- 1Department of Pediatrics and Child Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 2Department of Community Health Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Pediatrics and Child Health Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4Department of Maternity and Neonatal Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Background: Pre-maturity is the primary cause of neonatal mortality in the world. Although prematurity was the leading cause of neonatal mortality, the survival rate and its predictors may be varied from setting to setting and time to time due to different reasons. Therefore, this study aimed to assess the survival probability and predictors of mortality among preterm neonates at Felege Hiwot comprehensive specialized hospital.
Methods: This is a retrospective follow-up study that included 542 randomly selected preterm neonates admitted at Felege Hiwot comprehensive specialized hospital from the period of 2016-2020. Semi-parametric and parametric survival models were fitted to identify the survival probability of preterm neonates and its association with different predictors. The best fit model was selected using Akaike's information criteria, Bayesian information criteria and likelihood ratio criteria.
Results: The cumulative incidence and incidence rate of mortality among preterm neonates were 31 per 100 live births and 3.5 per 100 neonate days, respectively. From the adjusted cox-proportional-hazard model, predictors with higher preterm mortality risk include the presence of neonatal respiratory distress syndrome [AHR = 2.55, 95% CI: 1.23; 3.74], perinatal asphyxia [AHR = 4.26, 95% CI: 1.35; 6.79] and jaundice [AHR = 3.25, 95% CI: 2.14, 7.24]. However, admission weight of 1,500–2,499 g (AHR = 0.23, 95% CI: 0.11, 0.56) and ≥2,500 g (AHR = 0.12, 95% CI: 0.02; 0.32), early breastfeeding [AHR = 0.44, 95% CI: 0.36; 0.48] and kangaroo mother care [AHR = 0.11, 95% CI: 0.03; 0.15] were protective factors of preterm mortality.
Conclusion: The cumulative incidence of mortality among preterm neonates was consistent with the national incidence of preterm mortality. Factors such as respiratory distress syndrome, perinatal asphyxia, breastfeeding, kangaroo mother care, admission weight, and jaundice are significant predictors of survival. Therefore, considerable attention such as intensive phototherapy, optimal calorie feeding, oxygenation, and good thermal care should be given for admitted preterm neonates.
Background
The World Health Organization (WHO) defines preterm birth as a baby born before 37 completed weeks of gestation (1). It can be categorized into extremely preterm (<28 weeks of gestation), very preterm (28 to <32 weeks of gestation), and moderate preterm (32 to <37 weeks of gestation) (2, 3). Preterm birth is the primary cause of neonatal mortality and the second cause of under-five mortality in the world (4). Every year, an average of one million neonates and under-five children die of its complications (5). The survival rate in high and low-income countries is considerably varied with 9 and only 1 survivor out of the 10 preterm babies, respectively (2). The mortality among Africans showed twelve times higher compared to Europeans. In Sub-Saharan Africa, preterm birth is the second main cause of under-five mortality, accounting for 12.1% of deaths, and more than 50% of neonatal death in the East Africa is attributable to preterm birth (6). Furthermore, the survivor's are usually at a higher risk of morbidity and life-long physical, neurological, visual, learning, and hearing disabilities (7–11).
Among the neonates, the protective and risk factors associated with mortality including intra-natal related factors, post-natal related factors, maternal obstetrics related factors, neonatal related factors, and maternal socio-demographic related factors have been identified previously (8, 12, 13).
The majority of premature babies can be saved by feasible and cost-effective care using the continuity of midwifery-led care that could reduce the risk of prematurity by around 24 % (2). In doing so, Ethiopia has been incorporated the new WHO recommendations for improving preterm birth outcomes in the clinical standard (14). Moreover, the country has proposed a national strategy for newborn and child survival from 2015 to 2020 to end all preventable newborn and child deaths by 2035 (15). On the other hand, the Sustainable Development Goal three (SDG-3) offers a great weight to decrease neonatal mortality with a target of 12 neonatal deaths per 1,000 live births by 2030 (16). Despite these and many other efforts, the prevalence of neonatal mortality among preterm neonates is high. Different studies have been done on preterm neonates in Ethiopia; however, the majority of them used <4 year's data (17–20), and all of these studies did not investigate the independent predictors (time of breastfeeding initiated, gestational age and admission/birth weight) of preterm survival (17–23). Furthermore, some studies employed a cross-sectional type of study design, usually unable to make a causal inference (17, 20). Therefore, the goal of this study was to identify predictors (time of breastfeeding initiated, admission/birth weight, and gestational age) of survival among preterm neonates using 5 years retrospective follow-up study design.
Methods
Study Design and Setting
A retrospective follow-up study was carried out on preterm neonates admitted to the neonatal intensive-care unit at Felege Hiwot comprehensive specialized hospital. The hospital is located in Bahir Dar city, 552 km away from Addis Ababa, the capital city of Ethiopia. It is a public hospital serving a total of about 5 million populations. Most of the mothers who delivered in the hospital (>85%) have at least four antenatal care follow-up (24). The hospital has an annual delivery rate of about 6,000. Though the usual deliveries are from the hospital, it also serves some deliveries referred from the district, general, and referral hospitals in Amhara region. Nearly 3.4% of the total deliveries in the hospital were reported with neonatal mortality. Neonatal admissions in 2020 were 4,584, of whom the majorities (about 61%) were preterm neonates, including both the out born and inborn neonates. Preterm admission criteria were gestational age <34 weeks at birth or any preterm neonates with complications (having at least one newborn danger sign). About three-fourths of the overall neonatal admissions are from hospital deliveries (inborn). Out born from the different parts of Amhara region including the city are also admitted to the NICU. Out born neonates are transported from lower-level hospitals to the hospital NICU by ambulance with neonatal care providers. The neonatal intensive-care unit has five different room's namely preterm neonate rooms, term room, kangaroo mother care room, septic neonate room, and mother side room. In the NICU, there are different equipment including oxygen tubing, incubators, photo-therapy machine, CPAP machines, heaters and radiant warmers. Furthermore, there are therapeutic interventions such as intubation, vasopressin, IV antibiotics, gavage feeding, and parenteral nutrition. However, preterm services like surfactant administration and plastic wrapping aren't available in the study setting (24).
Source and Study Population
All preterm neonates admitted to neonatal intensive care unit at Felege Hiwot comprehensive specialized hospital were considered as a source population whereas all preterm neonates who were admitted to NICU at Felege Hiwot comprehensive specialized hospital from a period of January 1, 2016, to December 30, 2020, were the study population. The gestational age was determined by first-trimester ultrasound results for mothers with available reports or Ballard score for those mothers without the reports.
Eligibility Criteria
Preterm neonates with incomplete medical records (i.e., if the outcome, date of admission, date of death/censored, and the gestational age are not recorded) and gross congenital malformation were excluded.
Sample Size Determination
A total sample size of 542 preterm neonates was obtained using STATA version 14 statistical software based on the cox-proportional hazard regression, taking a Crude Hazard Ratio (CHR) of different predictors such as home delivery (CHR = 2.14), hyaline membrane disease (CHR = 4.38), KMC (CHR = 0.41), crying immediately after birth (CHR = 0.25) and hypoglycemia (CHR = 2.1) as effect size (20), 5% marginal error, a standard error of 0.5, study power of 80% and a reasonable estimate for the proportion of preterm mortality from the prior study was 28.8% (25). Meanwhile, to check the adequacy of the sample size used, a post-hoc power analysis was computed and the sample size was adequate to represent the study population with post-hoc power value of 90%.
Sampling Technique and Procedures
Medical Record Numbers (MRN) of the eligible preterm neonates charts were first coded by the Health Information Management system (HMIS) focal persons. Then, the eligible preterm neonate's were included by a computer-generated random selection of the coded neonatal medical charts (Figure 1).
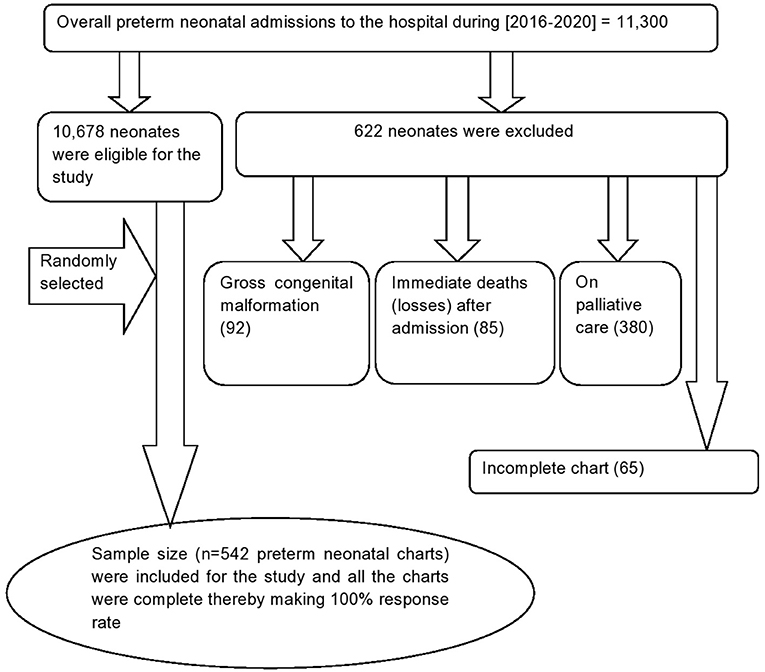
Figure 1. Flow diagram to show the overall admission of preterm birth Felege Hiwot comprehensive specialized hospital, Northwest Ethiopia, 2016-2020.
Data Collection Procedures and Quality Assurance
The data were collected by four trained clinical nurses under the supervision of a public health officer. Abstractors used a standardized case report form to retrieve the following information from medical charts: maternal socio-demographics, antenatal history, delivery history, neonatal complications, and treatments. Assessment of inter-rater reliability on 43 items on the case report form revealed a Cronbach alpha of 0.81. Furthermore, the case report form was validated through pre-testing on 28 preterm neonatal charts (5% of the sample size) 2 weeks before the actual data collection time at the same hospital.
Variables in the Study
Event
Eligible preterm neonate who died during the follow-up time (from date of admission until discharge).
Censored
Preterm neonates who were referred to other health institutions or discharged with parental refusal or alive during discharge were considered as censored.
The Time Origin
Admission of preterm neonates to NICU at Felege Hiwot comprehensive specialized hospital.
Follow-Up Time
From the time of admission until either an event or censorship occurs.
Time to Event
The time elapsing from the date of admission at NICU of the study hospital to the occurrence of death before discharge, which can be calculated by subtracting the date of death from the date of admission.
Obstetrics complications are considered if the mother had one or more of the following problems such as Premature Rupture of Membranes (PROM), corioamnioitis, eclampsia/pre-eclampsia and polyhaydraminos (15, 26).
Neonatal complications are considered if the neonate had one or more of the following problems such as Perinatal Asphyxia (PNA), Necrotizing Enterocolitis (NEC), jaundice, hypothermia, sepsis, hypoglycemia, and Respiratory Distress Syndrome/Hyaline Membrane Disease (RDS/HMD) (7, 11, 15).
Other variables such as Small for Gestational Age (SGA), Appropriate for Gestational Age (AGA), hypothermia, hypoglycemia, jaundice, antenatal visit, polyhaydraminous, PNA, NEC, PROM, chorioamnionitis, RDS/HMD, KMC, and use of antenatal steroids if the diagnosis was made at the admission of neonates to NICU.
Pre-maturity is defined as a baby born alive before 37 completed weeks of gestation (1).
Data Management and Analysis
The collected data were coded and double entered into Epi-info statistical software version 7.2.01 and exported to STATA version 14 statistical software for analysis. Since the proportion of missing data was not more than 5% for all variables, we used complete case-analysis. Descriptive statistics were carried out and summarized using means, medians, and proportions. The cumulative incidence of NICU mortality was determined to be the number of neonatal deaths per 100 live neonatal births while the incidence rate of preterm mortality among neonatal admissions to NICU was determined using Neonate-Day (ND) follow-up as a denominator for the entire cohort and for the group classified based on socio-demographic and other variables. The Kaplan-Meier curve was used for the analysis of the probabilities of preterm mortality. The log-rank test was used to compare survival curves between groups of explanatory variables. A life table was used to estimate the probability of survival at different time intervals in the follow-up time. Variables with a p-value <0.2 in bi-variable cox-proportional hazard models were entered into multi-variable cox-proportional hazard models to identify predictors of survival among preterm neonates. A 95% confidence interval of the hazard ratio was computed and variables with a p-value ≤ 0.05 in the multi-variable cox-proportional hazard model were considered as significant predictors of the incidence of mortality among preterm neonates.
Model Comparison and Goodness of Fit Test
The cox-proportional hazard model and other parametric survival analysis models (Gompertz Distribution, Wei-bull Distribution, and Exponential Distribution) were fitted by considering the baseline hazard distribution assumptions into account. Hence, the final fitted model, the cox-proportional hazard model was selected based on Akaike's information criteria, Bayesian information criteria, and likelihood ratio. The overall model fitness was assessed by using a cox-Snell residual test.
Ethical Approval and Consent to Participate
Ethical approvals were obtained from the University of Gondar, College of Medicine and Health Science, Institutional Health Research Ethics Review Committee (IHRERC). Informed consent was waived because the study was conducted using secondary data. To further secure the ethical perspective of the study, a permission letter was issued from Felege Hiwot comprehensive specialized hospital administration. Moreover, results were reported compositely in groups to secure confidentiality.
Results
Maternal Socio-Demographic and Obstetrics Related Characteristics
The mean maternal age was 26.64 (±5.24) years. Three-fifth (60.1%) of the mothers were urban residents, and a nearly equal number of pregnancies, 331 (61.1%) resulted in single tone birth. Five hundred ten (94.10%) mothers had antenatal care follow-up. One hundred fifty-five (28.60%) mothers delivered outside Felege Hiwot comprehensive specialized hospital (Table 1).
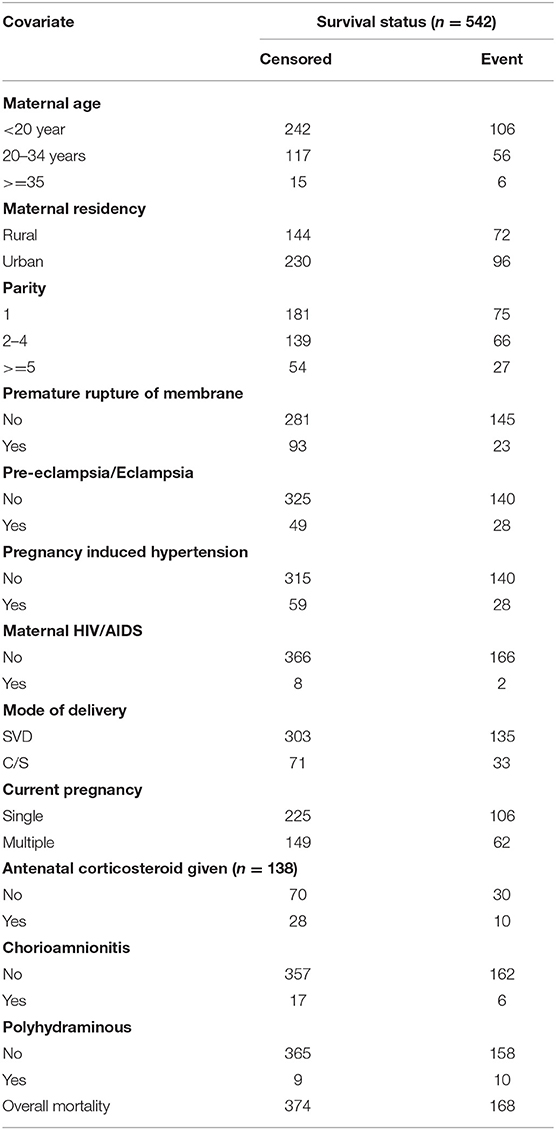
Table 1. Maternal socio-demographic and obstetrics related characteristics at FHCSH, during 2016-2020, (n = 542).
Socio-Demographic and Clinical Characteristics of Preterm Neonates
Out of 11,300 neonatal admissions to NICU in the hospital during the 5 years period [2016–2020], 622 preterm neonates were excluded for different reasons as illustrated in the flow diagram (Figure 1). About three-fifth [333 (61.44%)] of the preterm neonates were females. The mean gestational age at birth was 32.9 (95% CI = 32.67, 33.16) weeks, and about one-eighth (12.5%) of the preterm neonates were small for gestational age. More than half (306, 56.4%) of the preterm neonates have not initiated breastfeeding within the first hour of birth. More than two-thirds (374, 69%) of preterm neonates did not get KMC services. Besides, more than three quarters (420 77.49%) of the neonates have hypothermia (Table 2).
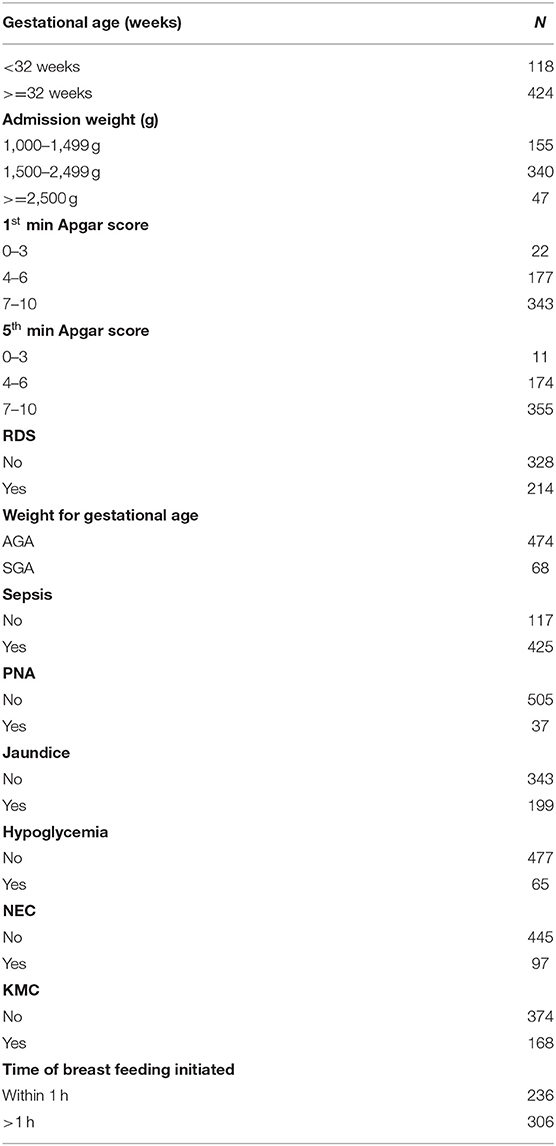
Table 2. Socio-demographic and clinical characteristics of preterm neonates at FHCSH, 2016-2020, (n = 542).
Incidence of Preterm Mortality
The cumulative incidence of neonatal mortality among preterm neonates was 31 per 100 live births. In the mean follow-up period of 40 days within the range of 32 to 48 follow-up months, the incidence rate of preterm mortality was 3.5 per 100 neonate days (95% CI = 1.9, 5.1). The median survival time was 30 days (95% CI = 6, 37) in a total of 4,820 neonates-days observation (Figure 2). In this cohort, the incidence rate of mortality among preterm neonates who were not under KMC service was 5.8 per 100 neonate days (95% CI = 3.8, 7.8). Furthermore, the incidence rate of mortality among preterm neonates who had PNA was 10 per 100 neonates days (95% CI = 7.5, 12.5). The incidence rate of preterm mortality was also found to be 6.2 per 100 neonate days (95% CI = 4.2, 8.2) among preterm neonates who initiated breastfeeding after 1 h of birth.
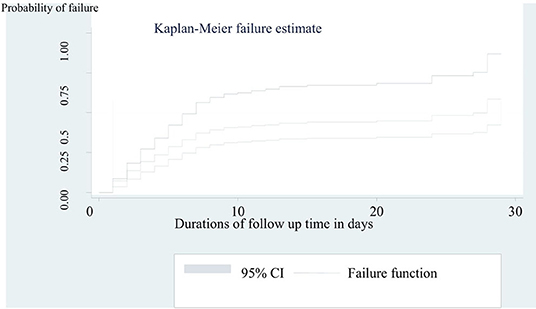
Figure 2. Kaplan-Meier curve to estimate failure of preterm neonates admitted to NICU at FHCSH from 2016 to 2020 (n = 542).
Comparison of Survival Experience
The overall survival across various groups was compared using a log-rank test. We found significant differences in the overall survival across various groups including PNA, time of breastfeeding initiated, admission weight, and KMC (Figures 3A–D). Accordingly, there was a clear statistically significant mortality difference between preterm neonates who initiate breastfeeding within 1 hour of birth [21 days (95% CI = 18, 23)] and their counterpart [18 days (95% CI = 17, 20)] (p = 0.0165). On the other hand, the mean survival time of preterm neonates who had not received KMC was significantly higher than 27 days (95 % CI = 25, 28) that of the received [15 days (95% CI = 14, 17)] (p = 0.000). A similar result has been recorded among preterm neonates who had not PNA (19 days 95% CI = 18, 21), compared to those who had PNA [7 days (95% CI = 5, 9)] (p = 0.0002). Moreover, the mean survival time among those who had admission weight of 1,000–1,499 g was significantly lower (14 days, 95% CI = 12, 17) than those with an admission weight of 1,500–2,499 g (21 days, 95% CI = 20, 23) and ≥2,500 g (24 days, 95% CI = 22, 27) (p = 0.000).
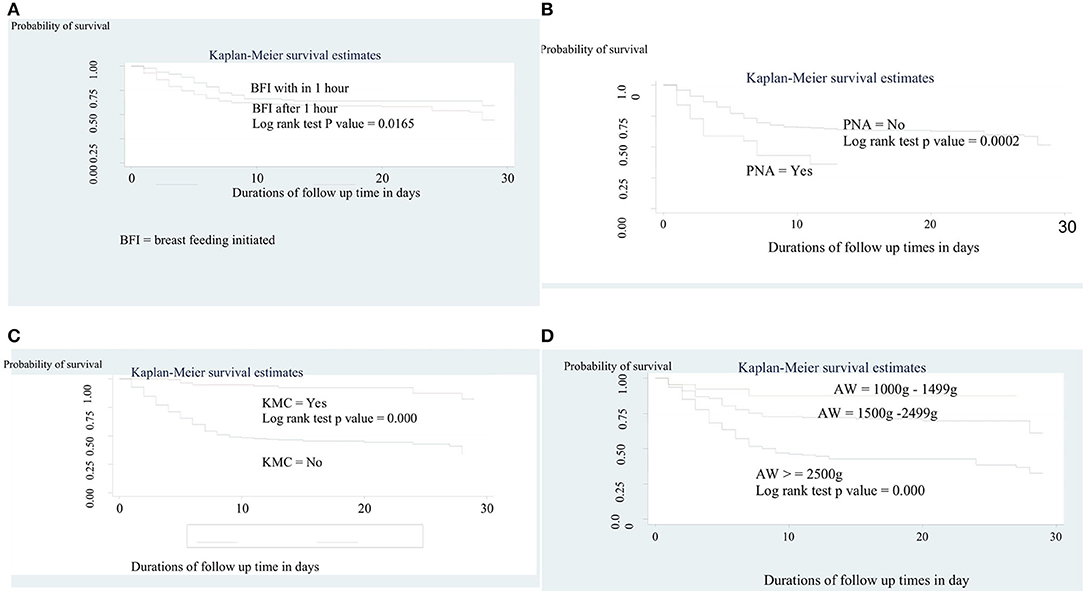
Figure 3. (A) The Kaplan-Meier survival curves by time of initiation of breast feeding among preterm neonates admitted to NICU at FHCSH, 2016-2020 (n = 542). (B) The Kaplan-Meier survival curves by KMC service among preterm neonates admitted to NICU at FHCSH, 2016-2020 (n = 542). (C) The Kaplan-Meier survival curves by PNA among preterm neonates admitted to NICU at FHCSH, during 2016-2020 (n = 542). (D) The Kaplan-Meier survival curves by admission weight among preterm neonates admitted to NICU at FHCSH, 2016-2020 (n = 542).
Assessing the Proportional Hazard Assumption
The global test of proportional-hazards assumption based on the Schoenfeld residuals revealed that all of the co-variates and full model satisfies the cox-proportional hazard assumption at p = 0.2425.
Model Selection
To identify predictors of survival among preterm neonates, semi-parametric and parametric proportional hazard models were fitted. The most parsimonious model was chosen using AIC, BIC, and LR, and the cox-proportional hazard model (AIC = 277.7724, BIC = 369.7376, log-likelihood = 114.8862) was found to be more efficient than other parametric models (Table 3). Interpretations and conclusions were thus based on the cox-proportional hazard model.
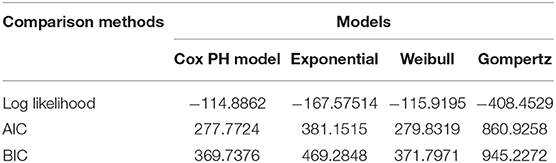
Table 3. Summary of Model comparison between semi-sox proportional hazard models and parametric Cox- Regression models using AIC, BIC and log likelihood.
The Goodness of Fit Test
It can be seen that the plot of the Nelson-Allen cumulative hazard function against Cox-Snell residuals is closest to 450 straight lines through the origin for the cox-proportional hazard model when compared to the parametric survival model. This suggested that the cox-proportional hazard model provided the best fit for our data set (Figure 4).
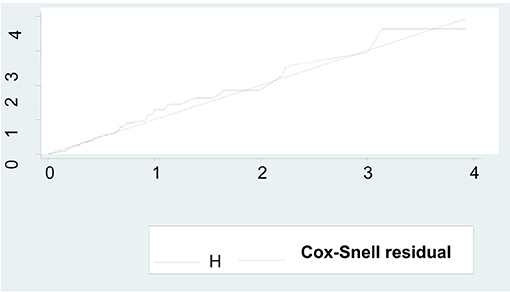
Figure 4. Model fitness by using Cox-Snell residual test among preterm neonates admitted to NICU at FHCSH, during 2016-2020 (n = 542).
Multi-Variable Analysis of Cox-Proportional Hazard Model
From the bi-variable cox-regression analysis, PNA, jaundice, KMC, gestational age, RDS, admission weight of 2,500 g and above, initiation of breastfeeding within 1 h of birth, and first minute Apgar score were significant predictors of preterm mortality. However, after adjusting for possible confounders in multi-variable cox-regression analysis, PNA, jaundice, KMC, RDS, initiation of breastfeeding within 1 h of birth, and admission weight of 2,500 g and above were independently and significantly associated with the incidence rate of preterm mortality.
The hazard of mortality among preterm neonates who had PNA was 4.3 times higher as compared with those who had not PNA (AHR = 4.26, 95% CI: 1.35; 6.79). In addition, the risk of mortality among preterm neonates diagnosed with RDS was 2.6 times higher as compared to those who had not RDS (AHR = 2.55, 95% CI:1.23; 3.74). The risk of mortality among preterm neonates who had jaundice was 3.3 times higher than their counterparts (AHR = 3.25, 95% CI: 2.14, 7.24).
Preterm neonates who had admission weight of 1,500–2,499 g (AHR = 0.26, 95% CI: 0.11, 0.56) and ≥2,500 g (AHR = 0.12, 95% CI: 0.02; 0.32) had 74 and 88% less hazard of mortality as compared with those who had admission weight of 1,000–1,499 g.
Preterm neonates who initiated breastfeeding within 1 h of birth had 56% less hazard of mortality as compared with their counterparts (AHR = 0.44, 95% CI: 0.36; 0.48). Providing KMC for preterm neonates reduce the risk of mortality by 89% as compared with their counterparts (AHR = 0.11, 95% CI: 0.03; 0.15) (Table 4).
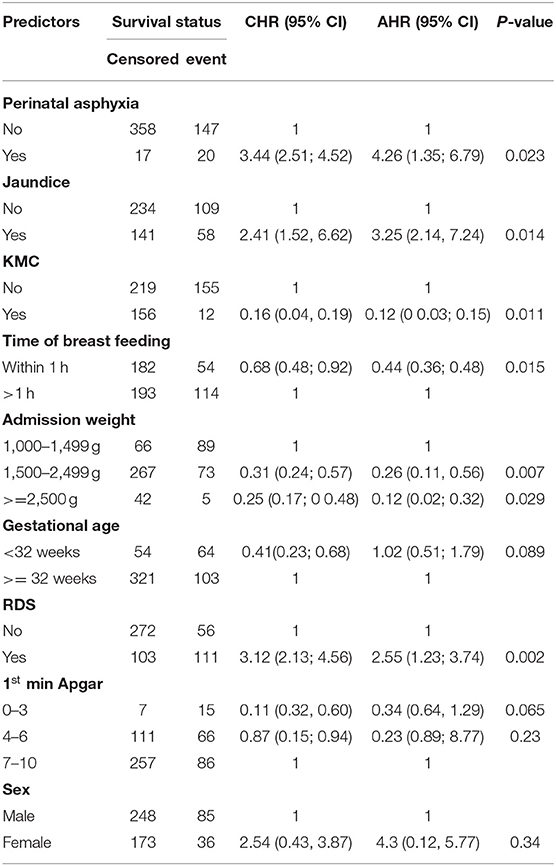
Table 4. Adjusting for sex, bi-variable and multivariable Cox regression analysis of predictors of incidence of mortality among preterm neonates admitted to NICU at FHCSH from 2016 to 2020 (n = 542).
When gestational age below 32 weeks and admission weight (1,000–1,499 g) were entered together in the final model (were controlled each other) as presented in Table 4, gestational age wasn't significant. This may be due to co-linearity between these variables (standard error = 2.13). Thus, on separate entry to the final model, while adjusting for sex; gestational age below 32 weeks became significant. Adjusting for sex, preterm neonates whose gestational age below 32 weeks had 5.8 times more hazard of mortality (AHR = 5.78, 95% CI, 4.03; 8.23) as compared to those whose gestational age was above 32 weeks when admission weight (1,000–1,499 g) was not entered to the model (Table 5).
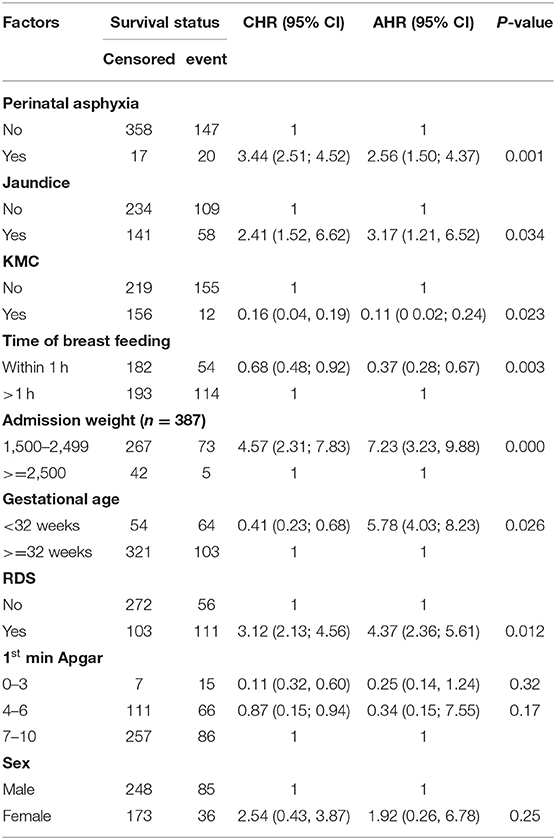
Table 5. Significance of gestational age (<32 weeks) in the multivariable cox proportional hazard model when the model is adjusted for sex and admission weight (1,000–1,499 g) was not entered to the model.
Furthermore, adjusting for sex and leaving gestational age from the model, preterm neonates whose admission weight (1,000–1,499 g) had 4.7 times more hazard of mortality (AHR = 4.65, 95% CI = 2.27, 6.70) as compared to those whose admission weight was ≥2,500 g (Table 6).
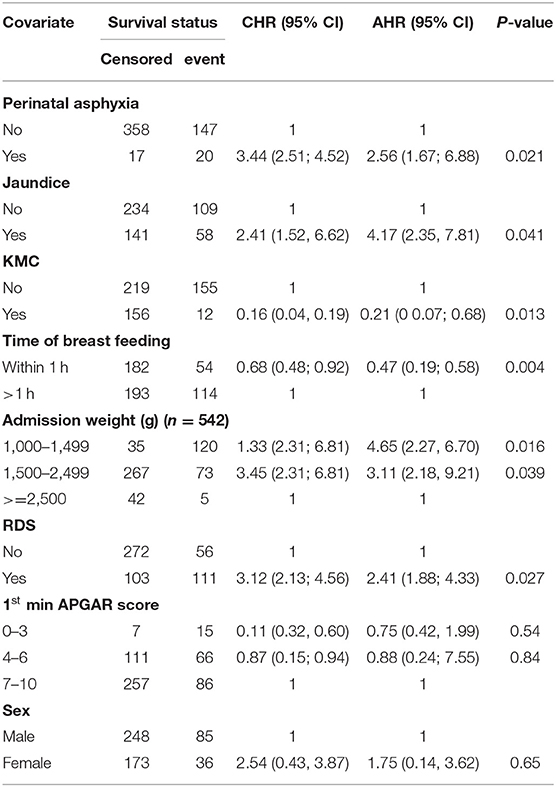
Table 6. Significance of admission weight (1,000–1,499 g) in the multivariable cox proportional hazard model when the model was adjusted for sex and gestational age (<32 weeks) was not entered to the model.
Discussion
This retrospective follow-up study aimed to assess the incidence rate of mortality and its predictors among preterm neonates admitted to NICU at Felege Hiwot comprehensive specialized hospital. In this study, the incidence rate of preterm mortality was 3.5 per 100 neonate days. Moreover, the cumulative incidence of preterm neonatal mortality was 30.81% which is consistent with the study conducted in Gondar, Addis Ababa, Nigeria, East Africa, and Iran (12, 14, 20, 27, 28). However, this finding was higher than the study conducted in Nairobi hospital in Kenya, Malawi, and Mulago National referral hospital in central Uganda (29–31). The possible reasons could be difference in the study participants between the studies. Thus, the study participants in our study were both inborn and out born preterm neonates which might have increased the time-lapse for starting appropriate neonatal care. However, the study in Malawi (29) included out born babies when they were <48 h. In addition, the majority of the study participants in Mulago National referral hospital in central Uganda were inborn preterm neonates (30). On the other hand, the disparity in the study period contributed to the variation of the findings between the studies.
But, the finding in our study was lower than the study conducted in Felege Hiwot comprehensive specialized hospital, Jimma, Eritrea, Bangladesh, and India (18, 19, 32–34). This discrepancy may be due to differences in study populations which include preterm neonates who had gestational age 28 weeks and above with different categories of birth weight in this study but the study populations were preterm neonates who had gestational age 26 weeks and above in Jimma (19) and very low birth weight preterm neonates in India (32). Moreover, the study design in Felege Hiwot comprehensive specialized hospital and Eritrea (18, 33) was cross-sectional in sharp contrast to the retrospective follow-up in our study. The duration of follow-up time was only seven days in Bangladesh (34) and forty in our study.
On the other hand, the median survival time in this study was 30 days (95% CI = 6, 37) which is consistent with the study conducted in Black lion specialized hospital (28 days) (35), University of Gondar comprehensive specialized hospital (21 days) (21), and Mizan Tepi teaching hospital (15 days) (17). The similarity in the quality of service provided between the hospitals may contribute to the consistency. Therefore, to enhance the survival rate of preterm birth different specialty neonatal care has to be provided during the first month of the neonatal period.
From the adjusted cox-regression analysis, the hazard of mortality among preterm neonates who had perinatal asphyxia was 4.3 times higher than those who had no perinatal asphyxia. This finding was congruent with the study conducted in Gondar, Jimma and Ethiopia suggested that perinatal asphyxia was the commonest cause of mortality among premature neonates (19, 20, 36). This is related to intra-partum hypoxia, which contributed to difficulties in the transition to the extra-uterine environment. Hence, for preterm neonates who had prenatal asphyxia, lifesaving essential and extra essential care like neonatal resuscitation and safe oxygen use should be encouraged. Besides, advanced neonatal care such as surfactant replacement has to be provided for preterm neonates with RDS secondary to PNA (37). However, it is not routine practice in our study setting. But, an Indian study has shown that perinatal asphyxia was not a significant predictor of neonatal mortality among preterm neonates. This could be due to differences in study populations because our study was entirely on preterm neonates whereas the study in Indian include only very low birth weight neonates (32).
Preterm neonates who had admission weight of 1,500–2,499 g and ≥2,500 g had 74 and 88% less hazard of mortality as compared to those who had admission weight of 1,000–1,499 g, respectively. This result is supported by the study conducted in Gondar, Addis Ababa, Eritrea, East Africa, Iran, China, western Nepal, and the Eastern Mediterranean region (14, 27, 28, 35, 38–41). This congruence may be due to the fact that low birth weight preterm neonates have low levels of brown fat that predisposes them to hypoglycemia and hypothermia thereby increasing their risk of mortality. Physiologically, these neonates have immature organ development (especially the lungs), have poor feeding skills, metabolic deregulation, and are at a higher risk of infections, all of which could increase susceptibility for different preterm birth complications and death. In the study setting, neonatal complications such as hypothermia and hypoglycemia are high. However, KMC was poorly practiced in the study setting. Therefore, WHO thermal care for preterm neonates such as KMC, radiant warmers, and/or incubators are recommended (42) to prevent hypothermia and hypoglycemia-related mortality of preterm neonates.
Preterm neonates who had jaundice were at a higher hazard of mortality than those who had not jaundiced. This may be due to adverse outcomes due to either neurotoxicity or over-treatment of photo-therapy or exchange transfusion (43). Moreover, jaundice considered for this study was pathological type as operationalized in the methods section and hence, the jaundiced preterm neonates were more likely to develop kernicterus because the hypoxia from respiratory distress and perinatal asphyxia accelerates communication of the preterm neonate's un-conjugated hyper-bilirubin with their neuronal tissues thereby increases the likelihood of acute bilirubin encephalopathy (kernicterus) (7, 11). Besides, some of the pathologically jaundiced preterm neonates admitted to the hospital were attributed to ABO and/Rh incompatibility with severe anemia as ABO/Rh sensation was a common phenomenon in the study setting during the study period (24). As a result, regular and timely monitoring of bilirubin level is helpful to prevent treatment-related complications of jaundice (neurotoxicity or over-treatment of photo-therapy or exchange transfusion) and to identify the type of treatment provided for preterm neonates with jaundice. In addition, a screening test for bilirubin level has to be performed for preterm neonates who had PNA and/or RDS.
The hazard of mortality among preterm neonates who had RDS was 2.6 times higher as compared with their counterparts. This finding was similar to the study conducted in Gondar, Jimma, Ethiopia, Iran, and India (19, 20, 28, 35, 44, 45). Neonatal RDS is often accompanied by life-threatening complications such as pneumothorax, pulmonary hypertension, Cor-Pulmonary and metabolic deregulations which in turn increase the possibility of preterm mortality (46–48). Hence, to reduce the risk of RDS, antenatal corticosteroids are WHO-recommended practice for women at risk for preterm labor and/or in preterm labor (13).
In our study, receiving KMC service and early breastfeeding were protective factors of preterm mortality. Comparison of mortality risk regarding KMC and early breastfeeding services was made among preterm neonates who were relatively stable (denominator). Despite eligibility for KMC and early breastfeeding, some neonates weren't given these cares because of probably different constraints in the study setting and even from the parental side, which in turn necessitates another investigation (24). The hazard of death among preterm neonates who received KMC was decreased by 89%. This may be due to the fact that KMC service facilitates skin-to-skin contact, optimal breastfeeding, and prevention of apnea and colonization of the neonate's skin with mutualistic maternal skin microbial flora. These clinical significances of KMC help prevent the neonates from being hypothermia, hypoglycemic, distressed, and developing infection thereby increasing their survival (33, 35, 45, 49, 50). Therefore, preterm infants whose birth weight is <2,000 g and have relatively stable vital signs should always receive KMC service to optimize their survival.
The hazard of death among preterm neonates who initiated breastfeeding within 1 h of birth was 56% less as compared to those who initiated later. This finding was supported by the study conducted in Gondar and India (20, 32). This may be due to the fact that early initiation of breastfeeding decreases the risk of hypoglycemia, infection, and hypothermia (49, 51–54). For this reason, the relatively stable preterm neonate should be initiated breastfeeding as early as possible after gut priming is once tolerated (7, 11).
Strength and Limitation of the Study
This study included study populations from a tertiary center, which handled large number of high-risk preterm neonates. Since the data were accessed from secondary source; some important predictors such as socio-economic and nutritional statuses were missed which are significant predictors of mortality among preterm neonates. Furthermore, the study area covers only Felege Hiwot comprehensive specialized hospital that limit's representations to other hospitals found in Amhara region and Ethiopia.
Conclusion
The cumulative incidence of mortality among preterm neonates was consistent with the national incidence of preterm neonatal mortality. Factors such as respiratory distress syndrome, perinatal asphyxia, breastfeeding, kangaroo mother care, admission weight, and jaundice are significant predictors of survival. Therefore, considerable attention such as intensive photo-therapy, optimal calorie feeding, oxygenation, exogenous post-natal surfactant administrations and good thermal care should be given for admitted preterm neonates. Moreover, the study didn't investigate the true causality of some predictors like maternal socio-economic and nutritional status on the incidence of preterm mortality. Therefore, we recommend a multi-center prospective cohort study to show the public health importance of these and other predictors on preterm mortality.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
DB worked on conceiving and designing the study, training and supervising the data collectors, interpreting the result, and preparing the manuscript. The co-authors namely AW, WW, HH, and WB played their roles in analyzing and interpreting the result. Moreover, the co-authors wrote the manuscript. All authors were involved in reading and approving the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
First and foremost, we want to thank the Almighty GOD for providing us with the optimal strength and health during this research work. We would also like to acknowledge Debre Tabor University, Gondar University, Felege Hiwot Comprehensive Specialized Hospital administration, data collectors, supervisors, health management information system workers and NICU staffs for their respective unreserved efforts during this research work.
Abbreviations
AHR, adjusted hazard ratio; ANC, antenatal care; AOR, adjusted odd ratio; ARR, adjusted relative risk; CI, confidence interval; CPAP, continuous positive airway pressure; CS, cesarean section; HMD, hyaline membrane disease; HR, hazard ratio; KMC, kangaroo mother care; LBW, low birth weights; NEC, necrotizing entro colitis; NICU, neonatal intensive care unit; OR, odd ratio; PIH, pregnancy induced hypertension; PNA, peri natal asphyxia; PROM, premature rupture of membrane; RDS, respiratory distress syndrome; RR, relative risk; SVD, spontaneous vaginal delivery; UNICEF, united nation international children's emergency fund; WHO, world health organization.
References
1. WHO. The Prevention of Perinatal Mortality And Morbidity: Report of a WHO Expert Committee [Meeting Held in Geneva From 25 November to 2 December 1969] (1970).
2. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388:3027–35. doi: 10.1016/S0140-6736(16)31593-8
3. Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
4. Walani SR. Global burden of preterm birth. Int J Gynecol Obstet. (2020) 150:31–3. doi: 10.1002/ijgo.13195
5. Howson CP, Kinney MV, McDougall L. Lawn JEJRh. Born too soon: preterm birth matters. Reprod Health. (2013) 10:1–9. doi: 10.1186/1742-4755-10-S1-S1
6. WHO Mathers C. Global Strategy For Women's, Children's And Adolescents' Health (2016-2030). World health Organization (2016).
7. Federal Ministry of Health of Ethiopia AA. Neonatal Intensive Care Unit (NICU) Training Management Protocol (2014).
8. Gebreslassie HG, Gebregziabher B, Hailu T, Gebreegziabiher G. Patterns of preterm neonatal death and associated factors in ayder referral hospital neonatal unit (five years record review), Tigray Region, North Ethiopia. Int J Contemp Pediatr. (2018). p. 10060–5. Available online at: https://www.journalijdr.com/sites/default/files/issue-pdf/6370.pdf (accessed November 30, 2021).
9. Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, et al. Visual and hearing impairments after preterm birth. Pediatrics. (2018) 142:e20173888. doi: 10.1542/peds.2017-3888
10. Majewska J, Zajkiewicz K, Wacław-Abdul K, Baran J, Szymczyk D. Neuromotor development of children aged 6 and 7 years born before the 30th week gestation. BioMed Res Int. (2018) 2018:2820932. doi: 10.1155/2018/2820932
11. Vaughn VC, McKay RJ, Behrman RE, Nelson WE. Textbook of Pediatrics. 11th ed. Philadelphia, PA: WB Saunders Company (1979).
12. Kunle-Olowu OE, Peterside O, Adeyemi OO. Prevalence and outcome of preterm admissions at the neonatal unit of a tertiary health centre in Southern Nigeria. Open J Pediatr. (2014) 4:67–75. doi: 10.4236/ojped.2014.41009
14. Temesgen M, Worku B, Regassa Y, Mekasha A. Survial of preterm infants admitted to Tikur Anbessa Hospital Nicu, Addis Ababa. Ethiop J Pediatr Child Health. (2014) 10:68–78.
15. Federal democratic republic of Ethiopia Ministry of Health BLBpimancMDSaR. BEmONC LRP (2018). Best Practice in Maternal and Newborn Care Maternal Death Surveillance and Response.
16. Child EWE. The Global Strategy for Women's, Children's and Adolescents Health. New York, NY: Every Woman Every Child (2015).
17. Bereka B, Demeke T, Fenta B, Dagnaw Y. Survival status and predictors of mortality among preterm neonates admitted to Mizan Tepi university teaching hospital, South West Ethiopia. Pediatric Health Med Ther. (2021) 12:439. doi: 10.2147/PHMT.S319774
18. Tamene A, Abeje G, Addis Z. Survival and associated factors of mortality of preterm neonates admitted to Felege Hiwot specialized hospital, Bahir Dar, Ethiopia. SAGE Open Med. (2020) 8:2050312120953646. doi: 10.1177/2050312120953646
19. Wesenu M, Kulkarni S, Tilahun T. Modeling determinants of time-to-death in premature infants admitted to neonatal intensive care unit in Jimma university specialized hospital. Ann Data Sci. (2017) 4:361–81. doi: 10.1007/s40745-017-0107-2
20. Yismaw AE, Gelagay AA, Sisay MM. Survival and predictors among preterm neonates admitted at university of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. Ital J Pediatr. (2019) 45:1–11. doi: 10.1186/s13052-018-0597-3
21. Aynalem YA, Mekonen H, Akalu TY, Gebremichael B, Shiferaw WS. Preterm neonatal mortality and its predictors in Tikur anbessa specialized hospital, addis Ababa, Ethiopia: a retrospective cohort study. Ethiop J Health Sci. (2021) 31:43–54. doi: 10.4314/ejhs.v31i1.6
22. Gebreheat G, Teame H. Survival and mortality of preterm neonates in a neonatal intensive care unit in Northern Ethiopia: a retrospective cohort study. Sci Rep. (2022) 12:1–8. doi: 10.1038/s41598-021-04521-z
23. Girma B, Nigussie J. Magnitude of preterm hospital neonatal mortality and associated factors in northern Ethiopia: a cross-sectional study. BMJ Open. (2021) 11:e051161. doi: 10.1136/bmjopen-2021-051161
25. Anderson JG, Baer RJ, Partridge JC, Kuppermann M, Franck LS, Rand L, et al. Survival and major morbidity of extremely preterm infants: a population-based study. Pediatrics. (2016) 138:e20154434. doi: 10.1542/peds.2015-4434
26. Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS. Williams Obstetrics. 24th ed. New York, NY: Mcgraw-Hill (2014).
27. Marchant T, Willey B, Katz J, Clarke S, Kariuki S, Kuile Ft, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. (2012) 9:e1001292. doi: 10.1371/journal.pmed.1001292
28. Basiri B, Esna Ashari F, Shokouhi M, Sabzehei MK. Neonatal mortality and its main determinants in premature infants hospitalized in neonatal intensive care unit in Fatemieh hospital, Hamadan, Iran. J Compr Pediatr. (2015) 6. doi: 10.17795/compreped-26965
29. Ahlsén AK, Spong E, Kafumba N, Kamwendo F, Wolff K. Born too small: who survives in the public hospitals in Lilongwe, Malawi? Arch Dis Child Fetal Neonatal Ed. (2015) 100:F150–4. doi: 10.1136/archdischild-2013-305877
30. Abdallah Y, Namiiro F, Mugalu J, Nankunda J, Vaucher Y, McMillan D. Is facility based neonatal care in low resource setting keeping pace? a glance at Uganda's national referral hospital. Afr Health Sci. (2016) 16:347–55. doi: 10.4314/ahs.v16i2.2
31. Patel A, Kandasamy Y. Outcome of premature neonates born in a tertiary neonatal intensive care unit in Nairobi, Kenya. J Pediatr Neonatal Individ Med. (2017) 6:e060113. doi: 10.7363/060113
32. Basu S, Rathore P, Bhatia B. Predictors of mortality in very low birth weight neonates in India. Singapore Med J. (2008) 49:556-60.
33. Shah S, Zemichael O, Meng HD. Factors associated with mortality and length of stay in hospitalised neonates in Eritrea, Africa: a cross-sectional study. BMJ Open. (2012) 2:e000792. doi: 10.1136/bmjopen-2011-000792
34. Shah R, Mullany LC, Darmstadt GL, Talukder RR, Rahman SM, Mannan I, et al. Neonatal mortality risks among preterm births in a rural B angladeshi cohort. Paediatr Perinat Epidemiol. (2014) 28:510–20. doi: 10.1111/ppe.12145
35. Yehuala S, Teka Z. Survival analysis of premature infants admitted to neonatal int ensive care unit (NICU) in Northwest Ethiopia using semi-parametric frailty model. J Biom Biostat. (2015) 6:1. doi: 10.4172/2155-6180.1000223
36. Deressa A, Cherieb A, Negash B. Factors associated with induced preterm birth and its immediate outcome in Addis Ababa public hospitals, Ethiopia. Neonatal Pediatr Med. (2018) 4:2. doi: 10.4172/2572-4983.1000170
37. Lawn JE, Davidge R, Paul VK, von Xylander S, de Graft Johnson J, Costello A. et al. Born too soon: care for the preterm baby. Reprod Health. (2013) 10:1–19. doi: 10.1186/1742-4755-10-S1-S5
38. Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. (2016) 16:1–8. doi: 10.1186/s12887-016-0716-5
39. Ashish K, Wrammert J, Nelin V, Ewald U, Clark R, Målqvist M. Level of mortality risk for babies born preterm or with a small weight for gestation in a tertiary hospital of Nepal. BMC Public Health. (2015) 15:1–9. doi: 10.1186/s12889-015-2232-1
40. Nayeri F, Emami Z, Mohammadzadeh Y, Shariat M, Sagheb S, Sahebi L. Mortality and morbidity patterns of very low birth weight newborns in eastern Mediterranean region: a meta-analysis study. J Pediatr Rev. (2019) 7:67–76. doi: 10.32598/jpr.7.2.67
41. Desta BN, Assefa N, Damte TD, Hordofa LO. Neonatal mortality and its risk factors in eastern Ethiopia: A prospective cohort study in Kersa health and demographic surveillance system (Kersa HDSS). Mortality (2016) 13:19. doi: 10.1186/s40748-016-0035-8
42. Vogel JP, Oladapo OT, Manu A, Gülmezoglu AM, Bahl R. New WHO recommendations to improve the outcomes of preterm birth. Lancet Glob Health. (2015) 3:e589–90. doi: 10.1016/S2214-109X(15)00183-7
43. Bhutani VK, Wong RJ, Stevenson DK. Hyperbilirubinemia in preterm neonates. Clin Perinatol. (2016) 43:215–32. doi: 10.1016/j.clp.2016.01.001
44. Tripathy SK, Chatterjee K, Behera N. Mortality and morbidity of very low birth weight and extremely low birth weight babies in neonatal period. Int J Contemp Pediatr. (2019) 6:645. doi: 10.18203/2349-3291.ijcp20190704
45. Muhe LM, McClure EM, Nigussie AK, Mekasha A, Worku B, Worku A, et al. Major causes of death in preterm infants in selected hospitals in Ethiopia (SIP): A prospective, cross-sectional, observational study. Lancet. (2019) 7:e1130–8. doi: 10.1016/S2214-109X(19)30220-7
46. Pugni L, Pietrasanta C, Acaia B, Merlo D, Ronchi A, Ossola MW, et al. Chorioamnionitis and neonatal outcome in preterm infants: a clinical overview. J Matern Fetal Neonat Med. (2016) 29:1525–9. doi: 10.3109/14767058.2015.1053862
47. McPherson C, Wambach JA. Prevention and treatment of respiratory distress syndrome in preterm neonates. Neonatal Netw. (2018) 37:169–77. doi: 10.1891/0730-0832.37.3.169
48. Bansal S, Arora A, Bansal S, Gupta M, Singh P. Pattern of morbidity and mortality in preterm newborns in a tertiary care teaching hospital. J Evol Med Dent Sci. (2015) 4:11976–82. doi: 10.14260/jemds/2015/1729
49. Phukan D, Ranjan M, Dwivedi L. Impact of timing of breastfeeding initiation on neonatal mortality in India. Int Breastfeed J. (2018) 13:27. doi: 10.1186/s13006-018-0162-0
50. Amin T, Nur AN. Morbidity and mortality outcome in late preterm neonates at a tertiary care hospital. J Armed Force Med College Bangladesh. (2016) 12:44–7. doi: 10.3329/jafmc.v12i1.39966
51. Desta BN, Kassa NA, Damte TD, Hordofa LO. Incidence and risk factors of neonatal mortality in Eastern Ethiopia, a prospective cohort study in Kersa health and demographic surveillance system (Kersa HDSS). Epidemiol Biostatist Public Health. (2016) 13. doi: 10.2427/11938
52. Mengesha HG, Wuneh AD, Lerebo WT, Tekle TH. Survival of neonates and predictors of their mortality in Tigray region, Northern Ethiopia: prospective cohort study. BMC Pregnancy Childbirth. (2016) 16:202. doi: 10.1186/s12884-016-0994-9
53. Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N. Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed. (2018) 103:F208–15. doi: 10.1136/archdischild-2017-312748
Keywords: Ethiopia, predictors, survival, preterm, neonates
Citation: Belay DM, Worku WZ, Wondim A, Hailemeskel HS and Bayih WA (2022) Predictors of Survival Among Preterm Neonates Admitted to Felege Hiwot Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Pediatr. 10:800300. doi: 10.3389/fped.2022.800300
Received: 22 October 2021; Accepted: 03 February 2022;
Published: 10 March 2022.
Edited by:
Hamid Y. Hassen, Mizan-Tepi University, EthiopiaReviewed by:
Liknaw Bewket Zeleke, Debre Markos University, EthiopiaZelalem Anteneh, Bahir Dar University, Ethiopia
Copyright © 2022 Belay, Worku, Wondim, Hailemeskel and Bayih. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Demeke Mesfin Belay, ZGVtZWtlbWVzZmluNjVAeWFob28uY29t
 Demeke Mesfin Belay
Demeke Mesfin Belay Workie Zemene Worku2
Workie Zemene Worku2 Amare Wondim
Amare Wondim Habtamu Shimels Hailemeskel
Habtamu Shimels Hailemeskel Wubet Alebachew Bayih
Wubet Alebachew Bayih