
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 04 March 2022
Sec. General Pediatrics and Pediatric Emergency Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.802977
This article is part of the Research Topic Pediatric Uveitis View all 7 articles
Purpose: Pediatric uveitis is the leading cause of acquired child blindness, due to unremitting inflammation and long-term steroid exposition. Biotherapies with anti-tumor necrosis factor alpha (anti-TNFα) are effective in controlling inflammation for severe pediatric uveitis in recent studies. Major concern of anti-TNFα prescription is the balance between the severity of the disease and side effects of the drug. The aim of the present study is to describe a cohort of children with severe uveitis and to highlight the risk factors for a pejorative development that led to the prescription of anti-TNFα drugs.
Method: A retrospective case-control study was carried out on children with uveitis associated with systemic inflammatory disease or idiopathic uveitis, with a minimum follow-up of 5 years. Anti-TNFα-treated patients (case) were studied and compared with patients who were not requiring anti-TNFα (control). Univariate logistic regression analyses were performed to compare both groups and determine the risk factors for anti-TNFα therapy.
Results: Seventy-three cases of pediatric uveitis were included, 13 cases and 60 controls. The risk factors associated with increased odds of anti-TNFα therapy were initial systemic disorder associated with uveitis [OR = 11.22 (1.37–91.85), p = 0.0241), family history of autoimmune diseases [OR = 9.43 (2.27–39.15), p = 0.0020], uveitis diagnosis before the age of 6 [OR = 4.05 (1.16–14.13), p = 0.0284], eye surgery [OR = 26.22 (2.63–261.77), p = 0.0054], ocular complications at the first slit lamp exam [OR = 67.11 (3.78–1191.69), p = 0.0042], low visual acuity at diagnosis (≥0.3 logMAR) [OR = 11.76 (2.91–47.62), p = 0.0005] and especially low binocular acuity at diagnosis (≥0.3 logMAR) [OR = 8.75 (1.93–39.57), p = 0.0048], panuveitis [OR = 9.17 (2.23–37.60), p = 0.0021], having positive ANA [OR = 3.89 (1.07–14.11), p = 0.0391], and positive HLA B27 [OR = 9.43 (2.27–39.16), p = 0.0020].
Conclusion: Those risk factors could be used to establish a new follow-up and treatment schedule for severe uncontrolled uveitis. This could help to better predict the best time to start anti-TNF therapy.
Pediatric uveitis is the leading cause of acquired child blindness with a prevalence of 30 cases per 100,000 inhabitants. It is a rare disease, with about 500 new cases per year in France, but stakes are huge. The main causes of childhood uveitis are systemic inflammatory diseases (5–47%) followed by infectious etiologies (2–39%). In a relatively large part (25–60% of cases depending on series), clinical and biological work-up does not allow to find the causes of the ocular inflammation: It is then an idiopathic uveitis (1–4). Treatment differs depending on the etiologies. Inflammatory uveitis is treated in the acute phase with corticosteroids (topically or systemically, depending on the severity) (5, 6). For all chronic cases, damage is due to unremitting inflammation and long-term steroid exposition. That is why, controlling eye inflammation and sparing corticosteroids are major issues in pediatric uveitis management (7, 8).
Concerning corticosteroid sparing, there is no established guideline to an appropriate therapeutic behavior, and therapeutic decision is often guided by the personal experiences of the ophthalmologist and the pediatrician (9–11). Methotrexate and more recently anti-tumor necrosis factor alpha (TNFα) have allowed a marked improvement in the functional prognosis of severe inflammatory pediatric uveitis, as demonstrated in the ADJUVITE and SYCAMORE trials (12, 13). Prescribing anti-TNFα treatment raises two main questions. The first is the choice of the molecule, according to the expected efficacy on ocular and associated damage and acceptability for families (routes of administration and periodicity). The second is the optimal time to start treatment. Treatment should be started early enough to prevent complications, but in a reasonable way to limit side effects. The identification of initial factors of severity and/or of pejorative evolution would, thus, be very useful to guide the prescription of anti-TNFα drugs.
The aim of the present study is to describe a cohort of children with severe uveitis associated with inflammatory or idiopathic diseases and to highlight the risk factors for a pejorative development that led to the prescription of anti-TNFα drugs.
A retrospective case-control study was carried out in Strasbourg University Hospital (France). From 2000 to 2018, the inclusion criteria were the presence of related inflammatory disease or idiopathic chronic symptomatic uveitis (persistent uveitis with relapse in <3 months after discontinuing treatment) in a child (age at onset >13 and age <18 years at the end of the follow-up) with a minimum follow-up of 5 years. For all the included patients (presenting with chronic uveitis resistant to first-line local treatment, the treatment consisted of systemic corticotherapy started at 1 mg/kg/day for 2 weeks. Methotrexate (dose appropriate for the weight of the child) was initiated in all patients in the study. Clinical data and uveitis classification were based on the SUN Working Group recommendations (14). Recruitment was carried out using internal hospital databases as well as health insurance data. Infectious uveitis, posttraumatic, demyelinating diseases, postsurgical inflammation, and masquerade syndrome were excluded.
According to the clinical course, patients included were then separated into two groups. The group of cases was defined by chronic uveitis requiring the prescription of anti-TNFα (etanercept, infliximab, or adalimumab) due to insufficient control of inflammation and corticosteroid dependence (need for corticosteroid therapy >5 mg per day after a 3-month period of methotrexate or greater than one drop per day for a period of more than 1 month). Controls were patients with an objective uveitis detected at the slit lamp examination and who did not required anti-TNFα therapy during the 5-year follow-up. The clinical and biological data of the cases and controls were then compared in order to define the elements favoring the prescription of an anti-TNFα treatment. The protocol was approved by the Local Ethics Committee (Strasbourg, France) in accordance to the tenets of the Declaration of Helsinki.
Demographic data (gender, date of birth, age at diagnosis), patient history (personal and familial medical conditions), initial ophthalmological state (initial visual acuity, intraocular pressure, Tyndall effect, keratic precipitates, posterior segment abnormalities), ocular complications [posterior synechiae, cataract, band keratopathy, optic disc edema, cystoid macular edema (macular thickness >320 μm with inner retinal cysts), vasculitis, retinochoroiditis lesions], characteristics of uveitis (uni- or bilateral; acute, recurrent, or chronic evolution; anterior, posterior, intermediate, or panuveitis), etiologic assessment data [full blood count (FBC), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), kidney and liver function, serum angiotensin-converting enzyme (ACE), antinuclear antibodies (ANA), rheumatoid factor (RF), antineutrophil cytoplasmic antibodies (ANCA), anti-DNA, complement test, serum immunoglobulin count, HLA B27, herpes simplex virus (HSV)–Toxoplasma–Toxocara–varicella-zoster virus (VZV) serologies, chest x-ray, tuberculin skin test, pulmonary function testing depending on the clinic presentation] and treatments in place and their introduction sequences were screened. Patients had a clinical examination every 3 months with systematic collection of data from slit lamp examination, the fundus, and OCT. Biological data were collected at least during the initial assessment and then every 2 years (three collections per patient). Data were retrospectively collected in the digital files of patients (Dx-Care®) for the newest cases and in paper files for the oldest.
We examined distributions for all variables of interest by determining the frequencies, means, and measures of variance. To evaluate the statistical significance of the unadjusted associations between case (anti-TNFα treatment) and control (no anti-TNFα treatment) status and the characteristics of the participants, we used either Fisher's exact tests or Pearson's chi-square tests for categorical variables. We used unconditional logistic regression to compute adjusted odds ratios (ORs) and 95% confident interval (CI) of independent variables. A value of p < 0.05 was determined to be significant. The statistical software used was GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
Seventy-three patients with pediatric uveitis were included, and among them were 33 uveitis related to systemic inflammatory disease (45%) and 40 idiopathic uveitis (55%). Because of uncontrolled eye inflammation, treatment by anti-TNFα was decided for 13 (18%) patients (cases). Of the patients, 60 (82%) remained stable without anti-TNFα (controls). Demographic features were similar in both patient groups (Table 1). The repartition of the etiologies in each group is described in Table 2.

Table 1. Demographic and clinical data for the 13 anti-tumor necrosis factor alpha (TNFα)-treated patients (Case) and the 60 patients without anti-TNFα (control).
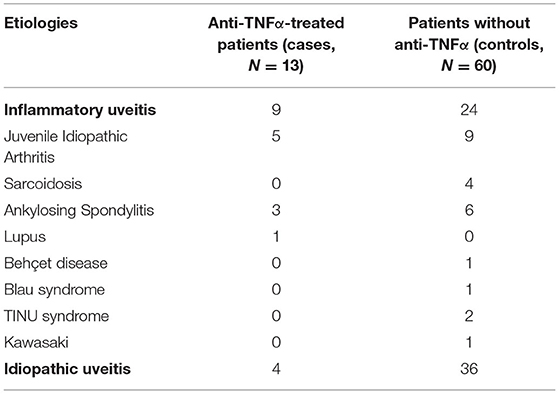
Table 2. Etiologic data for the 13 anti-TNFα treated patients (cases) and the 60 no anti-TNFα patients (control).
Only 23 patients (31%) were already known for having a systemic disease that could lead to uveitis. The mean duration of uveitis evolution before the introduction of anti-TNFα was 16 months. The mean duration of oral steroid impregnation over the 5-year follow-up was 22.4 months.
Comparative data with univariate ORs for the risk factors relative to the 13 anti-TNFα-treated patients (case) and 60 patients without anti-TNFα (control) are provided in Table 3.
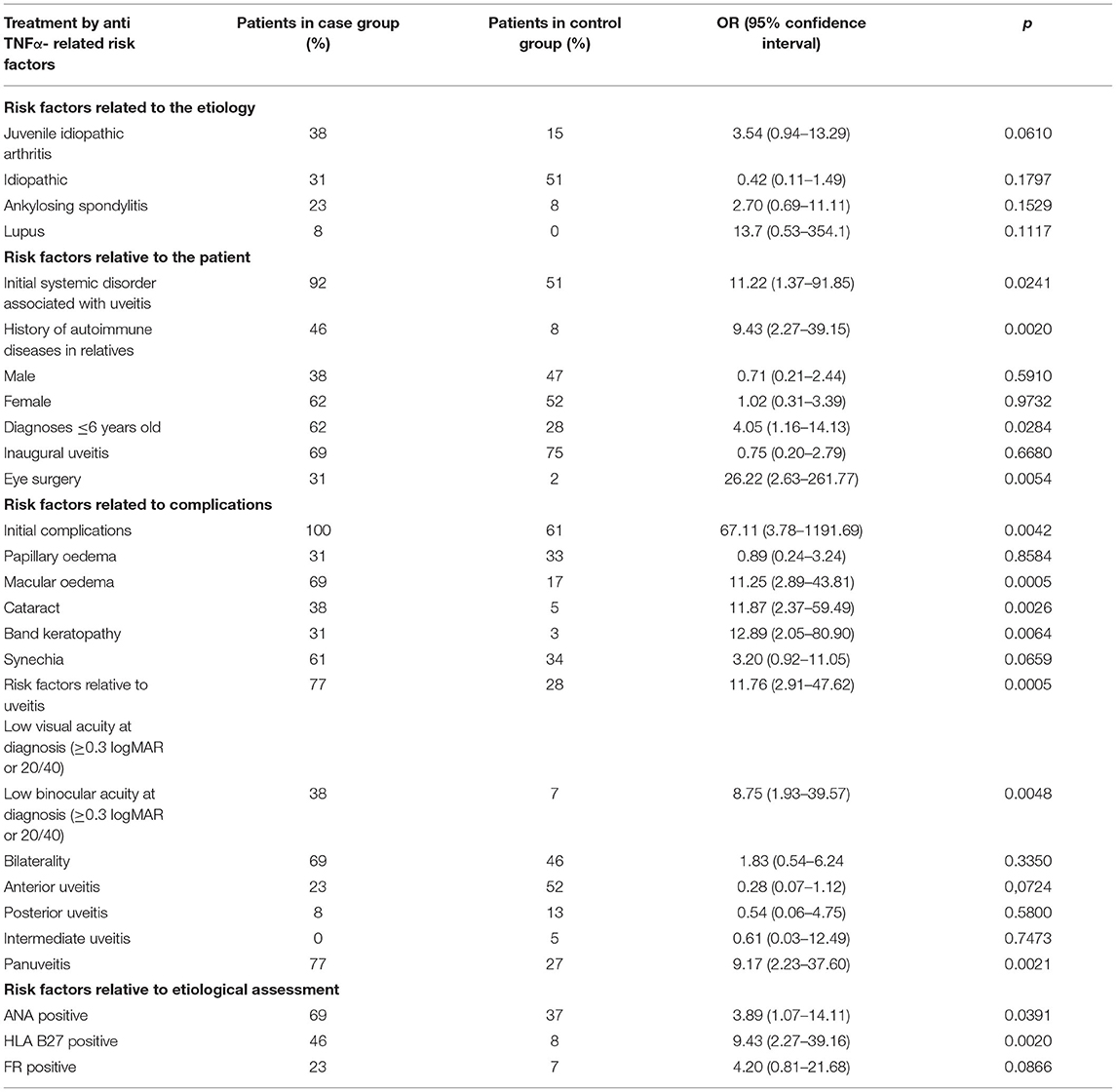
Table 3. Comparative data with univariate ORs for the risk factors relative to the 13 anti-TNFα-treated patients (case) and 60 patients without anti-TNFα (control).
The risk factors associated with the greatest increased odds of anti-TNFα therapy were as follows: initial systemic disorder associated with uveitis [OR = 11.22 (1.37–91.85), p = 0.0241], family history of autoimmune diseases [OR = 9.43 (2.27–39.15), p = 0.0020], uveitis diagnosis before the age of 6 [OR = 4.05 (1.16–14.13), p = 0.0284], eye surgery [OR = 26.22 (2.63–261.77), p = 0.0054], and ocular complications at the first slit lamp exam [OR = 67.11 (3.78–1191.69), p = 0.0042]. Among those complications, some were more likely to be associated with anti-TNFα treatment: macular edema [OR = 11.25 (2.85–43.81), p = 0.0005], cataract [OR = 11.87 (2.37–59.49), p = 0.0026], and band keratopathy [OR = 12.89 (2.05–80.90 p = 0.0064]. Low visual acuity at diagnosis (≥0.3 logMAR) [OR = 11.76 (2.91–47.62), p = 0.0005] and especially low binocular acuity at diagnosis (≥0.3 logMAR) [OR = 8.75 (1.93–39.57), p = 0.0048] were associated to a higher need of biotherapy. Panuveitis seemed to be more often associated with anti-TNFα treatment [OR = 9.17 (2.23–37.60), p = 0.0021]. Finally, having positive ANA [OR = 3.89 (1.07–14.11), p = 0.0391] and positive HLA B27 [OR = 9.43 (2.27–39.16), p = 0.0020] seemed to increase the risk of having an anti-TNFα therapy.
This study made it possible to collect the files of 73 children with chronic anterior uveitis with a minimum follow-up of 5 years. The prolonged follow-up of children with chronic uveitis is relatively rare in the literature. As uveitis is a rare disease, a retrospective recruitment was preferred, although bias are possible (notably missing data). The comparative analysis of the clinical and laboratory data of the cases and of the controls made it possible to highlight factors increasing the risk of resorting to anti-TNF immunotherapy. Moreover, the collection of data, having been carried out before the prescription of anti-TNFα, allows a quantification of the evolution risk of the various factors present from the inclusion consultation.
Results match with common knowledge in this field. For example, JIA is more associated with anti-TNFα therapy than other etiologies because diagnosis is often made very late due to the limited and discrete symptoms and clinical signs in children. Prescribing habits in JIA uveitis are quite easily oriented toward anti-TNFα studies having shown an important efficacy of this treatment (8, 15). Positive ANA is also associated with anti-TNFα therapy [OR = 3.89 (1.07–14.11), p = 0.0391], and it is established that they are a risk factor of developing uveitis when having JIA (16, 17). According to Zuber et al., antirheumatic drugs (DMARDs) and corticosteroids were more frequently clinically ineffective in HLA-B27 positive than negative patients (23.1 vs. 15.2%; p = 0.09). HLA-B27-positive patients with enthesitis-related arthritis received biological therapy more frequently than HLA-B27-negative ones (20.4% vs. 0, respectively; p = 0.09) (18). Our result corroborate this statement as positive HLA-B27 has an OR = 9.43 (2.27–39.16), p = 0.0020. Studies also have reported an increased risk of several systemic autoimmune diseases among relatives of patients with a systemic autoimmune disease (5, 19). Therefore, we looked for family history of autoimmune diseases in the midst of our patients and found a significant match [OR = 9.43 (2.27–39.15), p = 0.0020]. Consequently, it is important to ask parents about medical conditions in relatives at the first examination because if there are some, probabilities of inflammatory uveitis, with an underlying autoimmune disease, are higher.
As far as clinical criterion is concerned, uveitis diagnosis under the age of 6 years increased the risk of anti-TNFα therapy in our study [OR = 4.05 (1.16–14.13), p = 0.0284]. It is explained by the fact that diagnosis is often delayed (fewer symptoms, less complaints), so inflammation will evolve uncontrolled over a longer period. That is why the severity at onset is increased, complications are already set up, and steroid dependence is higher, which lead to anti-TNFα therapy decision. Logically, ocular complications at the first slit lamp exam are a significant risk factor too [OR = 67.11 (3.78–1191.69), p = 0.0042]. Among those complications, macular edema [OR = 11.25 (2.85–43.81), p = 0.0005], cataract [OR = 11.87 (2.37–59.49), p = 0.0026], and band keratopathy [OR = 12.89 (2.05–80.90), p = 0.0064] are the most significant being associated with anti-TNFα therapy; those occur in severest uveitis with chronic inflammation. Furthermore, low visual acuity at diagnosis (≥ 0.3 logMAR) [OR = 11.76 (2.91–47.62), p = 0.0005] and especially low binocular acuity at diagnosis (≥0.3 logMAR) [OR = 8.75 (1.93–39.57), p = 0.0048] reflect aggressive manifestations of uveitis less steroid response. We also find that having eye surgery [OR = 26.22 (2.63–261.77), p = 0.0054] is a significant risk factor; it may consolidate the need of early diagnosis in pediatric uveitis. However, we only had one patient with eye surgery in the control group. This result should be taken carefully. All these factors should be considered as elements of gravity, which must lead to the discussion of an aggressive immunotherapy at the onset of the disease, especially when children show initial eye complications.
Bringing out decision factors for initiating anti-TNFα therapy should help to establish guidelines in pediatric uveitis management. Indeed, children in our study have a mean duration of uveitis evolution before introducing anti-TNFα of 16 months and a mean duration of oral steroid impregnation, during the 5-year follow-up, of 22 months, which is obviously too much. Several studies have shown that anti-TNFα is not only efficient in controlling severe children eye inflammation (8, 12, 13), but it is also safe to use, with a low rate of severe side effects now well-known and treatable (20–24). In the future, we may imagine analyzing these risk factors at the first consultation to estimate the risk of having a severe uveitis, which may need prompt biotherapy. Thus, high-risked children could be monitored more carefully, and anti-TNFα could be introduced sooner, which could allow limitation or reduction of steroid therapy. By doing so, sight damage due to uncontrolled inflammation and to corticosteroid exposition should be reduced, and visual prognoses should be improved. Finally, it may be interesting to design a prospective study to investigate if introducing anti-TNFα sooner can effectively control sight damage.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Comité d'Ethique, Université de Strasbourg, France. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AS, DO, and A-CR contributed to conception and design of the study. DO and A-CR organized the database and wrote the first draft of the manuscript. AS and DO performed the statistical analysis. AS, TB, JT, and CS wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chang MH, Shantha JG, Fondriest JJ, Lo MS, Angeles-Han ST. Uveitis in children and adolescents. Rheum Dis Clin North Am. (2021) 47:619–41. doi: 10.1016/j.rdc.2021.07.005
2. BenEzra D, Cohen E, Maftzir G. Uveitis in children and adolescents. Br J Ophthalmol. (2005) 89:444–8. doi: 10.1136/bjo.2004.050609
3. Smith JA, Mackensen F, Sen H, Leigh JF, Watkins AS, Pyatetsky D, et al. Epidemiology and course of disease in childhood uveitis. Ophthalmology. (2009) 116:1544–51. doi: 10.1016/j.ophtha.2009.05.002
4. Rahimi M, Oustad M, Ashrafi A. Demographic and Clinical Features of Pediatric Uveitis at a Tertiary Referral Center in Iran. Middle East Afr J Ophthalmol. (2016) 23:237–40. doi: 10.4103/0974-9233.186096
5. Maccora I, Sen ES, Ramanan AV. Update on noninfectious uveitis in children and its treatment. Curr Opin Rheumatol. (2020) 32:395–402. doi: 10.1097/BOR.0000000000000723
6. Choi J, Hawley DP, Ashworth J, Edelsten C, Bossuyt ASAM. An update on the modern management of paediatric uveitis. Br J Ophthalmol. (2019) 103:1685–9. doi: 10.1136/bjophthalmol-2019-314212
7. Touhamin S, Diwo E, Sève P, Trad S, Bielefeld P, Sène D, et al. Expert opinion on the use of biological therapy in non-infectious uveitis. Expert Opin Biol Ther. (2019) 19:477–90. doi: 10.1080/14712598.2019.1595578
8. Quartier P. Juvenile idiopathic arthritis-associated chronic uveitis: recent therapeutic approaches. J Clin Med. (2021) 10:2934. doi: 10.3390/jcm10132934
9. Dick AD, Rosenbaum JT, Al-Dhibi HA, Belfort R, Brézin AP, Chee SP, et al. Guidance on Noncorticosteroid Systemic Immunomodulatory Therapy in Noninfectious Uveitis: Fundamentals Of Care for UveitiS (FOCUS). Initiative Ophthalmology. (2018) 125:757–73. doi: 10.1016/j.ophtha.2018.03.005
10. Constantin T, Foeldvari I, Anton J, de Boer J, Czitrom-Guillaume S, Edelsten C, et al. Consensus-based recommendations for the management of uveitis associated with juvenile idiopathic arthritis: the SHARE initiative. Ann Rheum Dis. (2018) 77:1107–17. doi: 10.1136/annrheumdis-2018-213131
11. Solebo AL, Rahi JS, Dick AD, Ramanan AV, Ashworth J, Edelsten C. Areas of agreement in the management of childhood non-infectious chronic anterior uveitis in the UK. Br J Ophthalmol. (2020) 104:11–6. doi: 10.1136/bjophthalmol-2018-313789
12. Quartier P, Despert A, Poignant V, Elie E, Kone-Paut I, Belot A, et al. Adjuvite: a double-blind, randomized, placebo-controlled trial of adalimumab in juvenile idiopathic arthritis associated uveitis. Ann Rheum Dis. (2018) 77:1003–11. doi: 10.1136/annrheumdis-2017-212089
13. Ramanan AV, Dick AD, Benton D, Compeyrot-Lacassagne S, Dawoud D, Hardwick B, et al. A randomised controlled trial of the clinical effectiveness, safety and cost-effectiveness of adalimumab in combination with methotrexate for the treatment of juvenile idiopathic arthritis associated uveitis (SYCAMORE Trial). Trials. (2014) 15:14. doi: 10.1186/1745-6215-15-14
14. The Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol. (2005) 140:509–16. doi: 10.1016/j.ajo.2005.03.057
15. Nagy A, Mátrai P, Hegyi P, Alizadeh H, Bajor J, Czopf L, et al. The effects of TNF-alpha inhibitor therapy on the incidence of infection in JIA children: a meta-analysis. Pediatr Rheumatol Online J. (2019) 18:17. doi: 10.1186/s12969-019-0305-x
16. Ravelli A, Felici E, Magni-Manzoni S, Pistorio A, Novarini C, Bozzola E, et al. Patients with antinuclear antibody-positive juvenile idiopathic arthritis constitute a homogeneous subgroup irrespective of the course of joint disease. Arthritis Rheum. (2005) 52:826–32. doi: 10.1002/art.20945
17. Angeles-Han ST, Rabinovich CE. Uveitis in children. Curr Opin Rheumatol. (2016) 28:544–9. doi: 10.1097/BOR.0000000000000316
18. Zuber Z, Turowska-Heydel D, Sobczyk M, Chudek J. Prevalence of HLA-B27 antigen in patients with juvenile idiopathic arthritis. Reumatologia. (2015) 53:125–30. doi: 10.5114/reum.2015.53133
19. Cooper GS, Miller FW, Pandey JP. The role of genetic factors in autoimmune disease: implications for environmental research. Environ Health Perspect. (1999) 107:693–700. doi: 10.1289/ehp.99107s5693
20. Papadopoulou M, Zetterberg M, Oskarsdottir S, Andersson Grönlund A. Assessment of the outcome of ophthalmological screening for uveitis in a cohort of Swedish children with juvenile idiopathic arthritis. Acta Ophthalmol. (2017) 95:741–7. doi: 10.1111/aos.13388
21. Lerman MA, Burnham JM, Chang PY, Daniel E, Foster CS, Hennessy S, et al. Response of pediatric uveitis to tumor necrosis factor-α inhibitors. J Rheumatol. (2013) 40:1394–403. doi: 10.3899/jrheum.121180
22. Simonini G, Druce K, Cimaz R, Macfarlane GJ, Jones GT. Current evidence of anti-tumor necrosis factor α treatment efficacy in childhood chronic uveitis: A systematic review and meta-analysis approach of individual drugs. Arthritis Care Res (Hoboken). (2014) 66:1073–84. doi: 10.1002/acr.22214
23. Jari M, Shiari R, Salehpour O, Rahmani K. Epidemiological and advanced therapeutic approaches to treatment of uveitis in pediatric rheumatic diseases: a systematic review and meta-analysis. Orphanet J Rare Dis. (2020) 15:41. doi: 10.1186/s13023-020-1324-x
Keywords: uveitis (MeSH), anti-TNF agent, children, risk factors, juvenile idiopathic arthritis associated uveitis, idiopathic uveitis in children
Citation: Osswald D, Rameau A-C, Terzic J, Sordet C, Bourcier T and Sauer A (2022) Risk Factors Leading to Anti-TNF Alpha Therapies in Pediatric Severe Uveitis. Front. Pediatr. 10:802977. doi: 10.3389/fped.2022.802977
Received: 27 October 2021; Accepted: 13 January 2022;
Published: 04 March 2022.
Edited by:
Séverine Guillaume-Czitrom, Assistance Publique Hopitaux De Paris, FranceReviewed by:
Kaisu Kotaniemi, Private Practitioner, Abingdon, United StatesCopyright © 2022 Osswald, Rameau, Terzic, Sordet, Bourcier and Sauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnaud Sauer, YXJuYXVkLnNhdWVyQGNocnUtc3RyYXNib3VyZy5mcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.