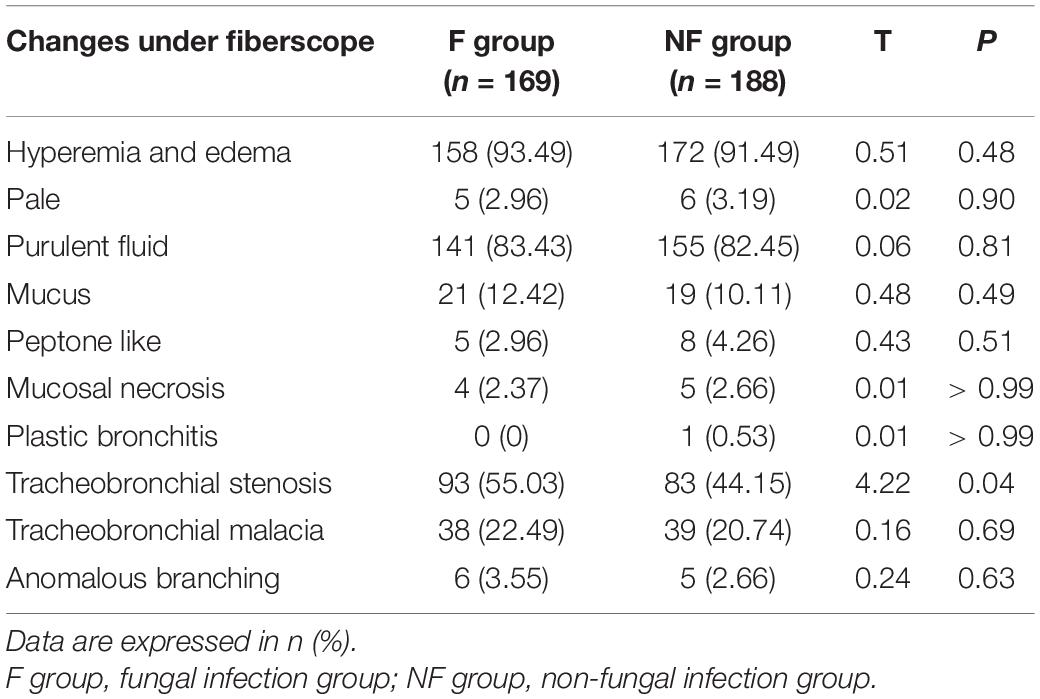
Pulmonary fibroscopy findings were compared between both groups (Table 8). Changes in the bronchoscopy results observed in the F group could contain bronchial congestion, edema, paleness, necrosis, and bleeding. The lavage fluid could contain pus and blood and have a sticky, jelly, or foamy consistency. Tracheobronchial stenosis was more common in the F group than in the NF group (55.0 vs. 44.1%, P = 0.04). There was no significant difference in the properties of lavage fluid and tracheomalacia between the two groups.
Discussion
Invasive fungal infection is an important cause of morbidity and mortality in children, especially infants and premature neonates in a hospital setting. However, prompt and definitive diagnosis is challenging because of non-specific symptoms and inadequate detection tests. Although the present study was not a formal diagnostic study that could suggest replacing the gold standard for fungal infection, the results might suggest that using antifungal therapies combined with tests like serum and BALF BDG and GM could enable a timely diagnosis of pulmonary fungal infections. An early diagnosis should improve the prognosis of pediatric patients, but prospective studies are necessary for confirmation. Here, we analyze these options with the aim of providing a reference for clinicians as well as future studies.
There was no significant difference between the F and NF groups with respect to preterm birth, malnutrition, congenital heart disease, and pulmonary dysplasia, among others. The duration of hospital and PICU stay of patients with fungal infections was significantly longer, which is consistent with previous studies (8); however, this may have been due to the time required for GM detection.
At our hospital, the GM detection results were obtained in 3–4 days and BDG in 2 days. It is suggested that fungal detection and assessments should be conducted at the earliest time possible to reduce the duration of hospital stay and thereby reduce the spread of nosocomial infections. Fever was a common symptom in patients with fungal infections. Moist rales, coarse rales, wheezing, and dyspnea were common clinical symptoms in patients with fungal infections than in those with non-fungal infections; this appears to be similar to the observations by Wu et al. (24). Therefore, if the fever is unexplained and conventional therapy proves to be challenging to treat pulmonary rales and dyspnea, a diagnosis of fungal infection should be promptly considered.
Our results revealed a significantly higher incidence of sepsis in the F group (Table 1), suggesting that fungal sepsis should be considered in addition to bacterial sepsis during diagnosis. Furthermore, the levels of non-specific indicators such as C3, albumin, prealbumin, hydroxybutyrate, lactate dehydrogenase, prothrombin, monocyte ratio, and abnormal lymphocyte ratio were higher in the F group than in the NF group (Table 5); and the APACHE score was significantly lower in the F group. These data are consistent with those by Li et al. (25). Therefore, it is suggested that fungal infection should be considered in addition to bacterial infection during clinical diagnosis since it can have serious inflammatory reactions and clinical manifestations. Prompt identification of the pathogen is vital in cases of severe sepsis.
Singhi et al. (26) conducted a prospective study with 186 PICU patients and found that 45 (69%) had Candida colonization. Oropharyngeal (52%) and rectal (43%) colonization were more common than that of the skin (34%). The species identified were Candida tropicalis (34.2%), C. parafilariasis (28.8%), and C. albicans (14.4%). Twenty patients (30.2%) had candidemia; among these, 18 (90%) showed the presence of colonization, and 15 (75%) had active Candida infection. It is suggested that patients with sepsis and septic shock who have been treated in the PICU for > 5 days should be promptly tested for Candida colonization, especially children with central venous catheters. Sepsis, septic shock, and fungal infection may interact and induce either of the illnesses.
In this study, the mortality rates in the F and NF groups were 1.18% (2/169) and 1.06% (2/188), respectively. This is not consistent with the mortality rates of 14.4% reported by Warris et al. (27) and 29.4% reported by Chakrabarti et al. (28) in India. However, there were no patients with tumors or transplants at our hospital; therefore, we are unable to entirely represent the global data for mortality rates. Moreover, these low rates may be due to increased awareness and technological improvements in fungal diagnostic methods in recent years and early antifungal treatment. Another reason for this could be that we excluded patients with a hospital stay of < 3 days; thus, we automatically excluded the number of deaths. Therefore, prompt diagnosis and treatment of fungal infection can significantly reduce mortality and improve the treatment rate.
Similar to other studies, our study showed that the absolute values and specific positive rates of BDG and GM in the serum and BALF of patients with fungal infections were significantly higher than those of patients without fungal infections, suggesting that these parameters remain suitable for the clinical diagnosis of fungal infections (29). Tong et al. believe that continuous observation of G and GM testing can guide clinical practice to some extent (29). In our study, positive rates of BDG and GM in BALF (50.38 and 39.05%, respectively) were significantly higher than those in serum (21.71 and 11.83%, respectively), and the simultaneous positive rates of BDG in serum and BALF were 11.83% (22/169) and 5.33% (9/188), respectively, which were significantly higher in the F group than in the NF group. It supports the argument that BDG and GM are both suitable in the clinical diagnosis of fungal infections, especially when combined with existing detection methods such as chest CT, which has a high diagnostic value (30).
Although new detection methods such as PCR, high-throughput pathogen sequencing, and mass spectrometry (31, 32) have been used for the diagnosis of fungal infection in recent years, they have limitations in clinical use because of cost among other factors. However, some studies have found that the combination of chest CT and new detection methods can greatly improve the detection rate (13, 15). Although there is no specificity in CT manifestations of fungal infections, it can guide clinicians to determine the presence of a fungal infection. However, previously reported manifestations such as crescent signs, halo signs, and cavity and other signs (12, 13) specific to fungal infections were not common in our group of patients; these findings of our study are consistent with those by Burgos et al. (31). In contrast, non-specific manifestations such as wedge shadow and subpleural reticulation were common in the F group. However, in this group, the author observed that a combination of the patients’ history, physical characteristics, chest CT changes, and serum and BALF BDG and GM tests could help detect fungal infections. Moreover, specific factors such as hospitalization before admission, history of antibiotic treatment and hormone, and treatment outcome of pulmonary rales with antibiotics often suggest the presence of fungal infection. CT diagnosis of children with pulmonary fungal infections is complex, and the lesions are widely distributed, showing pleomorphism, multifocality, and variability (7). For the same disease with different shadows, the same shadow of different diseases should be carefully identified. Whenever necessary, more advanced detection methods such as positron emission tomography/magnetic resonance imaging (15) should be performed to increase the accuracy of diagnosis, for timely diagnosis and treatment, and to reduce complications and mortality.
In Table 7, fibroscopy results showed that secretions were not specific in the F group, and fungal infection could not be clinically detected only from sputum. However, the incidence of tracheobronchial stenosis in the F group was significantly higher than that in the NF group (55.0 vs. 44.1%), suggesting that tracheobronchial infection can be accompanied by stenosis or be associated with fungal infection. Marchioni et al. (33) analyzed the changes in the bronchi lumen of 2,075 adults for 10 years. Among these, 22% showed tracheobronchial stenosis, with Aspergillus being the secondary pathogen of endobronchial diseases requiring medical treatment. Fifty-three cases of tracheobronchial fungal infection (tracheobronchial mucormycosis) in adults showed tumor-like changes, and the most common characteristics were broncho-obstruction due to tumor, mucosal necrosis, uneven mucosa, or a mass and bronchial stenosis with mucosal hyperplasia or mucosal heterogeneity (34). The causes of tracheobronchial stenosis in children with mycotic infection of the trachea and bronchus may be related to the inflammatory injury caused by the infection (35) or invasive procedures such as tracheal intubation (36) and bronchoscopy (37), among others. Though children did not report infection with tracheobronchial fungi after bronchofibroscopy in this study, care still should be taken while performing clinical procedures to avoid tracheal stenosis and re-invasion.
Our study is promising, but it has some limitations. Most fungal diagnoses are clinical diagnoses, and diagnostic procedures, including fungal culture-based methods, have low yield. Furthermore, ours is a retrospective study; therefore, although our findings are significant, the data obtained with our limited sample size may be insufficient. Not all patients were tested for BDG and GM after treatments, and the data were too incomplete for analysis. A larger sample size analyzing prospective studies may provide robust data. Moreover, there is no test clearly indicating the presence of colonization or active fungal infection, which may lead to other complications caused by excessive antifungal treatment.
Conclusion
The factors associated with fungal infections should be considered when evaluating pediatric patients. PICU pneumonia patients with fungal infection have specific clinical and laboratory features compared with those without fungal infection, including higher rates of BALF, serum BDG, GM positivity and tracheobronchial stenosis. Using antifungal therapies combined with tests like serum and BALF BDG and GM could enable a timely diagnosis of pulmonary fungal infections, possibly improving prognosis. Future prospective studies should examine the diagnosis and prognosis of PICU pneumonia patients with fungal infection.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Maternal and Child Health Hospital of Hubei Province (approval number: 2021 IECLW008). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
CH and SX collected the data on all fungal infections. HX analyzed and explained the data of patients with fungi. CH was a major contributor to the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PICUs, pediatric intensive care units; NICUs, neonatal intensive care units; F group, fungal infection group; NF group, non-fungal infection group; BDG, 1,3-beta-D-glucan; GM, galactomannan; BALF, bronchoalveolar lavage fluid; CT, computed tomography; PCR, polymerase chain reaction; APACHE, acute physiology and chronic health evaluation.
References
1. Steinbach WJ, Fisher BT. International collaborative on contemporary epidemiology and diagnosis of invasive fungal disease in children. J Pediatric Infect Dis Soc. (2017) 6:S1–2. doi: 10.1093/jpids/pix039
2. Ahangarkani F, Shokohi T, Rezai MS, Ilkit M, Mahmoodi Nesheli H, Karami H, et al. Epidemiological features of nosocomial candidaemia in neonates, infants and children: a multicentre study in Iran. Mycoses. (2020) 63:382–94.
3. Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. (2007) 20:133–63.
4. Pana ZD, Roilides E, Warris A, Groll AH, Zaoutis T. Epidemiology of invasive fungal disease in children. J. Pediatric Infect Dis Soc. (2017) 6:S3–11. doi: 10.1093/jpids/pix046
5. Wisplinghoff H, Ebbers J, Geurtz L, Stefanik D, Major Y, Edmond MB, et al. Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents. (2014) 43:78–81. doi: 10.1016/j.ijantimicag.2013.09.005
6. Jantarabenjakul W, Yodkitudomying C, Chindamporn A, Suchartlikitwong P, Anugulruengkitt S, Pancharoen C, et al. Pediatric and neonatal invasive candidiasis: species distribution and mortality rate in a Thai tertiary care hospital. Pediatr Infect Dis J. (2021) 40:96–102.
7. Wen XM, Ren N, Xu XH, Huang X. Distribution and drug resistance of pathogens causing nosocomial infections in national nosocomial infection surveillance network. J Chin Hosp Infect Sci. (2002) 16:241–4.
8. Bhattacharjee P. Epidemiology and antifungal susceptibility of Candida species in a tertiary care hospital, Kolkata, India. Curr Med Mycol. (2016) 2:20–7. doi: 10.18869/acadpub.cmm.2.2.5
9. Boch T, Spiess B, Cornely OA, Vehreschild JJ, Rath PM, Steinmann J, et al. Diagnosis of invasive fungal infections in haematological patients by combined use of galactomannan, 1,3-β-D-glucan, Aspergillus PCR, multifungal DNA-microarray, and Aspergillus azole resistance PCRs in blood and bronchoalveolar lavage samples: results of a prospective multicentre study. Clin Microbiol Infect. (2016) 22:862–8. doi: 10.1016/j.cmi.2016.06.021
10. Singh S, Singh M, Verma N, Sharma M, Pradhan P, Chauhan A, et al. Comparative accuracy of 1,3 beta-D glucan and galactomannan for diagnosis of invasive fungal infections in pediatric patients: a systematic review with meta-analysis. Med Mycol. (2021) 59:139–48. doi: 10.1093/mmy/myaa038
11. Arastehfar A, Daneshnia F, Hafez A, Khodavaisy S, Najafzadeh MJ, Charsizadeh A, et al. Antifungal susceptibility, genotyping, resistance mechanism, and clinical profile of Candida tropicalis blood isolates. Med Mycol. (2020) 58:766–73. doi: 10.1093/mmy/myz124
12. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the Mycoses study group education and research consortium. Clin Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
13. Katragkou A, Fisher BT, Groll AH, Roilides E, Walsh TJ. Diagnostic imaging and invasive fungal diseases in children. J Pediatr Infect Dis Soc. (2017) 6:S22–31. doi: 10.1093/jpids/pix055
14. Thomas KE, Owens CM, Veys PA, Novelli V, Costoli V. The radiological spectrum of invasive aspergillosis in children: a 10-year review. Pediatr Radiol. (2003) 33:453–60. doi: 10.1007/s00247-003-0919-4
15. Varotto A, Orsatti G, Crimì F, Cecchin D, Toffolutti T, Zucchetta P, et al. Radiological assessment of paediatric fungal infections: a pictorial review with focus on PET/MRI. In vivo. (2019) 33:1727–35. doi: 10.21873/invivo.11663
16. Yao Q, Zhou QH, Shen QL, Qiao ZW, Wang XC, Hu XH. Imaging findings of pulmonary manifestations of chronic granulomatous disease in a large single center from Shanghai, China (1999-2018). Sci Rep. (2020) 10:19349. doi: 10.1038/s41598-020-76408-4
17. Efrati O, Sadeh-Gornik U, Modan-Moses D, Barak A, Szeinberg A, Vardi A, et al. Flexible bronchoscopy and bronchoalveolar lavage in pediatric patients with lung disease. Pediatr Crit Care Med. (2009) 10:80–4. doi: 10.1097/PCC.0b013e31819372ea
18. Guegan H, Robert-Gangneux F, Camus C, Belaz S, Marchand T, Baldeyrou M, et al. Improving the diagnosis of invasive Aspergillosis by the detection of Aspergillus in broncho-alveolar lavage fluid: comparison of non-culture-based assays. J Infect. (2018) 76:196–205. doi: 10.1016/j.jinf.2017.11.011
19. De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases Mycoses study group (EORTC/MSG) consensus group. Clin Infect Dis. (2008) 46:1813–21. doi: 10.1086/588660
20. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. (2011) 53:e25–76. doi: 10.1093/cid/cir531
21. Katragkou A, Roilides E. Best practice in treating infants and children with proven, probable or suspected invasive fungal infections. Curr Opin Infect Dis. (2011) 24:225–9. doi: 10.1097/QCO.0b013e3283460e22
22. Editorial Committee of Chinese Journal of Pediatrics, Respiratory Group of the Pediatrics Society of Chinese Medical Association. Guidelines for diagnosis and treatment of invasive pulmonary fungal infection in children (2009 edition). Chin J Ped. (2009) 47:96–8.
23. Bao J, Wei H. Advances in diagnosis and treatment of invasive pulmonary fungal infection in children. Chin J Pract Ped. (2017) 34:359–62.
24. Wu TT, Liu Y, Su TF, Liu LL, Xu SQ. Clinical characteristics of invasive fungal infection in children. Chin J Nosocomial Infect. (2015) v.25:5239–41.
25. Liang H, Wang M. MET oncogene in non-small cell lung cancer: mechanism of MET dysregulation and agents targeting the HGF/c-met axis. Onco Targets Ther. (2020) 13:2491–510. doi: 10.2147/OTT.S231257
26. Singhi S, Rao DS, Chakrabarti A. Candida colonization and candidemia in a pediatric intensive care unit. Pediatr Crit Care Med. (2008) 9:91–5.
27. Warris A, Pana ZD, Oletto A, Lundin R, Castagnola E, Lehrnbecher T, et al. Etiology and outcome of candidemia in neonates and children in Europe: an 11-year multinational retrospective study. Pediatr Infect Dis J. (2020) 39:114–20. doi: 10.1097/inf.0000000000002530
28. Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Jillwin J, Iyer R, et al. Characteristics, outcome and risk factors for mortality of paediatric patients with ICU-acquired candidemia in India: a multicentre prospective study. Mycoses. (2020) 63:1149–63. doi: 10.1111/myc.13145
29. Tong T, Shen J, Xu Y. Serum galactomannan for diagnosing invasive aspergillosis in pediatric patients: a meta-analysis. Microb Pathog. (2018) 118:347–56. doi: 10.1016/j.micpath.2018.03.059
30. Qiu KY, Liao XY, Huang K, Xu HG, Li Y, Fang JP, et al. The early diagnostic value of serum galactomannan antigen test combined with chest computed tomography for invasive pulmonary aspergillosis in pediatric patients after hematopoietic stem cell transplantation. Clin Transplant. (2019) 33:e13641. doi: 10.1111/ctr.13641
31. Burgos A, Zaoutis TE, Dvorak CC, Hoffman JA, Knapp KM, Nania JJ, et al. Pediatric invasive aspergillosis: a multicenter retrospective analysis of 139 contemporary cases. Pediatrics. (2008) 121:e1286–94. qr
32. Hamula CL, Hughes K, Fisher BT, Zaoutis TE, Singh IR, Velegraki A. T2Candida provides rapid and accurate species identification in pediatric cases of candidemia. Am J Clin Pathol. (2016) 145:858–61. doi: 10.1093/ajcp/aqw063
33. Marchioni A, Casalini E, Andreani A, Cappiello G, Castaniere I, Fantini R, et al. Incidence, etiology, and clinicopathologic features of endobronchial benign lesions: a 10-year consecutive retrospective study. J Bronchology Interv Pulmonol. (2018) 25:118–24. doi: 10.1097/lbr.0000000000000460
34. He R, Hu C, Niu R. Analysis of the clinical features of tracheobronchial fungal infections with tumor-like lesions. Respiration. (2019) 98:157–64. doi: 10.1159/000496979
35. Nafstad P, Magnus P, Jaakkola JJ. Early respiratory infections and childhood asthma. Pediatrics. (2000) 106:E38. doi: 10.1542/peds.106.3.e38
36. Johnson RF, Saadeh C. Nationwide estimations of tracheal stenosis due to tracheostomies. Laryngoscope. (2019) 129:1623–6. doi: 10.1002/lary.27650
Keywords: tracheal stenosis, bronchoalveolar lavage fluid, bronchoscopy, invasive fungal infection, child, hospitalized
Citation: Huang C, Xiao S, Cheng Y, Li Y, Xia Z, Tang W, Shi B, Qin C, Xu H and Shu X (2022) Clinical, Laboratory, Radiological, Bronchoscopic, and Outcome Characteristics of Pulmonary Fungal Infection in Children in PICU in Central China: A Case Series. Front. Pediatr. 10:822043. doi: 10.3389/fped.2022.822043
Received: 25 November 2021; Accepted: 23 March 2022;
Published: 25 April 2022.
Edited by:
Bülent Taner Karadağ, Marmara University, TurkeyReviewed by:
Charl Verwey, University of the Witwatersrand, South AfricaVladimir Pohanka, Slovak Medical University, Slovakia
Copyright © 2022 Huang, Xiao, Cheng, Li, Xia, Tang, Shi, Qin, Xu and Shu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Xu, aGJzZnkyMDIwcGljdUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Chengjiao Huang†
Chengjiao Huang† Hui Xu
Hui Xu