- 1Department of Orthopaedics, Xiangya Hospital, Central South University, Changsha, China
- 2School of Life Sciences, Central South University, Changsha, China
- 3Hunan Key Laboratory of Animal Models for Human Diseases, School of Life Sciences, Central South University, Changsha, China
- 4Hunan Key Laboratory of Medical Genetics, School of Life Sciences, Central South University, Changsha, China
Preaxial polydactyly (PPD) is a common congenital abnormality with an incidence of 0.8–1.4% in Asians, characterized by the presence of extra digit(s) on the preaxial side of the hand or foot. PPD is genetically classified into four subtypes, PPD type I–IV. Variants in six genes/loci [including GLI family zinc finger 3 (GLI3), ZPA regulatory sequence (ZRS), and pre-ZRS region] have been identified in PPD cases. Among these loci, ZRS is, perhaps, the most special and well known, but most articles only reported one or a few cases. There is a lack of reports on the ZRS-variant frequency in patients with PPD. In this study, we recruited 167 sporadic or familial cases (including 154 sporadic patients and 13 families) with PPD from Central-South China and identified four ZRS variants in four patients (2.40%, 4/167), including two novel variants (ZRS131A > T/chr7:g.156584439A > T and ZRS474C > G/chr7:g.156584096C > G) and two known variants (ZRS428T > A/chr7:g.156584142T > A and ZRS619C > T/chr7:g.156583951C > T). ZRS131A > T and ZRS428T > A were detected in PPD I cases and ZRS474C > G and ZRS619C > T combinedly acted to cause PPD II. The detectable rate of ZRS variants in PPD I was 1.60% (2/125), while PPD II was significantly higher (9.52%, 2/21). Three bilateral PPD cases harbored ZRS variants (13.64%, 3/22), suggesting that bilateral PPD was more possibly caused by genetic etiologies. This study identified two novel ZRS variants, further confirmed the association between ZRS and PPD I and reported a rare PPD II case resulted from the compound heterozygote of ZRS. This investigation preliminarily evaluated a ZRS variants rate in patients with PPD and described the general picture of PPD in Central-South China.
Introduction
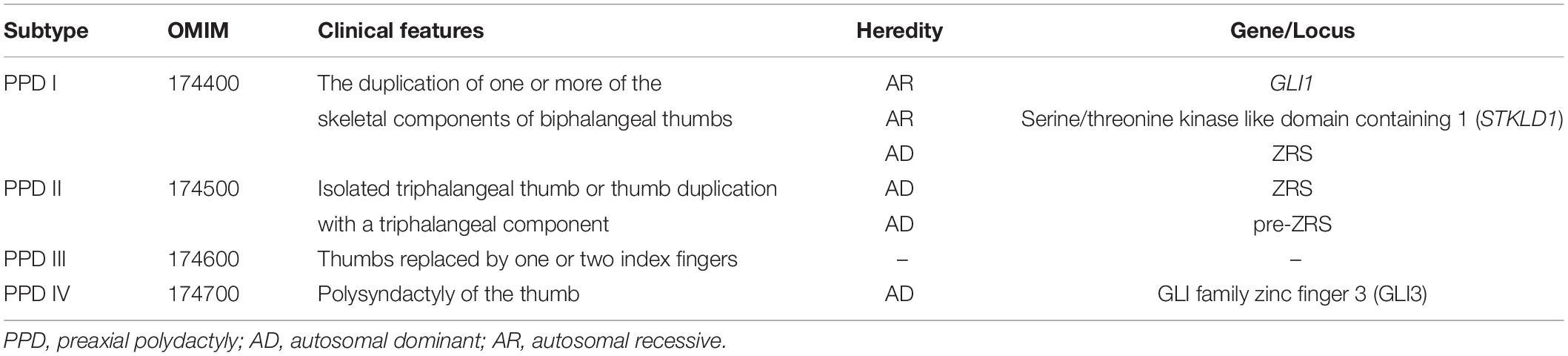
Preaxial polydactyly (PPD) is a common congenital abnormality with an incidence of 0.8–1.4% in Asians, characterized by the presence of extra digit(s) on the preaxial side of the hand or foot (1). Severity varies from mere broadening of the distal phalanx with slight bifurcation at the tip to full duplication of the thumb, including the metacarpals (2). PPD is genetically classified into four subtypes, PPD type I–IV (Table 1) (3). PPD I (OMIM 174400) indicates the duplication of one or more of the skeletal components of biphalangeal thumbs, which is the most common subtype in many populations (2). PPD II (OMIM 174500) refers to isolated triphalangeal thumbs or the thumb duplication with triphalangeal components (4). PPD III (174600) is also known as index finger polydactyly. Thumbs of PPD III cases are replaced by one or two index fingers (5). PPD IV (174700) is polysyndactyly of the thumb (6).
Currently, only six genes/loci [GLI1, GLI3, serine/threonine kinase like domain containing 1 (STKLD1), ZPA regulatory sequence (ZRS), pre-ZRS region, and a deletion of 240 kb from the sonic hedgehog signaling molecule (SHH) promoter] have been identified in isolated PPD cases and ZRS is, perhaps, the most special and well known (7–12). ZRS, the zone of polarizing activity (ZPA) (located in the posterior region of the limb bud) regulatory sequence, is a limb-specific enhancer of SHH, which is located nearly 1 Mb from SHH and within intron 5 of Limb development membrane protein 1 (LMBR1) (4). ZRS can promote the expression of SHH in ZPA during the limb development. SHH diffuses from ZPA (posterior mesoderm) to anterior region of limb bud and there is no SHH in anterior region. The graded distribution of SHH determines the finger pattern. ZRS variants would alter the expression of SHH and cause limb deformities. ZRS variants and duplications had been shown to cause PPD I, PPD II, Werner mesomelic syndrome (WMS) (OMIM 188770), and other limb deformities (such as mirror-image polydactyly and radial ray deficiency) (12–16). The correlation between PPD and ZRS is definite, but most articles only reported one or a few cases, especially in PPD II cases. There is a lack of reports on the ZRS-variant frequency in patients with PPD.
In this study, we recruited 167 sporadic or familial cases with PPD from Central-South China. We identified four ZRS variants in four PPD cases (4/167, 2.40%), including two novel variants (ZRS131A > T/chr7:g.156584439A > T and ZRS474C > G/chr7:g.156584096C > G) and two known variants (ZRS428T > A/chr7:g.156584142T > A and ZRS619C > T/chr7:g.156583951C > T). This study preliminarily investigated the ZRS variant rate in patients with PPD living in Central-South China, expanded the spectrum of ZRS variants, furthered our understanding of PPD, and contributed to genetic diagnosis and counseling of patients with PPD.
Materials and Methods
Patients and Subjects
This study was approved by the Review Board of Xiangya Hospital of Central South University. A total of 167 sporadic or familial PPD cases admitted to the Department of Orthopaedics of Xiangya Hospital were recruited. They were all from Central-South China, especially Hunan province. Almost subjects were preschoolers and informed consent forms were obtained from the patients and their guardians. All the subjects and their guardians consented to participate in this study and to publication of the images. Blood was collected from patients and their blood relations.
Deoxyribonucleic Acid Extraction
Peripheral blood samples were collected from patients and their family members to extract genomic DNA by the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, United States).
Variant Screening
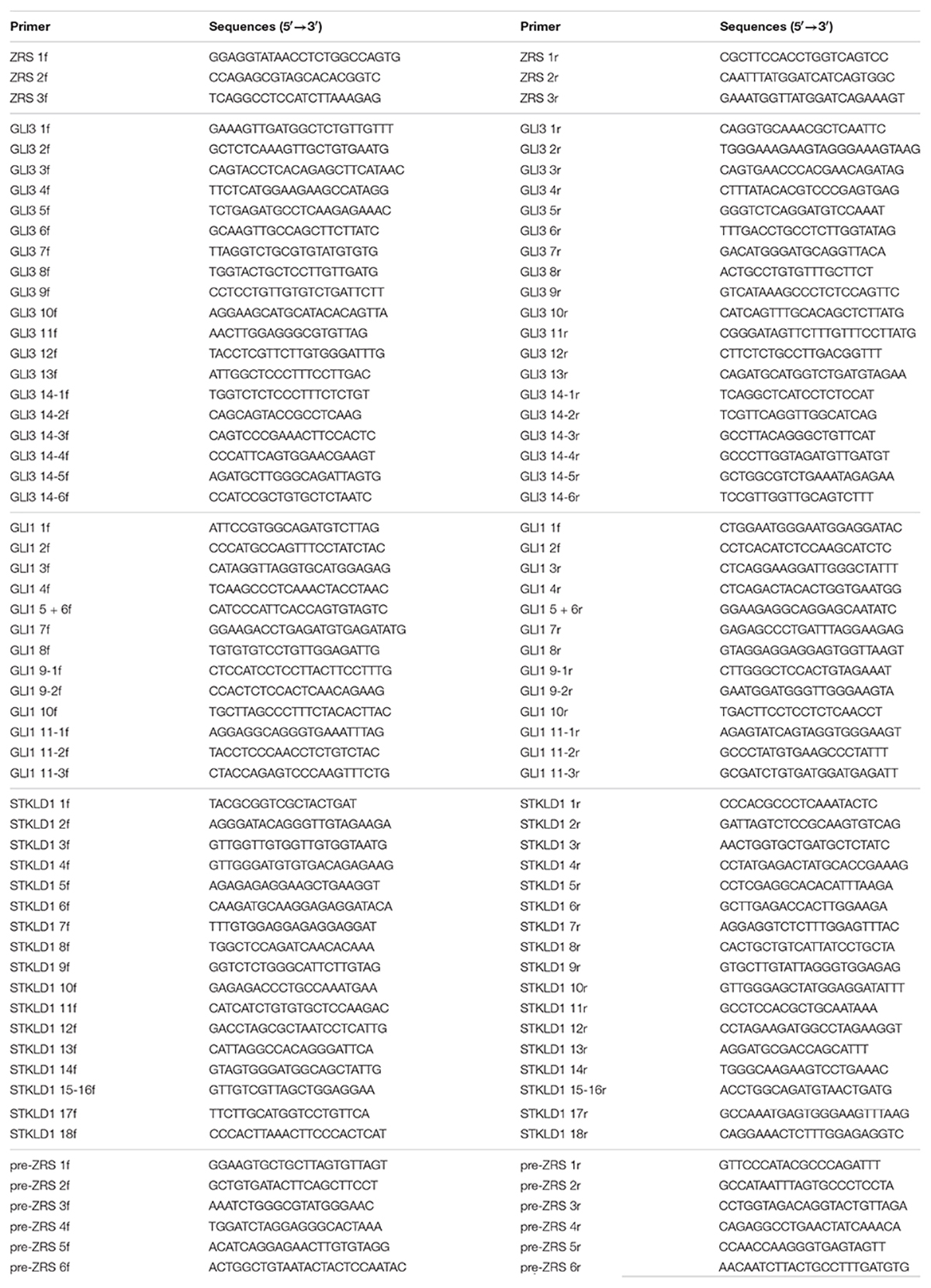
The highly conserved 774-bp region of the ZRS (chr7: 156583796-156584569, hg19) was obtained from the National Center for Biotechnology Information (NCBI) database1 and primers were designed by Integrated DNA Technologies (IDT)2 (Table 2) (17). PCR was operated to amplify the target sequences by CFX384 Touch PCR Amplifier (Bio-Rad, Hercules, CA, United States). PCR product sequences were determined using the ABI 3100 Genetic Analyzer (Thermo Fisher Scientific, Waltham, MA, United States) by Sanger sequencing performed by Boshang Biotechnology Co., Ltd. (Shanghai, China). For patients who were identified ZRS variants, further genetic screening (using PCR and Sanger sequencing) was used to detect whether they harbored pathogenic variants in GLI3 (NM_000168.6, NP_000159.3), GLI1 (NM_005269.3, NP_005260.1), STKLD1 (NM_153710.5, NP_714921.4), or pre-ZRS. Their primer pairs were also designed by IDT (Table 2).
Prediction of Pathogenicity
MutationTaster3 was applied for predicting the pathogenicity of variants. GnomAD4 was used to annotate the minimum allele frequency (MAF) of variants. ZRS sequences of species from Evgeny et al. (18) were used to compare the conservation of variant sites (18).
Results
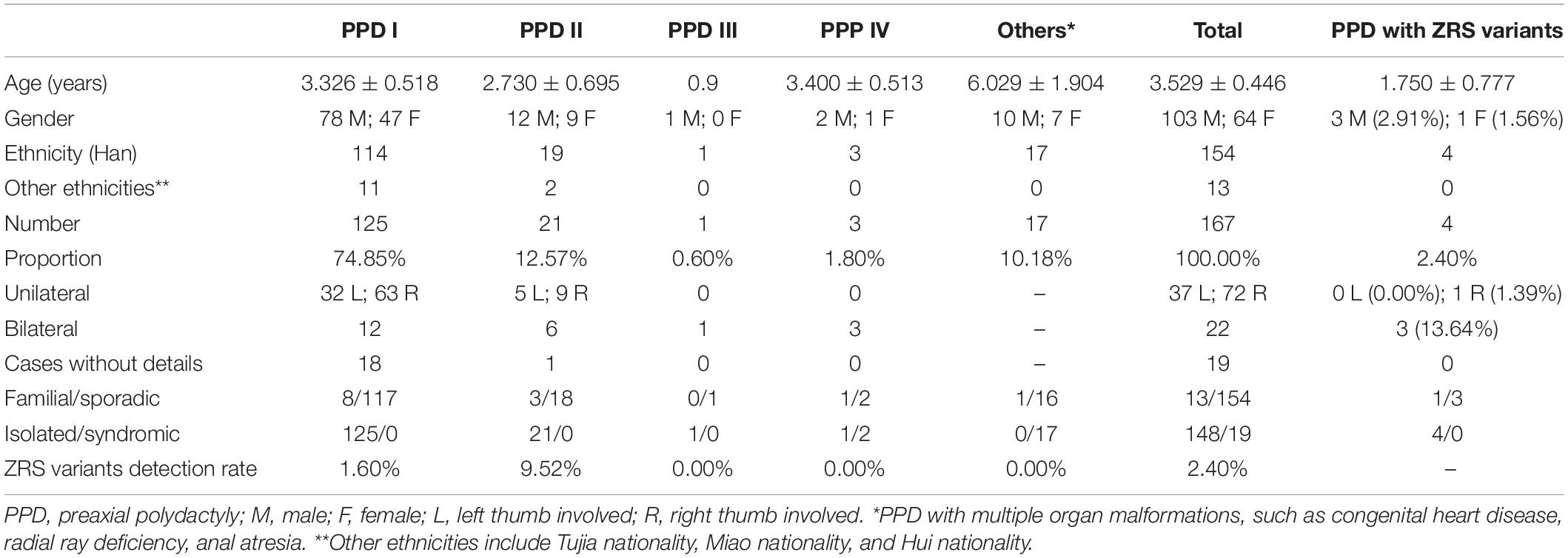
We recruited 167 cases with PPD from Central-South China, including 154 sporadic patients and 13 families, named as PPD001–PPD167 depending on the order of recruitment. Among these cases, almost subjects (154/167, 92.22%) were Han Chinese and 148 patients had isolated PPD (Table 3). Based on PPD subtypes to divide subjects, 125 patients (74.85%) revealed PPD I, 21 patients (12.57%) had PPD II, and only four cases exhibited PPD III (1/167, 0.60%) or PPD IV (3/167, 1.80%). The rest of PPD subjects (17 cases, 10.18%) presented other organ malformations, such as congenital heart disease, radial ray deficiency, and anal atresia. There were 103 male patients (61.68%) and 64 female patients (38.32%). Most subjects were younger than 3 years old. Except 19 cases without clinical details, the overwhelming majority of PPD I/II was unilateral (109/127, 85.83%), in which PPD, on the right hand, accounted for almost two-thirds (72/109, 66.06%; Table 3).
In accordance with the flow diagram (Figure 1), we identified four ZRS variants in four patients (PPD003, PPD029, PPD116, and PPD154; Table 4). The detectable rate of ZRS variants in PPD I was 1.60% (2/125), while PPD II was significantly higher (2/21, 9.52%). Three of these four patients were with bilateral thumbs involvement, occupying 13.64% of bilateral PPD (3/22). None ZRS variant was identified in patients with left PPD, although they were more than one-third total subjects (37.72%, 63/167).
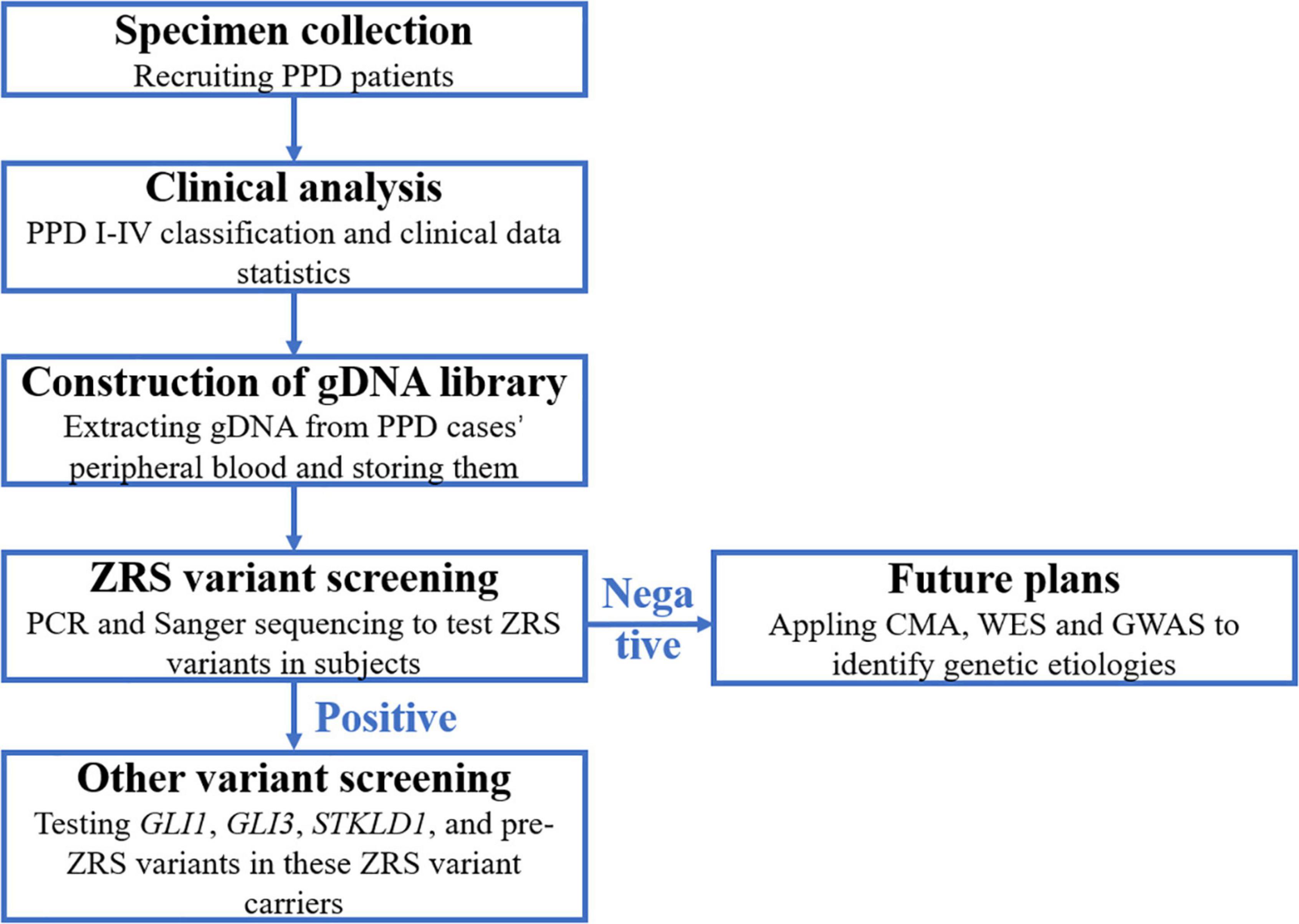
Figure 1. The flow diagram of this study. PPD, preaxial polydactyly; CMA, chromosomal microarray analysis; WES, whole-exon sequencing; GWAS, genome-wide association study.
PPD029 Family
The proband of PPD029 (III:1) was a 4-year-old girl, who presented bilateral triphalangeal thumbs (Figures 2A–C). She harbored compound heterozygous variants in ZRS (ZRS474C > G/chr7:g.156584096C > G and ZRS619C > T/chr7:g.156583951C > T) without GLI3, GLI1, STKLD1, or pre-ZRS variants (Figure 2D). ZRS474C > G was inherited from her father (II:2) and another variant was from her mother (II:3). Other family members without variants or with only one variant were unaffected.
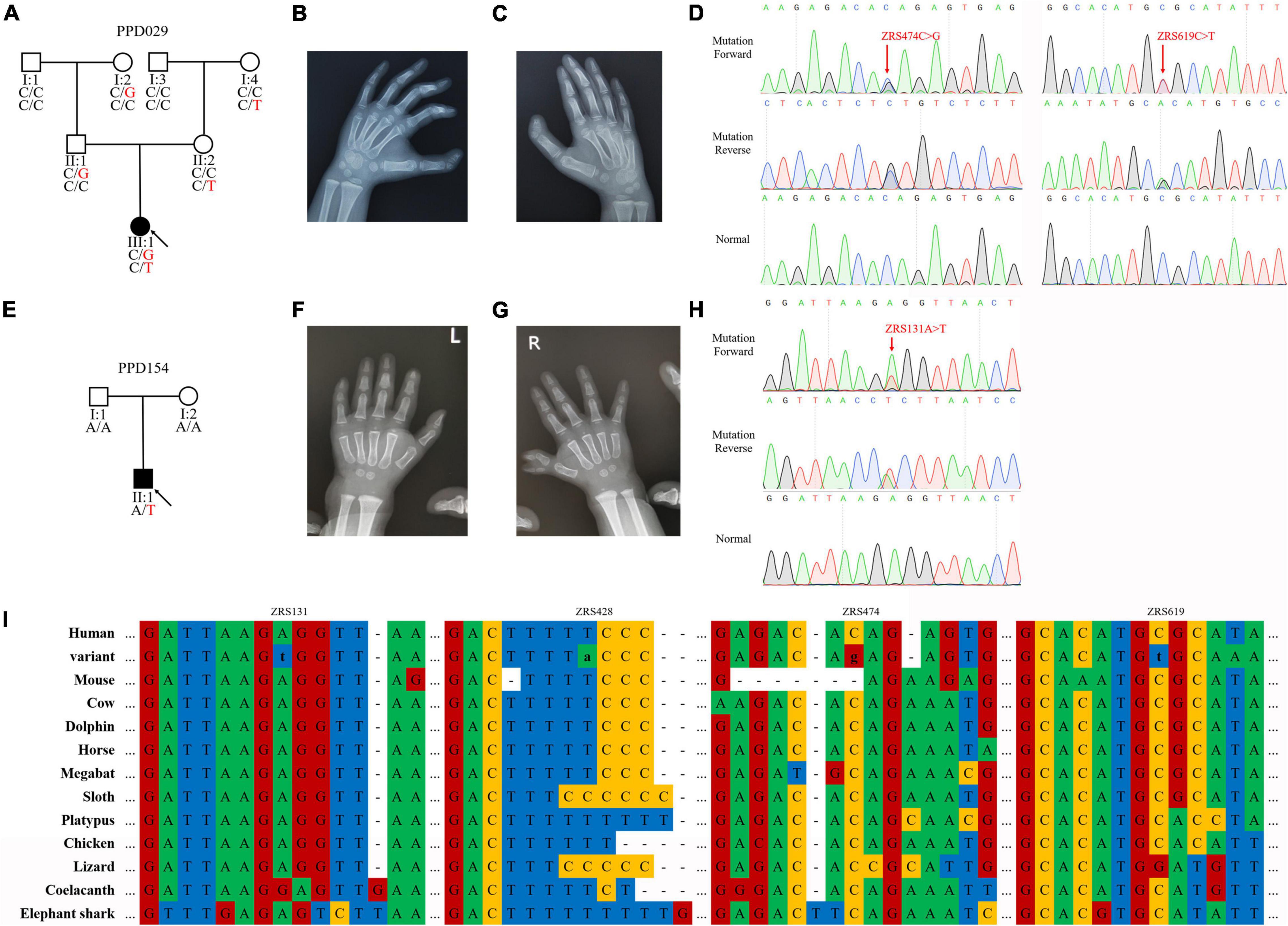
Figure 2. ZPA regulatory sequence (ZRS) variants identified in preaxial polydactyly (PPD) families. (A,E) Pedigrees of PPD families (PPD029 and PPD154). “PPD029” and “PPD154” were PPD family numbers. Squares indicate male family members and circles indicate female family members. The black symbols represent the affected members and arrows indicate probands. Genotypes are identified by letters and a slash, with red representing variants. (B,C,F,G) Symptoms of patients. PPD029 had bilateral triphalangeal thumbs and PPD154 exhibited PPD I on the right hand. (D,H) Sequencing results of ZRS variants using Sanger sequencing. (I) Species conservation analysis of mutant base sites in ZRS.
PPD154 Family
The proband of PPD154 (II:1) was a boy with PPD I on the right hand (Figures 2E–G). He was admitted to our hospital for operative treatment at his age of 1.5 years. We identified a de novo variant in ZRS (ZRS131A > T/chr7:g.156584439A > T) in this patient and did not find suspicious variants in GLI3, GLI1, STKLD1, and pre-ZRS (Figure 2H). His parents were unaffected.
PPD003 Family and PPD116 Family
Known ZRS variant (ZRS428T > A/chr7:g.156584142T > A) was identified in two families with PPD II (PDD003) or PPD I (PDD116). Four variants identified in this study were highly evolutionarily conserved and were predicted to be disease-causing by MutationTaster (Figure 2I and Table 4).
Discussion
Polydactyly is the most common limb malformation in China and PPD is over half (data from National Stocktaking Report on Birth Defect Prevention)5. PPD I is the most common subtype and PPD III is rarest (2, 19). In this study, 125 cases (74.85%, 125/167) had PPD I and only one patient (1.80%, 1/167) was diagnosed with PPD III. 17 patients with PPD (10.18%, 17/167) had other organ malformations, including congenital heart disease, radial ray deficiency, and anal atresia. These complications were relatively frequent in patients with PPD. Male patients with PPD are approximately twice as many as female (19). In this study, the proportion of male patients was 61.68% (103/167). This study showed that overwhelming majority of PPD I/II were unilateral (85.83%, 109/127), in which PPD, on the right hand, accounted for almost two-thirds (66.06%, 72/109), consistent with previous studies (19, 20).
In this study, we tested ZRS variants in 167 patients with PPD and identified unique variants (MAF ≤ 0.05) in four cases (2.40%, 4/167). The detectable rate of ZRS variants in PPD I was 1.60% (2/125), while PPD II was significantly higher (9.52%, 2/21). Indeed, most known ZRS variants are identified in PPD II cases [data from the human gene mutation database (HGMD)]6. In this study, three ZRS variants were associated with bilateral PPD and 13.64% bilateral PPD cases (3/22) harbored ZRS variants, suggesting that bilateral PPD was more possibly caused by genetic etiologies. Compared with that no ZRS variant was detected by Xiang et al. (20) in 82 Chinese patients with PPD I/II or Rao et al. (21) in 72 Chinese patients with PPD, our identification was fortunate (20, 21). For the remaining 163 cases, we planned to applied chromosomal microarray analysis (CMA), whole-exon sequencing (WES), and genome-wide association study (GWAS) to detect their genetic etiologies. Furthermore, environmental factors, such as alcohol, are causes of limb deformities (22).
Of these four variants, ZRS131A > T and ZRS474C > G were novel and ZRS428T > A and ZRS619C > T had been reported in patients with PPD II (4, 15). ZRS428T > A was identified in both the patients with PPD I (PPD116) and PPD II (PPD003), suggesting the variability of ZRS428T > A-related clinical phenotypes. ZRS131A > T was identified in a sporadic case with PPD I (PPD154). Generally, ZRS variants are associated with PPD II and ZRS was first linked with PPD I by Xu et al. (12). Our report may be the second case worldwide, further demonstrating the correlation between ZRS and PPD I.
PPD029 was a rare case. We found that the proband harbored the compound heterozygote of ZRS (ZRS474C > G and ZRS619C > T). Given that Jacob et al. (23) reported ZRS variant carriers with minor anomalies and underlined the importance of accurate clinical examination in mild triphalangeal thumb families, we carefully checked the phenotypes of her family members with one ZRS variant and did not find any limb defects (cannot completely exclude the possibility of an extremely subtle anomaly) (23). We reasoned that PPD in this family was attributed to combinedly acting by these two variants. PPD II is an autosomal dominant disease and our description indicated that PPD II individuals can be affected with a pattern of autosomal recessive inheritance. A previous study indicated that compared with a heterozygous variant in ZRS (ZRS402C > T), the homozygote led to more severe phenotypes, WMS, manifesting the superimposed effect of ZRS variants and our detection demonstrated again this phenomenon (24). ZRS619C > T had been reported by Mohammad et al. (15) in a Saudi Arabian family presented with variable preaxial deformities of the upper limbs including isolated triphalangeal thumb, PPD, preaxial syndactyly, and absent thumb and radius (15). Some family members suffered from renal agenesis and congenital heart disease. The variant (ZRS619C > T) showed obvious phenotypic heterogeneity in the Saudi Arabian family, whereas the variant was unable to alone trigger PPD in PPD029 family. It suggested that the pathogenicity of ZRS variants may be affected by ethnic difference, individual variation, and/or environmental factor.
ZPA regulatory sequence is a limb-specific enhancer of SHH, which induces the expression of SHH within ZPA (25). SHH expends from posterior mesoderm to anterior region of limb buds and lacks within the anterior-proximal. The expression gradient of SHH is crucial in establishing the number and the identity of the digits during anteroposterior patterning of the limb (26). Duplications involved ZRS or gain-of-function variants in ZRS would promote the expression of SHH in ZPA and then trigger the ectopic expression within the anterior region, where proliferation of mesenchymal cells is increased to cause PPD I/II (27). For four ZRS variants identified by us, their biological functions were not clarified and further studies needed to be performed. But, we predicted the pathogenicity of these four ZRS variants and analyzed their conservation. GnomAD showed that these variants were absent from controls or extremely rare. Thus, we highly suspected that these ZRS variants were their genetic etiologies, which should be further investigated.
Conclusion
In summary, we recruited 167 sporadic or familial cases with PPD from Central-South China and identified four ZRS variants (ZRS131A > T/chr7:g.156584439A > T, ZRS428T > A/chr7:g.156584142T > A, ZRS474C > G/chr7:g.156584096C > G, and ZRS619C > T/chr7:g.156583951C > T) in four patients with PPD (2.39%). Our description about epidemiological investigation of PPD helped us to understand the general picture of PPD in Central-South China. Our detection of two novel ZRS variants not only enrich the genetic map of PPD, but also contributed to genetic diagnosis and counseling of patients with PPD. Furthermore, we reported two patients with PPD I harboring ZRS variants further supporting the link between ZRS and PPD I and a PPD II case caused by the compound heterozygote in ZRS contributing to our understanding of PPD II and its genetic mechanism.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Review Board of Xiangya Hospital of Central South University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
LZ performed the acquisition, analysis, and interpretation of the data. J-YJ contributed to conception and design, carried out the analysis, and interpretation of the data. F-ML and YS carried out the analysis and interpretation of the data. P-FW contributed to conception and design and wrote the original draft. RX revised the draft and finally approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (2017ZX10103005-006), the National Natural Science Foundation of China (81970403, 82072194, 82170598, and 82102527), and Provincial Natural Science Foundation of Hunan (2021JJ40968).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the patients and their family members for their participation in this study and all the patient advisers for their assistance in clinical examination and blood specimen collection.
Footnotes
- ^ https://www.ncbi.nlm.nih.gov/gene/105804841
- ^ http://sg.idtdna.com/Primerquest/Home/Index
- ^ http://www.mutationtaster.org/
- ^ http://gnomad-sg.org/
- ^ https://wenku.so.com/d/8c145f3c9e9b4892c02bf7e70aa83d01
- ^ http://www.hgmd.cf.ac.uk/ac/search.php
References
1. Evanson BJ, Hosseinzadeh P, Riley SA, Burgess RC. Radial Polydactyly and the Incidence of Reoperation Using A New Classification System. J Pediatr Orthop. (2016) 36:158–60. doi: 10.1097/BPO.0000000000000395
2. Malik S. Polydactyly: phenotypes, genetics and classification. Clin Genet. (2014) 85:203–12. doi: 10.1111/cge.12276
3. Umair M, Ahmad F, Bilal M, Ahmad W, Alfadhel M. Clinical genetics of polydactyly: an updated review. Front Genet. (2018) 9:447. doi: 10.3389/fgene.2018.00447
4. Wu PF, Guo S, Fan XF, Fan LL, Jin JY, Tang JY, et al. A Novel ZRS Mutation in a Chinese Patient with Preaxial Polydactyly and Triphalangeal Thumb. Cytogenet Genome Res. (2016) 149:171–5. doi: 10.1159/000448820
5. Atasu M. Hereditary index finger polydactyly: phenotypic, radiological, dermatoglyphic, and genetic findings in a large family. J Med Genet. (1976) 13:469–76. doi: 10.1136/jmg.13.6.469
6. Al-Qattan MM, Shamseldin HE, Salih MA, Alkuraya FS. GLI3-related polydactyly: a review. Clin Genet. (2017) 92:457–66. doi: 10.1111/cge.12952
7. Fujioka H, Ariga T, Horiuchi K, Otsu M, Igawa H, Kawashima K, et al. Molecular analysis of non-syndromic preaxial polydactyly: preaxial polydactyly type-IV and preaxial polydactyly type-I. Clin Genet. (2005) 67:429–33. doi: 10.1111/j.1399-0004.2005.00431.x
8. Petit F, Jourdain AS, Holder-Espinasse M, Keren B, Andrieux J, Duterque-Coquillaud M, et al. The disruption of a novel limb cis-regulatory element of SHH is associated with autosomal dominant preaxial polydactyly-hypertrichosis. Eur J Hum Genet. (2016) 24:37–43. doi: 10.1038/ejhg.2015.53
9. Potuijt JWP, Baas M, Sukenik-Halevy R, Douben H, Nguyen P, Venter DJ, et al. A point mutation in the pre-ZRS disrupts sonic hedgehog expression in the limb bud and results in triphalangeal thumb-polysyndactyly syndrome. Genet Med. (2018) 20:1405–13. doi: 10.1038/gim.2018.18
10. Ullah A, Umair M, Majeed AI, Abdullah Jan A, Ahmad W. A novel homozygous sequence variant in GLI1 underlies first case of autosomal recessive pre-axial polydactyly. Clin Genet. (2019) 95:540–1. doi: 10.1111/cge.13495
11. Umair M, Bilal M, Ali RH, Alhaddad B, Ahmad F, Abdullah, et al. Whole-exome sequencing revealed a nonsense mutation in STKLD1 causing non-syndromic pre-axial polydactyly type A affecting only upper limb. Clin Genet. (2019) 96:134–9. doi: 10.1111/cge.13547
12. Xu C, Yang X, Zhou H, Li Y, Xing C, Zhou T, et al. A novel ZRS variant causes preaxial polydactyly type I by increased sonic hedgehog expression in the developing limb bud. Genet Med. (2020) 22:189–98. doi: 10.1038/s41436-019-0626-7
13. Lettice LA, Heaney SJ, Purdie LA, Li L, de Beer P, Oostra BA, et al. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum Mol Genet. (2003) 12:1725–35. doi: 10.1093/hmg/ddg180
14. Wieczorek D, Pawlik B, Li Y, Akarsu NA, Caliebe A, May KJ, et al. A specific mutation in the distant sonic hedgehog (SHH) cis-regulator (ZRS) causes Werner mesomelic syndrome (WMS) while complete ZRS duplications underlie Haas type polysyndactyly and preaxial polydactyly (PPD) with or without triphalangeal thumb. Hum Mutat. (2010) 31:81–9. doi: 10.1002/humu.21142
15. Al-Qattan MM, Al Abdulkareem I, Al Haidan Y, Al Balwi M. A novel mutation in the SHH long-range regulator (ZRS) is associated with preaxial polydactyly, triphalangeal thumb, and severe radial ray deficiency. Am J Med Genet A. (2012) 158A:2610–5. doi: 10.1002/ajmg.a.35584
16. Vanlerberghe C, Faivre L, Petit F, Fruchart O, Jourdain AS, Clavier F, et al. Intrafamilial variability of ZRS-associated syndrome: characterization of a mosaic ZRS mutation by pyrosequencing. Clin Genet. (2015) 88:479–83. doi: 10.1111/cge.12534
17. Gurnett CA, Bowcock AM, Dietz FR, Morcuende JA, Murray JC, Dobbs MB. Two novel point mutations in the long-range SHH enhancer in three families with triphalangeal thumb and preaxial polydactyly. Am J Med Genet A. (2007) 143A:27–32. doi: 10.1002/ajmg.a.31563
18. Kvon EZ, Kamneva OK, Melo US, Barozzi I, Osterwalder M, Mannion BJ, et al. Progressive Loss of Function in a Limb Enhancer during Snake Evolution. Cell. (2016) 167:633–642e611. doi: 10.1016/j.cell.2016.09.028
19. Xiang Y, Bian J, Wang Z, Xu Y, Fu Q. Clinical study of 459 polydactyly cases in China, 2010 to 2014. Congenit Anom. (2016) 56:226–32. doi: 10.1111/cga.12163
20. Xiang Y, Jiang L, Wang B, Xu Y, Cai H, Fu Q. Mutational screening of GLI3, SHH, preZRS, and ZRS in 102 Chinese children with nonsyndromic polydactyly. Dev Dyn. (2017) 246:392–402. doi: 10.1002/dvdy.24488
21. Rao C, Chen J, Peng Q, Mo Q, Xia X, Lu X. Mutational Screening of GLI3, SHH, and SHH ZRS in 78 Chinese Children with Nonsyndromic Polydactyly. Genet Test Mol Biomark. (2018) 22:577–81. doi: 10.1089/gtmb.2018.0096
22. Pauli RM, Feldman PF. Major limb malformations following intrauterine exposure to ethanol: two additional cases and literature review. Teratology. (1986) 33:273–80. doi: 10.1002/tera.1420330304
23. Potuijt JWP, Hoogeboom J, de Graaff E, van Nieuwenhoven CA, Galjaard RJH. Variable expression of subclinical phenotypes instead of reduced penetrance in families with mild triphalangeal thumb phenotypes. J Med Genet. (2020) 57:660–3. doi: 10.1136/jmedgenet-2019-106685
24. VanderMeer JE, Lozano R, Sun M, Xue Y, Daentl D, Jabs EW, et al. A novel ZRS mutation leads to preaxial polydactyly type 2 in a heterozygous form and Werner mesomelic syndrome in a homozygous form. Hum Mutat. (2014) 35:945–8. doi: 10.1002/humu.22581
25. Al-Qattan MM. Zone of polarizing activity regulatory sequence mutations/duplications with preaxial polydactyly and longitudinal preaxial ray deficiency in the phenotype: a review of human cases, animal models, and insights regarding the pathogenesis. Biomed Res Int. (2018) 2018:1573871. doi: 10.1155/2018/1573871
26. Tickle C, Towers M. Sonic Hedgehog Signaling in Limb Development. Front Cell Dev Biol. (2017) 5:14. doi: 10.3389/fcell.2017.00014
Keywords: ZRS, preaxial polydactyly type I, preaxial polydactyly type II, enhancer, SHH
Citation: Zeng L, Jin J-Y, Luo F-M, Sheng Y, Wu P-F and Xiang R (2022) ZPA Regulatory Sequence Variants in Chinese Patients With Preaxial Polydactyly: Genetic and Clinical Characteristics. Front. Pediatr. 10:797978. doi: 10.3389/fped.2022.797978
Received: 19 October 2021; Accepted: 29 March 2022;
Published: 16 May 2022.
Edited by:
Rong Qiang, Northwest Women’s and Children’s Hospital, ChinaReviewed by:
Sajid Malik, Quaid-i-Azam University, PakistanShuchao Pang, Jining Medical University, China
Muhammad Umair, King Abdullah International Medical Research Center (KAIMRC), Saudi Arabia
Copyright © 2022 Zeng, Jin, Luo, Sheng, Wu and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pan-Feng Wu, d3VwYW5mZW5nQGNzdS5lZHUuY24=; Rong Xiang, c2hpcmxlc21pbGVAY3N1LmVkdS5jbg==
 Lei Zeng1
Lei Zeng1 Fang-Mei Luo
Fang-Mei Luo Pan-Feng Wu
Pan-Feng Wu Rong Xiang
Rong Xiang