- 1Department of Pediatrics, Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2School of Clinical Medicine, Hangzhou Normal University, Hangzhou, China
- 3Department of Chemical Pathology, Prince of Wales Hospital, Hong Kong, Hong Kong SAR, China
Background: Parental smoking is the dominant source of passive smoke exposure in the pediatric population. The current randomized controlled trial (RCT) study aimed to evaluate the effectiveness of a multi-component smoking reduction intervention in parental smoking reduction and children's environmental tobacco smoke exposure reduction in clinical settings.
Methods: A single-blinded, 6-month randomized controlled trial recruited smoking parents (N = 210) of children who attended the pediatric wards or clinics at the Prince of Wales Hospital. Participants allocated to the intervention group (n = 105) received monthly motivational interviews on smoking reduction with emphasis on health hazards related to children's passive smoke exposure, 8-week nicotine replacement therapy, and referral to smoking cessation service if the parents preferred. The control group (n = 105) received simple verbal advice on smoking cessation. Primary outcomes were parental urine cotinine validated and self-reported ≥50% smoking reduction rates at 6 months.
Results: Smoking parents in the intervention group had significantly more biochemically validated ≥50% smoking reduction than the control: 27.1 vs. 10.0% (OR = 3.34, 95% CI: 1.16–9.62, P = 0.02). The rate of self-reported ≥50% smoking reduction was also significantly higher in the intervention group than the control: 51.9 vs. 20.2% (OR = 4.40, 95% CI: 2.38–8.12, P < 0.001). For secondary outcomes, the rate of parental self-reported smoking cessation was higher in the intervention arm: 10.5 vs. 1.0% (OR = 12.17, 95% CI: 1.54–96.07, P < 0.001), however, no differences were detected in biochemically validated cessation and changes in children's passive smoke exposure between the groups.
Conclusion: Monthly smoking reduction counseling together with nicotine replacement therapy is more effective than simple verbal cessation advice in the smoking reduction for parents of pediatric patients. However, this study did not demonstrate differences in smoking cessation or reduction in children's passive smoke exposure with a 6-month follow-up. Achievement of a smoke-free environment remains challenging.
Trial Registration: Clinicaltrials.gov, identifier: NCT03879889.
Introduction
The prevalence of environmental tobacco smoke (ETS) exposure in children is ~40% globally and in Hong Kong (1–4). ETS exposure accounts for about 1% of annual worldwide mortality, and 28% of the deaths belong to the pediatric age group (1). The impact of ETS on lung function and childhood lung diseases is well-known (5–7). Children exposed to ETS are also more likely to have a severe influenza infection, vascular disease, and exhibit higher rates of health service utilization (8–11).
Hong Kong implemented the public smoking ban policy in the year 2007. However, there are concerns that comprehensive smoke-free legislation without strong support for cessation could displace smoking into homes, increasing children's passive smoke exposure (12, 13). Reducing parental smoking remains the key to reduce children's exposure (14–17). Pediatricians and healthcare professionals have an important role in screening for the use of tobacco and ETS exposure and providing guidance and referral to reduce children's ETS exposure (18, 19). Furthermore, public awareness of ETS-related hazards remains suboptimal, especially for third-hand smoke (THS) which is the chemical residuals of tobacco smoke that cling to surfaces after the tobacco product is extinguished. It is important to deliver those relevant information to the public and particularly to smoking parents (19–21).
Encountering smoking parents during their children's healthcare visits serves as a golden opportunity to intervene (18, 19). However, there is a lack of randomized controlled trial (RCT) evidence supporting interventions for smoking parents of pediatric patients in clinical settings (15, 22). A community study demonstrated that proactive telephone counseling is an effective aid to promote smoking cessation among parents (23). Unfortunately, its effect on children's ETS exposure was not evaluated (23). Furthermore, study design using biochemical validations with a longer follow-up is recommended for ETS reduction intervention for children (22).
Importantly, unlike smokers who actively seek help for cessation, the majority of smoking parents are not prepared to quit (23). A comprehensive review suggests that reduction-to-quit interventions may be more effective when pharmacotherapy is used as an aid (24). Smoking reduction is an option to provide an intermediate step for complete cessation (16, 25, 26). Studies have shown that people who reduce their smoking are ultimately more likely to quit than people who do not (27–29). Thus, studies to investigate the effectiveness of smoking reduction intervention in parental tobacco use are needed. Moreover, emphasizing benefits to child health as an incentive may further motivate parents to change their smoking behaviors (16).
In the current RCT, a multi-component intervention was evaluated for its effectiveness on smoking reduction in parents of our pediatric patients. Our primary aims were to compare the parental urine cotinine validated and self-reported smoking reduction rates between the intervention and control groups to test the intervention's effectiveness in smoking reduction. Secondary aims were to test the intervention's effectiveness in smoking cessation and in children's ETS reduction.
Methods
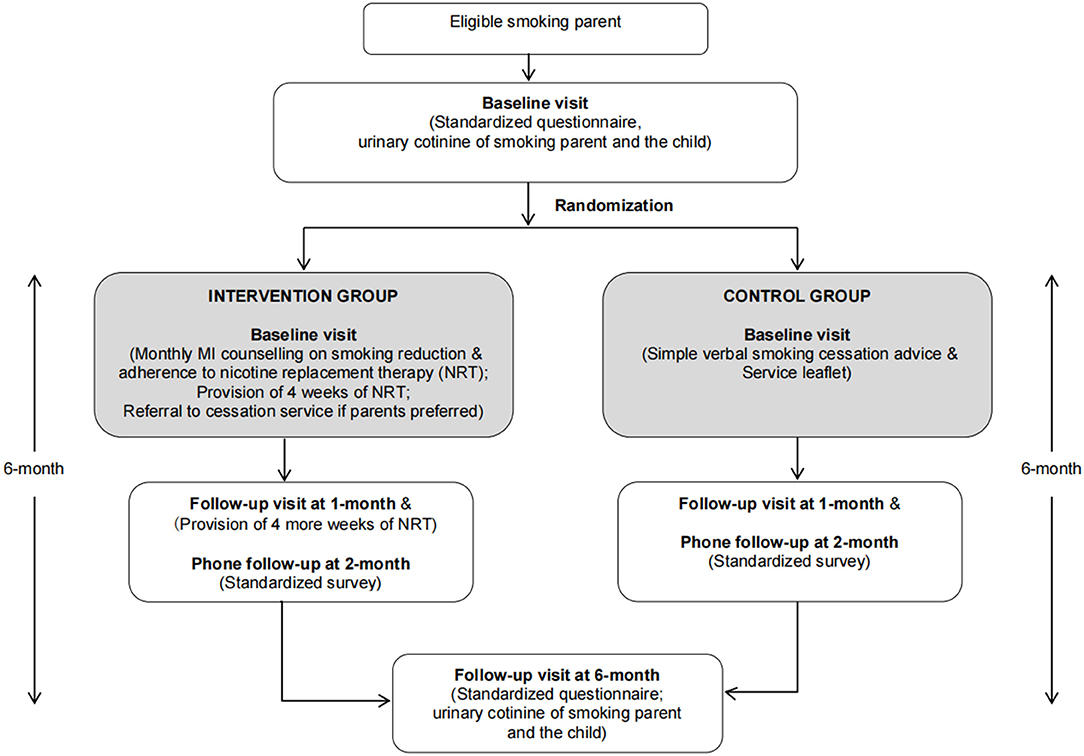
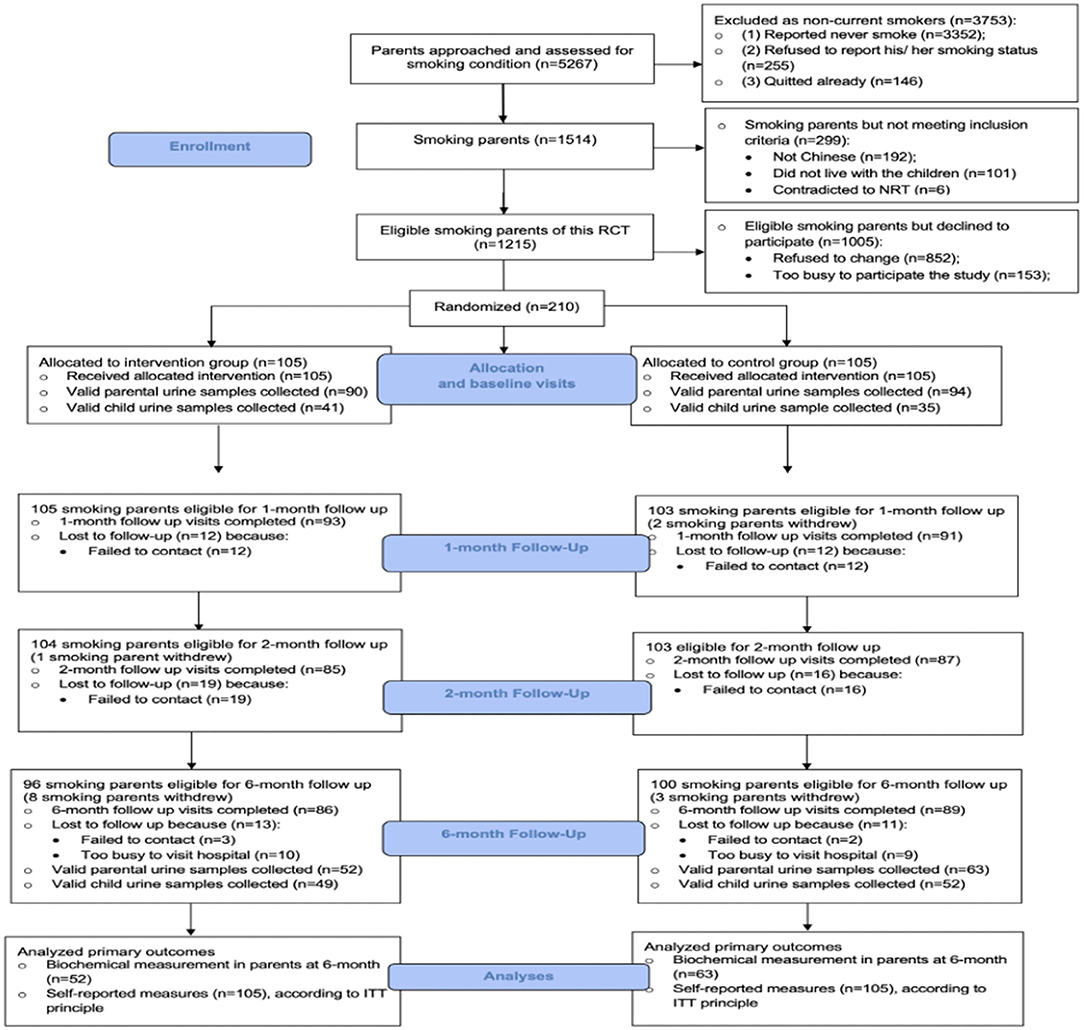
This 6-month RCT was carried out from January 2017 to July 2019. The flow diagram of the study design is shown in Figure 1. Recruited parents were randomly assigned to either intervention [monthly motivational interview (MI) counseling on smoking reduction and 8 weeks of nicotine replacement therapy (NRT)] or control group (simple verbal smoking cessation advice and service leaflet) at a 1:1 ratio. From each family, only one child (our pediatric patient) was recruited. If both parents of the family smoked, both of them were invited and randomized to the same group.
Ethics Approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Research Ethics Committee (CRE 2016.024-T). Clinical Trial Certificate was obtained from the Department of Health (Reference number: 100597). The trial was registered in clinicaltrials.gov (NCT03879889) and conducted in strict accordance with the protocol registered.
Participants
Parents of pediatric patients were approached and screened for eligibility at the pediatric clinics and in-patient wards of the Prince of Wales Hospital (PWH), the teaching hospital of The Chinese University of Hong Kong that serves a cluster population of 1.2 million. Those with smoking habit and living with a child aged <18 years who attended services at PWH were invited. Exclusion criteria were parents with children in foster care or with unclear custody, smoking patients with pediatric, parents who had a previous allergic reaction to NRT or other relative contraindications to NRT such as cardiovascular diseases and lactation. All the participating parents provided written informed consent, and we stopped the enrollment once we achieved our target sample size.
Intervention Group
The smoking reduction intervention included face-to-face counseling by trained research nurses and assistants on smoking reduction and NRT adherence, provision of free NRT, referral to cessation service if parents preferred, monthly phone follow-ups, and two in-person follow-up visits.
At recruitment, participants received a 20-min face-to-face individual smoking reduction counseling using the MI technique. In total, 4 weeks of nicotine patches were provided based on the participant's daily cigarette consumption. Parents who were not ready to quit immediately were encouraged to reduce cigarette consumption to at least half of their current consumption within the ensuing month; while for those who were ready to quit, cessation advice was given. Follow-ups were done monthly: week-2 (over phone), 1 month (follow-up clinic), 2 months (over phone), 3 months (over phone), 4 months (over phone), 5 months (over phone), and 6 months (clinic visit), for further counseling on smoking reduction and NRT adherence. Participants were invited to attend follow-up clinics at 1 month for receiving 4 more weeks of NRT, and at 6 months for trial effectiveness evaluation.
The intervention was designed based on the current evidence. MI is a patient-centered counseling that aims to explore and resolve ambivalence about behavioral change and has been widely adopted in smoking cessation and reduction research (30–32). This study counselors were trained by attending a workshop on smoking reduction counseling using the MI approach. To ensure the quality and fidelity, regular meetings were held every 2 weeks for case discussion, case evaluation, and continuing learning of MI. Feedbacks to improve counseling skills were provided and shared at the meetings, and we had written records of the interviews for later analyses and reviews.
Our MI was tailored by stages of change following the transtheoretical model. For the participants in the pre-contemplation stage, MI focused on the health hazards of tobacco use and ETS exposure in children, to help parents explore their motivations for smoking behavior change. For the contemplation stage, MI focused on helping parents to weigh the positive vs. negative ramifications of their smoking behavior and to resolve ambivalence. Guidance was provided to start developing plans for behavioral change. For the preparation stage, MI focused on the development of an action plan for smoking reduction, identification of potential barriers, and development of solutions. For action stage, MI focused on the progress follow-up of smoking reduction and adherence to NRT, identification of pitfalls, and modifications of action plan. For maintenance stage, MI focused on encouragement of the tobacco abstinence maintenance, identification of triggers to relapse, and development of resolutions.
Behavioral pharmacological interventions have been shown to be effective in reducing cigarette consumption (26, 33–35). Enhancement on NRT adherence was another key element of our intervention (36, 37). To increase the compliance, monthly phone follow-ups were implemented to reduce the number of hospital visits.
Control Group
Parents were given simple brief verbal advice on smoking cessation and an information leaflet detailing currently available smoking cessation services in the community. This approach is the usual practice in our unit when we identify smoking parents. Participants were invited to attend follow-up visits at 1 month and 6 months for evaluation.
Randomization, Blinding, and Allocation
A randomization list with block sizes of 8–12 was generated by a research staff not involved in this study. Details of the list were contained in a set of sealed, sequentially numbered envelopes. The envelopes were prepared by the same individual who generated the list and were opened by the counselors for group allocation. Each enrolled family was allocated to the next sequential number. Research personnel who performed data collection, outcome assessment, and analyses were unaware of the group allocation. However, enrolled families and counselors could not be blinded as the intervention had behavioral components, the study participants might have known whether they were in the intervention or the control group.
Data Collection
At baseline, 1-month, 2-month, and 6-month follow-ups, data were collected using standardized questionnaires. The baseline data included demographics, medical history, smoking history, cessation stages, and parental nicotine dependence levels based on the Fagerstrom Test for Nicotine Dependence (FTND) (15, 23, 34, 38). At follow-ups, the collected data were parental cigarette consumption, FTND, and whether the participants had received any additional smoking reduction/cessation treatment during the study. At baseline and 6-month visits, urine samples of the parents and children were collected.
Biochemical Validation and Outcome Measures
Urine cotinine is a valid biomarker of tobacco exposure and is used to validate self-reported measures (39). Moreover, a tobacco-specific alkaloid anabasine is used to distinguish pure NRT users from smokers: detectable cotinine but undetectable anabasine suggests that the participants took NRT only (39). Collected urine samples were stored at −20°C until analysis. Urinary samples were measured by ultra-performance liquid chromatography coupled online with the tandem mass spectrometer. The bioanalysis method has been validated according to US FDA Guidance for Industry on Bioanalytical Method Validation (2018) (40). The laboratory staff members were blinded to the group assignment. Individuals were considered as successful reducers if their urine cotinine level was ≤ 50% of their baseline level (34, 41), and successful quitters if their urine cotinine concentration was ≤ 115ug/L (34, 42).
Primary outcomes were: (1) urine cotinine validated parental ≥50% smoking reduction rate at 6 months; (2) self-reported parental successful smoking reduction rate at 6 months (self-reported ≥50% reduction of daily cigarette consumption compared with baseline). Secondary outcomes were (1) urine cotinine validated and self-reported parental smoking cessation rate at 6 months; (2) parental self-reported smoking reduction rate [(baseline–endline)/baseline] of cigarette consumption at 6 months; (3) change in children's urine cotinine level from baseline to 6 months.
The current RCT aimed to examine the effectiveness of a smoking reduction intervention, as it was reported that many smokers or smoking parents are not prepared to quit, and smoking reduction is an intermediate step for future complete cessation (23, 26). Therefore, we used ≥50% reduction of smoking as the primary outcomes and used smoking cessation outcomes as the secondary ones.
Sample Size Calculation
The sample size was calculated based on the estimated differences in the self-reported ≥50% smoking reduction between intervention and control groups. Upon the protocol design, data regarding biochemically validated smoking reduction among parental smoking were limited, while self-reported outcomes have been widely used. Therefore, we adopted the self-reported smoking reduction for the sample size calculation (15). Referring to a previous local community study that examined the effectiveness of 3-visit counseling plus 4-week NRT on smoking reduction, self-reported successful reduction was 51 and 26% for the intervention and control groups, respectively (34). The sample size required to demonstrate such a difference at a 5% level of significance with 90% power, and with an assumption of a 20% dropout rate, was 105 subjects in each arm (43).
Data Processing and Analysis
Successful smoking reduction and abstinence between groups were compared using logistic regression analyses (22, 23, 34). The comparisons between groups in the mean changes in urine cotinine levels (from baseline to 6 months), and tobacco consumption as well as FTND (from baseline to 1 month, 2 months, and 6 months) were performed using 2-way repeated measure ANOVA, while within-group differences were tested by paired Student's t-tests or the Mann–Whitney U test as appropriate. The natural log (ln)-transformations (LN) of the urine cotinine levels were performed for skewed data. In this study, there were 14 families where both parents smoked and participated, sensitivity analyses were done on one parent randomly selected from each pair.
All the analyses on self-reported outcomes were based on intention-to-treat (ITT) principle. Those lost to follow-up were counted as still smoking (unsuccessful reduction and unsuccessful cessation). For FTND scores and amount of smoking in cig/day at follow-ups, the latest available records were used if there were missing data. In addition, mixed effect models for repeated measures were adopted for fulfilling the missing data on FTND scores and amount of smoking, these additional analyses were done as sensitivity analyses. All the analyses were performed using statistical software packages SPSS (version 23.0 for Windows; SPSS Inc.). P < 0.05 were considered as significant.
Results
A total of 5,267 parents were approached and screened in our pediatric units. In total, 1,514 of them were current smokers, but 299 of those parents did not meet our inclusion criteria. Among the 1,215 eligible smoking parents, 210 smoking parents (response rate: 17.3%) consented and were randomized in this RCT study. A CONSORT diagram is shown in Figure 2.
The follow-up rates were > 80% at all evaluation time-points (1 month, 2 months, and 6 months). The retention rates were similar between the two groups. No significant adverse events occurred during the study. According to the parental self-reported results, none of them (both intervention and control groups) had received additional smoking reduction/cessation intervention outside of this study protocol during the study period.
Demographic and Clinical Characteristics
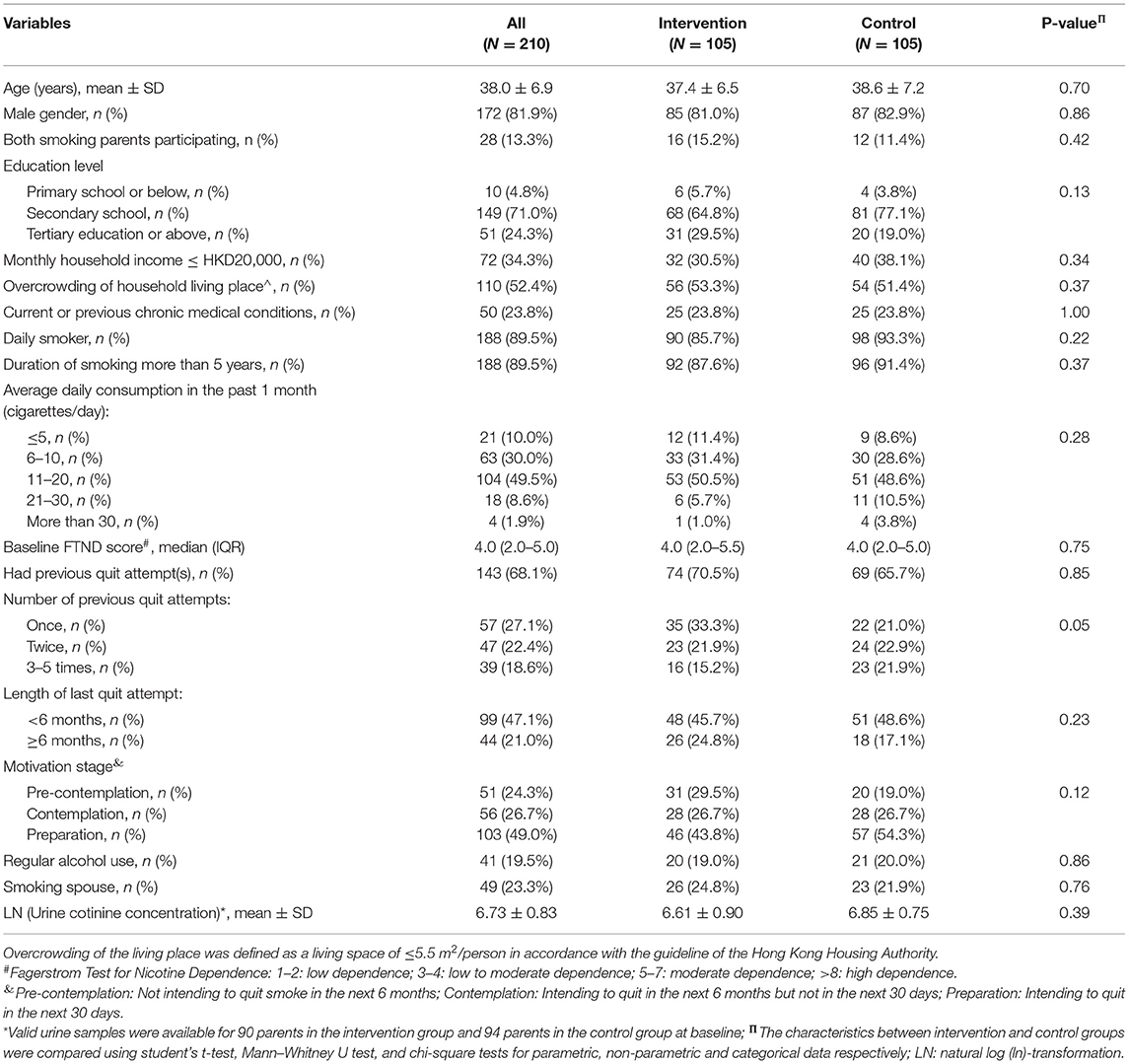
Baseline characteristics and smoking behavior of the parents are shown in Table 1. Most of the participants were middle-aged smoking fathers and were daily smokers with moderate nicotine dependence levels. The parental baseline urine cotinine concentrations were similar between groups, and the mean LN (urine cotinine concentrations) were 6.61 ± 0.90 and 6.85 ± 0.75 in the intervention and control subjects, respectively. There were no significant differences in the parental demographics and baseline smoking conditions between the two groups, except that the parents in the intervention group had a fewer number of previous quit attempts than those in the control group (P = 0.05).
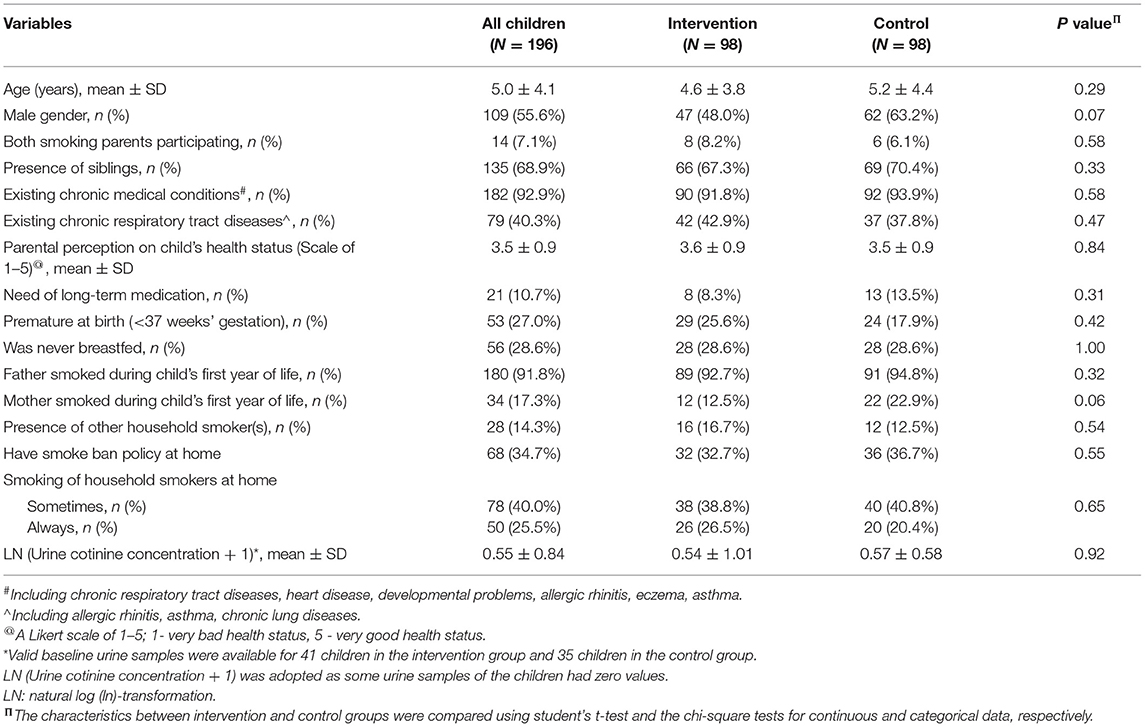
The demographic and clinical characteristics of the children are shown in Table 2. Most of them had existing chronic medical conditions. Children's baseline demographics and urine cotinine concentrations were also similar between the two groups.
Primary Outcomes
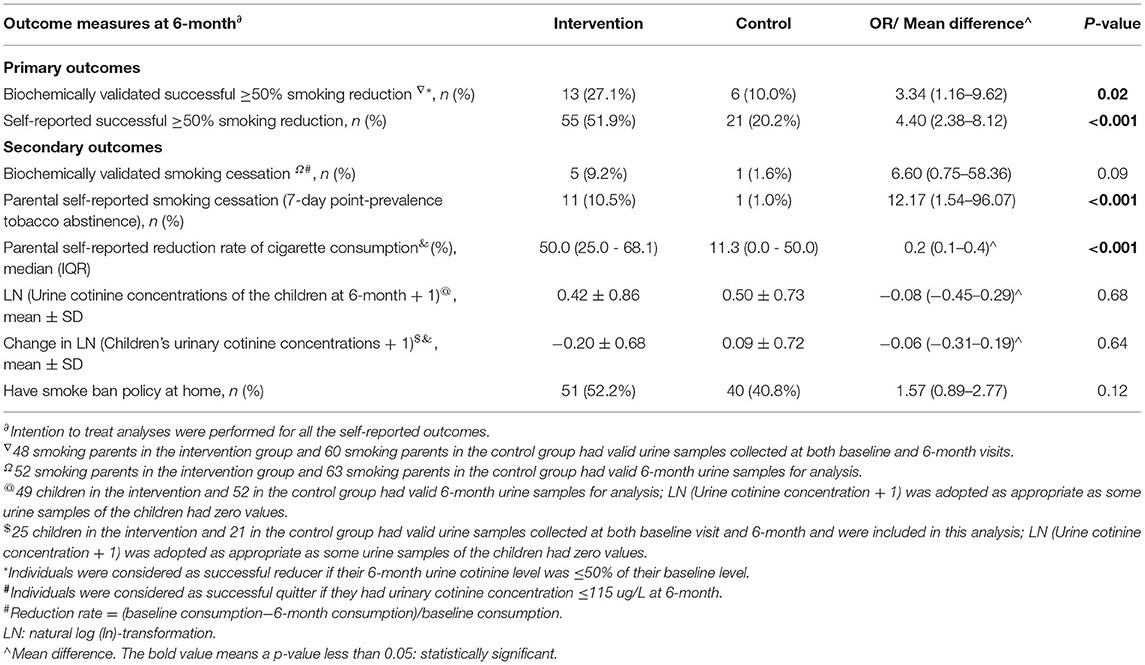
Results of urine cotinine validated outcomes are shown in Table 3. The urine cotinine validated parental successful ≥50% smoking reduction rate at 6 months in the intervention group was significantly higher than that of control: 27.1 vs. 10.0%. The intervention was shown to be effective in biochemically validated successful smoking reduction at the study end (OR = 3.34, 95% CI: 1.16–9.62, P = 0.02). The parental self-reported successful smoking reduction was also significantly higher in the intervention parents when compared with the control. Using ITT analysis, the self-reported successful smoking reduction rate at 6 months was 51.9% in the intervention group, which was significantly higher than the control of 20.2%, OR = 4.40, 95% CI: 2.38–8.12, P<0.001. The intervention was shown to be effective in smoking reduction according to our primary outcome analyses.
Secondary Outcomes
Parental self-reported rate of smoking cessation (10.5 vs. 1.0%, OR = 12.17, 95% CI: 1.54–96.07, P < 0.001) was significantly higher in the intervention group than the control (Table 3). As for urine cotinine validated smoking cessation, only 5 out of 11 parents in the intervention group and one in the control, who had self-reported successful smoking cessation, provided urine samples for validation at both baseline and 6-month visits. All these 6 parents had validated cessation status. There were no significant differences between the two groups regarding the validated smoking cessation in the univariate (P = 0.09). The parental self-reported reduction rate of daily cigarette consumption at 6 months was also significantly higher in the intervention group than the control (P < 0.001).
In children, cotinine concentrations in the majority of the samples (97.0%) were below the detection limit of 9.23 ng/L. Children in the intervention group had lower urinary cotinine concentrations than those in the control group at 6 months, but the difference did not reach statistical significance. Changes in urine cotinine concentrations from baseline to 6 months were similar in the two groups.
Other Outcome Measurements
The changes in parental cigarette consumption and FTND from baseline to each evaluation time point are shown in Tables 4, 5. Significantly greater reduction in both daily cigarette consumption and nicotine dependence level in the intervention group was documented throughout the study period.

Table 4. Parental self-reported reductions in cigarette consumption and Fagerstrom Test for Nicotine Dependence (FTND) (N = 210).
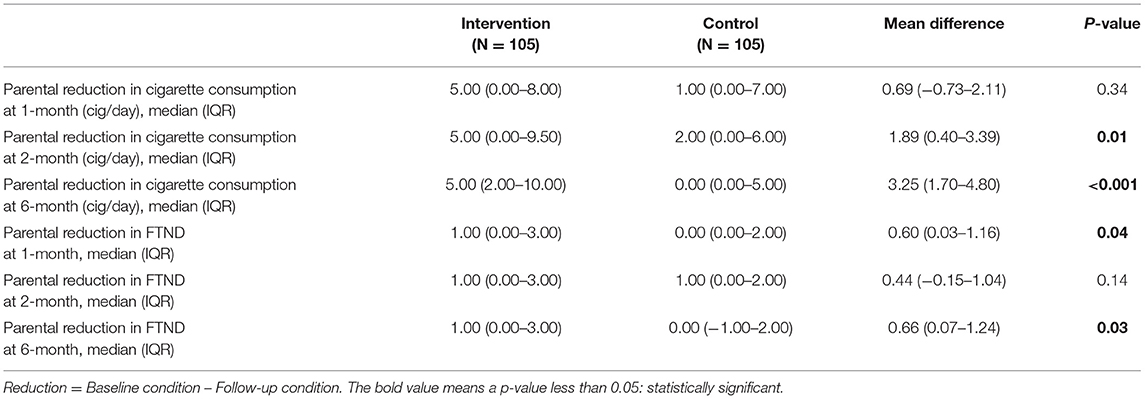
Table 5. Parental self-reported reductions in cigarette consumption and FTND (N = 210) (sensitivity analyses).
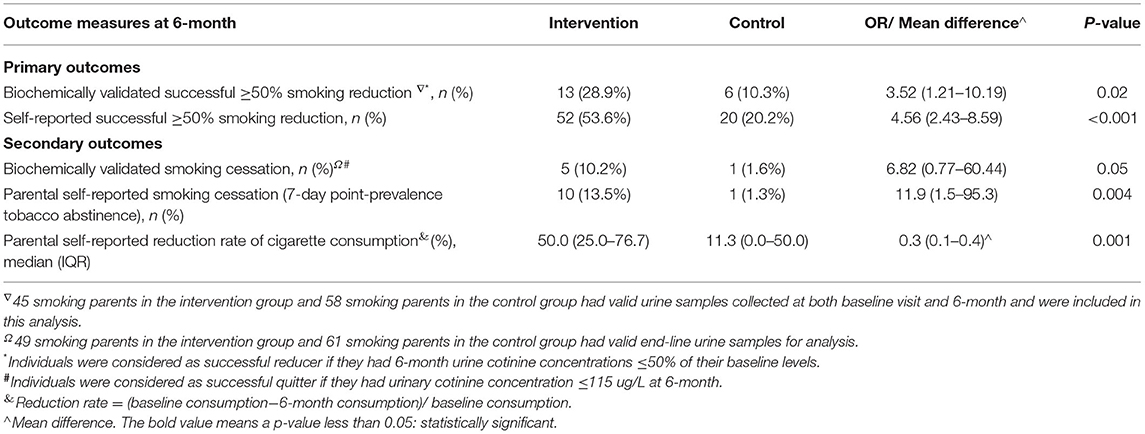
In 14 families (8 families in the intervention group and 6 families in the control group) where both smoking parents participated, sensitivity analyses on a randomly selected one yielded the same findings (Table 6).
Discussion
This study demonstrated the effectiveness of a multi-component intervention for smoking reduction in parents of patients with pediatric. One-quarter of the parents in the intervention group had biochemically validated smoking reduction and more than half had self-reported successful smoking reduction. The parental self-reported cessation rate was higher in the intervention group. However, no between-group difference was found in the urine cotinine validated cessation rate. This study has provided valuable support to the effectiveness of such intervention targeting smoking reduction for parents in clinical settings.
Both the urine cotinine validated (27.1 vs. 10.0%) and self-reported (51.9 vs. 20.2%) parental smoking reduction rates were significantly higher in the intervention group. Face-to-face individualized counseling on smoking reduction using the MI approach and free pharmacotherapy were combined in our intervention bundle. Many smokers have nicotine dependence and withdrawal symptoms are common during the cessation process (33). Pharmacotherapy helps to alleviate the symptoms and increases the chance to achieve and maintain abstinence (26, 35). A similar intervention package was adopted in a local community study for smokers who were unwilling to quit (34). The higher effectiveness in this study might be explained by the different target populations and settings that interventions are more likely to be effective when participants are recruited from healthcare settings compared to the community (33, 34). Moreover, about half of the participants recruited in this study were motivated to quit whereas the previous community study mainly recruited unmotivated smokers (34). In addition, our counseling highlighted the ETS and THS-related adverse effects on children, which might further motivate the parents to reduce tobacco use (16, 44). MI technique based on stage of change model was incorporated in our intervention (45). The technique can be easily acquired with increasing application in medical care settings (45). Counseling with a patient-centered approach encourages the participants to explore and resolve ambivalence about changing their behavior, increases self-efficacy, and helps to maintain behavioral change (30).
In this study, nearly half of the participants were in the pre-contemplation or contemplation stage at baseline, reflecting that they were not prepared to quit smoking in the next 30 days. Such barrier is common and therefore our intervention targeted reduction instead of cessation (34). An effective smoking reduction intervention is an option providing an intermediate step for complete cessation. Large (over 50%) and even moderate (25–50%) smoking reduction could increase the likelihood of future cessation (46, 47).
Nonetheless, the intervention was shown to have little impact on smoking cessation with a relatively short follow-up in this study. Although the self-reported cessation rates (10.5 vs. 1.0%) were higher in the intervention group, no statistically significant differences were found in the biochemically validated cessation rates (9.2 vs. 1.6%). Worthy to note that only less than half (5 out of 11) of the parents in the intervention group, who self-reported successful abstinence provided urine samples for analyses. According to two comprehensive reviews which aimed to explore whether smoking reduction interventions help to achieve better cessation, the average tobacco abstinence rates in the reduction intervention group was about 11%, compared with 6% in the control group (27, 28). Moreover, when compared with a previous local community study, their biochemically validated quit rates were 8.0% in the intervention group and 4.4% in the control (34). The cessation rates of our intervention group were close to the previously reported figures, while the cessation rates of the control group were much lower than expected. Smoking cessation is undoubtedly a more effective way to guarantee a smoke-free environment for children than smoking reduction. Further effort to achieve smoking cessation should be the ultimate aim of future research studies and policy development.
In this study, children's ETS exposure was validated using urine cotinine concentrations. However, the number of valid urine samples was limited as only 25.5% of the children in the intervention and 21.4% of those in the control group had valid samples collected at both baseline and the study end. The current RCT was not powered to detect the differences in children's ETS reduction between groups. Additionally, the majority of the children's urine cotinine concentrations were lower than the detection limit. Values below this level had limited accuracy and precision, casting doubt on its reliability. Children's urine cotinine concentrations in this study were relatively low when compared with other studies (48–50). One possible explanation is that most parents (87.7%) reported at the study baseline that they would keep certain distances from their children when they smoke. Further evaluation of parental smoking habits and its relation to children's ETS exposure would be needed.
Further trials with a larger sample size and longer follow-up period would be needed to evaluate the intervention's longer-term effectiveness in cessation, and its benefit on children's ETS exposure and health outcomes. The lengths of follow-up among previous similar research ranged from 1 month to 12 months, and the majority of them adopted a 6-month follow-up for their primary outcomes. Nonetheless, 6 months was relatively short and a study design that employs a longer follow-up such as 12 months would be preferred in future trials (15, 22). Being a common limitation in smoking reduction trials, our recruitment rate was moderate and was similar to previous local studies (34). This recruitment difficulty reflects that motivating smokers who are unready to change remains a major obstacle in tobacco control. For smokers who are not ready to change, smoking reduction may be an intermediate step to future cessation. However, we acknowledge the limitation of smoking reduction and in fact, smoking cessation would be the most effective way to achieve a smoke-free environment for children. Furthermore, biochemically verified smoking cessation has shown to be valid and more widely adopted outcomes than smoking reduction. Therefore, the effort to achieve parental smoking cessation would be the ultimate goal of future research. Another limitation with this study was the modest number of valid urine samples collected from smoking parents and particularly among the children subjects. Although the biochemical verification rate in this study was comparable with previous research, caution must be exercised in interpreting the biochemically validated outcomes (23, 34). In this study, the parents in the control group tended to have a higher number of previous quit attempts than those in the intervention group. However, the number of participants in each group who had previous quit attempts was similar. The exact effect of the baseline imbalance in the number of previous quit attempts on the study results was uncertain. Previous studies demonstrated that smokers who made quit attempts and failed were more likely to reduce cigarette consumption upon resumption of smoking (51). Moreover, some reported that for many smokers it could take 30 or more quit attempts before successful cessation (52, 53). There was a possibility that the parents in the control group were more likely to reduce consumption than those in the intervention group. Despite this potential limitation, this study was still able to demonstrate the effectiveness of the smoking reduction intervention.
Conclusion
To conclude, this study demonstrated the effectiveness of a multi-component intervention in reducing tobacco consumption of smoking parents. However, effectiveness was not observed in terms of smoking cessation and reduction in children's ETS exposure. Achievement of complete smoking cessation and a smoke-free environment remains a major challenge that requires further effort and research.
Data Availability Statement
The datasets used in the current study are available from the corresponding author upon reasonable request and with permission of The Chinese University of Hong Kong. However, restrictions apply and the data are not publicly available.
Ethics Statement
The studies involving human participants were reviewed and approved by the Joint Chinese University of Hong Kong-New Territories East Cluster Research Ethics Committee (CRE 2016.024-T). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SD designed the study, collected data, carried out the data analyses, drafted the initial manuscript, reviewed, and revised the manuscript. MC and RK contributed to the study design, supervised the biochemical analysis, interpreted the data, and critically reviewed the manuscript for important intellectual content. AL contributed to the study design and subject recruitment, interpreted the data, and critically reviewed the manuscript for important intellectual content. CA contributed to the study design and data analyses, interpreted the data, and critically reviewed the manuscript for important intellectual content. KC conceptualized and designed the study, recruited the subjects, supervised data collection and analyses, interpreted the data, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the study.
Funding
This study was supported by the Research Fellowship Scheme, Health and Medical Research Fund (Grant Number: 01150077) from the Food and Health Bureau, Hong Kong SAR, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the children and their parents for their participation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.798351/full#supplementary-material
References
1. Öberg M, Jaakkola MS, Woodward A, Peruga A, Prüss-Ustün A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. (2011) 377:139–46. doi: 10.1016/S0140-6736(10)61388-8
2. Leung TF, Chan IH, Liu TC, Lam CW, Wong GW. Relationship between passive smoking exposure and urinary heavy metals and lung functions in preschool children. Pediatr Pulmonol. (2013) 48:1089–97. doi: 10.1002/ppul.22801
3. Leung LT, Ho SY, Wang MP, Lo WS, Lam TH. Exposure to secondhand smoke from neighbours and respiratory symptoms in never-smoking adolescents in Hong Kong: a cross-sectional study. BMJ Open. (2015) 5:e008607. doi: 10.1136/bmjopen-2015-008607
4. Dai S, Chan KC. Household environmental tobacco smoke exposure in healthy young children in Hong Kong: prevalence and risk factors. PLoS ONE. (2020) 15:e0227733. doi: 10.1371/journal.pone.0227733
5. Jones LL, Hashim A, McKeever T, Cook DG, Britton J, Leonardi-Bee J. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res. (2011) 12:5. doi: 10.1186/1465-9921-12-5
6. Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest. (2016) 149:552–61. doi: 10.1378/chest.15-1858
7. Jayes L, Haslam PL, Gratziou CG, Powell P, Britton J, Vardavas C. SmokeHaz: systematic reviews and meta-analyses of the effects of smoking on respiratory health. Chest. (2016) 150:164–79. doi: 10.1016/j.chest.2016.03.060
8. Wilson KM, Wesgate SC, Pier J, Weis E, Love T, Evans K, et al. Secondhand smoke exposure and serum cytokine levels in healthy children. Cytokine. (2012) 60:34–7. doi: 10.1016/j.cyto.2012.06.236
9. Wilson KM, Pier JC, Wesgate SC, Cohen JM, Blumkin AK. Secondhand tobacco smoke exposure and severity of influenza in hospitalized children. J Pediatr. (2013) 162:16–21. doi: 10.1016/j.jpeds.2012.06.043
10. Raghuveer G, White DA, Hayman LL, Woo JG, Villafane J, Celermajer D, et al. Cardiovascular consequences of childhood secondhand tobacco smoke exposure: prevailing evidence, burden, and racial and socioeconomic disparities: a scientific statement from the American Heart Association. Circulation. (2016) 134:e336–59. doi: 10.1161/CIR.0000000000000443
11. Dai S, Chan KC. Associations of household environmental tobacco smoke exposure with utilisation of medical service and respiratory symptoms in healthy young children in Hong Kong. Tob Induc Dis. (2020) 18:02. doi: 10.18332/tid/114461
12. Nanninga S, Lhachimi SK, Bolte G. Impact of public smoking bans on children's exposure to tobacco smoke at home: a systematic review and meta-analysis. BMC Public Health. (2018) 18:749. doi: 10.1186/s12889-018-5679-z
13. Ho SY, Wang MP, Lo WS, Mak KK, Lai HK, Thomas GN, et al. Comprehensive smoke-free legislation and displacement of smoking into the homes of young children in Hong Kong. Tob Control. (2010) 19:129–33. doi: 10.1136/tc.2009.032003
14. Lando HA, Hipple BJ, Muramoto M, Klein JD, Prokhorov AV, Ossip DJ, et al. Tobacco is a global paediatric concern. Bull World Health Organ. (2010) 88:2 doi: 10.2471/BLT.09.069583
15. Behbod B, Sharma M, Baxi R, Roseby R, Webster P. Family and carer smoking control programmes for reducing children's exposure to environmental tobacco smoke. Cochrane Database Syst Rev. (2018) 1:CD001746. doi: 10.1002/14651858.CD001746.pub4
16. Rosen LJ, Noach MB, Winickoff JP, Hovell MF. Parental smoking cessation to protect young children: a systematic review and meta-analysis. Pediatrics. (2012) 129:141–52. doi: 10.1542/peds.2010-3209
17. Rosen LJ, Myers V, Hovell M, Zucker D, Noach MB. Meta-analysis of parental protection of children from tobacco smoke exposure. Pediatrics. (2014) 133:698–714. doi: 10.1542/peds.2013-0958
18. Farber HJ, Walley SC, Groner JA, Nelson KE. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. (2015) 136:1008–17. doi: 10.1542/peds.2015-3110
19. Drehmer JE, Walters BH, Nabi-Burza E, Winickoff JP. Guidance for the clinical management of thirdhand smoke exposure in the child health care setting. J Clin Outcomes Manag. (2017) 24:551–9.
20. Winickoff JP, Friebely J, Tanski SE, Sherrod C, Matt GE, Hovell MF, et al. Beliefs about the health effects of “thirdhand” smoke and home smoking bans. Pediatrics. (2009) 123:e74–79. doi: 10.1542/peds.2008-2184
21. Liao J, Abdullah AS, Nong G, Huang K, Lin L, Ma Z, et al. Secondhand smoke exposure assessment and counseling in the Chinese pediatric setting: a qualitative study. BMC Pediatr. (2014) 14:266. doi: 10.1186/1471-2431-14-266
22. Zhou YH, Mak YW, Ho GW. Effectiveness of interventions to reduce exposure to parental secondhand smoke at home among children in China: a systematic review. Int J Environ Res Public Health. (2019) 16:107. doi: 10.3390/ijerph16010107
23. Abdullah AS, Mak YW, Loke AY, Lam TH. Smoking cessation intervention in parents of young children: a randomised controlled trial. Addiction. (2005) 100:1731–40. doi: 10.1111/j.1360-0443.2005.01231.x
24. Lindson N, Klemperer E, Hong B, Ordóñez-Mena JM, Aveyard P. Smoking reduction interventions for smoking cessation. Cochrane Database Syst Rev. (2019) 9:CD013183. doi: 10.1002/14651858.CD006936.pub4
25. Wennike P, Danielsson T, Landfeldt B, Westin Å, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. (2003) 98:1395–402. doi: 10.1046/j.1360-0443.2003.00489.x
26. Moore D, Aveyard P, Connock M, Wang D, Fry-Smith A, Barton P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: systematic review and meta-analysis. BMJ. (2009) 338:b1024. doi: 10.1136/bmj.b1024
27. Fagerström KO. Can reduced smoking be a way for smokers not interested in quitting to actually quit? Respiration. (2005) 72:216–20. doi: 10.1159/000084057
28. Hughes JR, Carpenter MJ. Does smoking reduction increase future cessation and decrease disease risk? A qualitative review. Nicotine Tob Res. (2006) 8:739–49. doi: 10.1080/14622200600789726
29. Klemperer EM, Hughes JR. Does the magnitude of reduction in cigarettes per day predict smoking cessation? A qualitative review. Nicotine Tob Res. (2016) 18:88–92. doi: 10.1093/ntr/ntv058
30. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. (1995) 23:325–34. doi: 10.1017/S135246580001643X
31. Borrelli B, Endrighi R, Hammond SK, Dunsiger S. Smokers who are unmotivated to quit and have a child with asthma are more likely to quit with intensive motivational interviewing and repeated biomarker feedback. J Consult Clin Psychol. (2017) 85:1019. doi: 10.1037/ccp0000238
32. Lindson N, Thompson TP, Ferrey A, Lambert JD, Aveyard P. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. (2019) 7:CD006936. doi: 10.1002/14651858.CD006936.pub3
33. Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. (2016) 3:CD008286. doi: 10.1002/14651858.CD008286.pub3
34. Chan SS, Leung DY, Abdullah AS, Wong VT, Hedley AJ, Lam TH, et al. randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction. (2011) 106:1155–63. doi: 10.1111/j.1360-0443.2011.03363.x
35. Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. (2018) 113:1905–26. doi: 10.1111/add.14234
36. Lam TH, Abdullah AS, Chan SS, Hedley AJ. Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacology. (2005) 177:400–8. doi: 10.1007/s00213-004-1971-y
37. Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10 week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. (2008) 30:1852–8. doi: 10.1016/j.clinthera.2008.09.016
38. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. (1991) 86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x
39. Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, et al. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. (2002) 48:1460–71. doi: 10.1093/clinchem/48.9.1460
40. U.S. Department of Health and Human Services Food and Drug Administration. Bioanalytical Method Validation, Guidance for Industry. Center for Drug Evaluation and Research (CDER) and Center for Veterinary Medicine (CVM) (2018).
41. West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction. (2005) 100:299–303. doi: 10.1111/j.1360-0443.2004.00995.x
42. Kim S. Overview of cotinine cutoff values for smoking status classification. Int J Environ Res Public Health. (2016) 13:1236. doi: 10.3390/ijerph13121236
43. Campbell MJ, Julious SA, Altman DG. Estimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisons. BMJ. (1995) 311:1145–8. doi: 10.1136/bmj.311.7013.1145
44. Escoffery C, Bundy L, Carvalho M, Yembra D, Haardörfer R, Berg C, et al. Third-hand smoke as a potential intervention message for promoting smoke-free homes in low-income communities. Health Educ Res. (2013) 28:923–30. doi: 10.1093/her/cyt056
45. Lundahl B, Moleni T, Burke BL, Butters R, Tollefson D, Butler C, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. (2013) 93:157–68. doi: 10.1016/j.pec.2013.07.012
46. Broms U, Korhonen T, Kaprio J. Smoking reduction predicts cessation: longitudinal evidence from the Finnish adult twin cohort. Nicotine Tob Res. (2008) 10:423–7. doi: 10.1080/14622200801888988
47. Falba T, Jofre-Bonet M, Busch S, Duchovny N, Sindelar J. Reduction of quantity smoked predicts future cessation among older smokers. Addiction. (2004) 99:93–102. doi: 10.1111/j.1360-0443.2004.00574.x
48. Abdullah AS, Hua F, Khan H, Xia X, Bing Q, Tarang K, et al. Secondhand smoke exposure reduction intervention in Chinese households of young children: a randomized controlled trial. Acad Pediatr. (2015) 15:588–98. doi: 10.1016/j.acap.2015.06.008
49. Chen YT, Hsiao FH, Lee CM, Wang RH, Chen PL. Effects of a parent-child interactive program for families on reducing the exposure of school- aged children to household smoking. Nicotine Tob Res. (2016) 18:330–40. doi: 10.1093/ntr/ntv105
50. Walker N, Johnston V, Glover M, Bullen C, Trenholme A, Chang A, et al. Effect of a family-centered, secondhand smoke intervention to reduce respiratory illness in indigenous infants in Australia and New Zealand: a randomized controlled trial. Nicotine Tob Res. (2015) 17:48–57. doi: 10.1093/ntr/ntu128
51. Yong HH, Borland R, Siahpush M, Hyland A. How does a failed quit attempt among regular smokers affect their cigarette consumption? Findings from the International Tobacco Control Four-Country Survey (ITC-4). Nicotine Tob Res. (2008) 10:897–905. doi: 10.1080/14622200802023841
52. Chaiton M, Diemert L, Cohen JE, Bondy SJ, Selby P, Philipneri A, et al. Estimating the number of quit attempts it takes to quit smoking successfully in a longitudinal cohort of smokers. BMJ Open. (2016) 6:e011045. doi: 10.1136/bmjopen-2016-011045
Keywords: child, clinical settings, environmental tobacco smoke, health hazards, parental, smoke-free, smoking reduction, urine cotinine
Citation: Dai S, Chan MHM, Kam RKT, Li AM, Au CT and Chan KC (2022) Monthly Motivational Interview Counseling and Nicotine Replacement Therapy for Smoking Parents of Pediatric Patients: A Randomized Controlled Trial. Front. Pediatr. 10:798351. doi: 10.3389/fped.2022.798351
Received: 20 October 2021; Accepted: 14 March 2022;
Published: 13 April 2022.
Edited by:
Akihiro Nomura, Kanazawa University, JapanReviewed by:
Takaaki Ikeda, Yamagata University, JapanYusaku Morita, University of Occupational and Environmental Health Japan, Japan
Copyright © 2022 Dai, Chan, Kam, Li, Au and Chan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kate Ching-Ching Chan, a2F0ZWNoYW5AY3Voay5lZHUuaGs=
 Siyu Dai
Siyu Dai Michael Ho Ming Chan
Michael Ho Ming Chan Richard Kin Ting Kam
Richard Kin Ting Kam Albert Martin Li
Albert Martin Li Chun Ting Au
Chun Ting Au Kate Ching-Ching Chan
Kate Ching-Ching Chan