- 1Departments of Pediatrics, Graduate School of Medicine, University of Toyama, Toyama, Japan
- 2Biostatistics and Clinical Epidemiology, Graduate School of Medicine, University of Toyama, Toyama, Japan
- 3Department of Pediatrics, International University of Health and Welfare, Tokyo, Japan
Background: Isolated right ventricular hypoplasia (IRVH), not associated with severe pulmonary or tricuspid valve malformation, is a rare congenital myocardial disease. This study aims to evaluate the clinical status and outcome of IRVH.
Methods: A systematic search of keywords on IRVH was conducted. Studies were searched from MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Igaku Chuo Zasshi (Ichushi) published between January 1950 and August 2021.
Results: Thirty studies met the inclusion criteria. All of these studies were case reports and included 54 patients (25 males and 29 females). The median age of the patients was 2.5 years old (0–15.3 years). Of the 54 patients, 13 (24.1%) reported a family history of cardiomyopathy. Moreover, 50 (92.6%), 19 (35.2%), and 17 (31.5%) patients were diagnosed with cyanosis, finger clubbing, and dyspnea, respectively. Furthermore, 53 (98.2%) patients had a patent foramen ovale or an atrial septal defect (ASD). Z-score of the tricuspid valve diameter on echocardiogram was −2.16 ± 1.53, concomitant with small right ventricular end-diastolic volume. In addition, 29 (53.7%), 21 (38.9%), 7 (13.0%), and 2 (3.7%) patients underwent surgery, ASD closure, Glenn operation, and one and a half ventricular repair, respectively. Among them, nine (20.4%) patients expired, and the multivariable logistic regression analysis showed that infancy, heart failure, and higher right ventricular end-diastolic pressure were risk factors for death.
Conclusions: IRVH was diagnosed early in children with cyanosis and was associated with high mortality. This systematic review and pooled analysis provided evidence to assess the of IRVH degree in order to evaluate the clinical status and outcome of IRVH.
Introduction
Isolated right ventricular (RV) hypoplasia (IRVH), not associated with severe pulmonary or tricuspid valvar malformations, or ventricular septal defect (VSD), is a rare congenital myocardial disease. IRVH was first described in 1950 by Cooley et al. (1) and only limited case reports and case series have been published until recently (1–7). IRVH is also characterized by a small RV cavity, a patent foramen ovale (PFO) or an atrial septum defect (ASD), and a normal RV outflow tract concurrent with normally developed pulmonary valves (PV) (2). The clinical IRVH spectrum varies considerably from severe cyanosis, congestive heart failure, and early infant death to mild cyanosis (2, 8–14). This study aims to evaluate the clinical status and outcome of IRVH.
Materials and Methods
Eligibility
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines (Supplementary Table 1) (15). This study protocol conformed to the ethical guidelines of the Declaration of Helsinki 1964 and was approved by the Research Ethics Committee of the University of Toyama (approval no. R2021087), Toyama, Japan.
A systematic search was conducted utilizing IRVH-related keywords, regardless of the presence or absence of clinical outcomes. IRVH was defined as (1) not having pulmonary valve stenosis or pulmonary atresia, (2) not having tricuspid valve malformation, (3) not having congenital heart disease other than PFO or ASD, or (4) not having arrhythmogenic RV cardiomyopathy or Uhl's disease. The exclusion criteria were (1) studies that did not focus on IRVH, (2) articles that did not present original research (conference abstracts, editorials, or commentaries), (3) non-human studies (animal studies or in vitro experiments), (4) duplicated studies, and (5) any studies that the investigators deemed irrelevant to the objective.
Study Identification
MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials on the Ovid platform, and the Japanese literature database Igaku Chuo Zasshi (Ichushi) were searched for studies published in any language between January 1950 and August 2021. The experienced librarians at the National Center for Child Health and Development, who were also affiliated with Cochrane Japan, Tokyo, Japan, performed searches using the terms described in Supplementary Table 2.
Study Selection
Two investigators performed independent reviews of the articles. The titles and abstracts of all articles were read through during the initial screening, and articles that met the exclusion criteria were excluded. All articles were reviewed and identified for eligibility for secondary screening. A third reviewer moderated a face-to-face meeting whenever the two reviewers disagreed on an article's eligibility to determine its suitability.
Assessment of Risk of Bias in Included Studies
The following key domains were assessed following the guidance in the Cochrane Handbook (version 5.1.0) (16): (1) random sequence generation (selection bias), (2) allocation sequence concealment (selection bias), (3) blinding of participants and personnel (performance bias), (4) blinding of outcome assessment (detection bias), (5) incomplete outcome data (attrition bias), (6) selective outcome reporting (reporting bias), and (7) other biases. Two review authors (KH and SO) independently assessed the risk of bias of included studies. Disagreements were resolved by consensus. Study authors of eligible studies were contacted to resolve uncertainties and provide further data to reduce exclusion bias and minimize missing data.
Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD) or median [interquartile range (IQR)] values. Categorical variables were expressed as numbers and percentages. Continuous variables were compared using the unpaired t-test, non-parametric Mann–Whitney U-test, or one-way analysis of variance. However, categorical variables were compared using χ2 statistics or Fisher's exact test, as appropriate. Univariate regression tests were performed on all variables, and a multivariate logistic regression was performed on statistically significant variables (P < 0.05). The variables for inclusion were carefully selected, to ensure parsimony of the final models given the number of events. Statistical analyses were performed using the JMP software (version 13; SAS Institute, Cary, NC, USA). A p-value of < 0.05 was considered statistically significant.
Results
Literature Search and Characteristics of the Eligible Studies
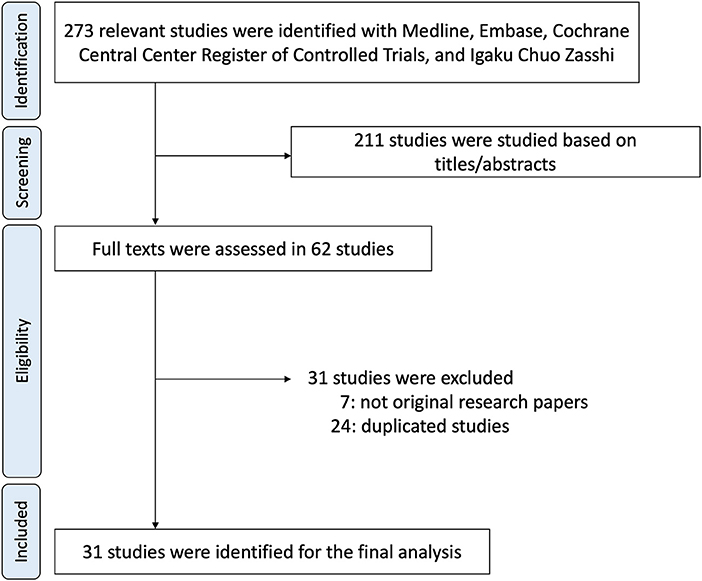
Four databases were utilized to identify 273 articles. Of the articles, 211 were excluded based on ineligibility determined by having titles and abstracts suggesting apparent ineligibility (Figure 1). Two investigators independently evaluated the entire contents of the remaining 62 articles and ultimately identified 31 articles as eligible for the study (2–8, 11–13, 17–38). All of these studies were case reports.
Risk of Bias
The risk of bias was assessed for all included studies (Supplementary Table 3).
Case Demographics
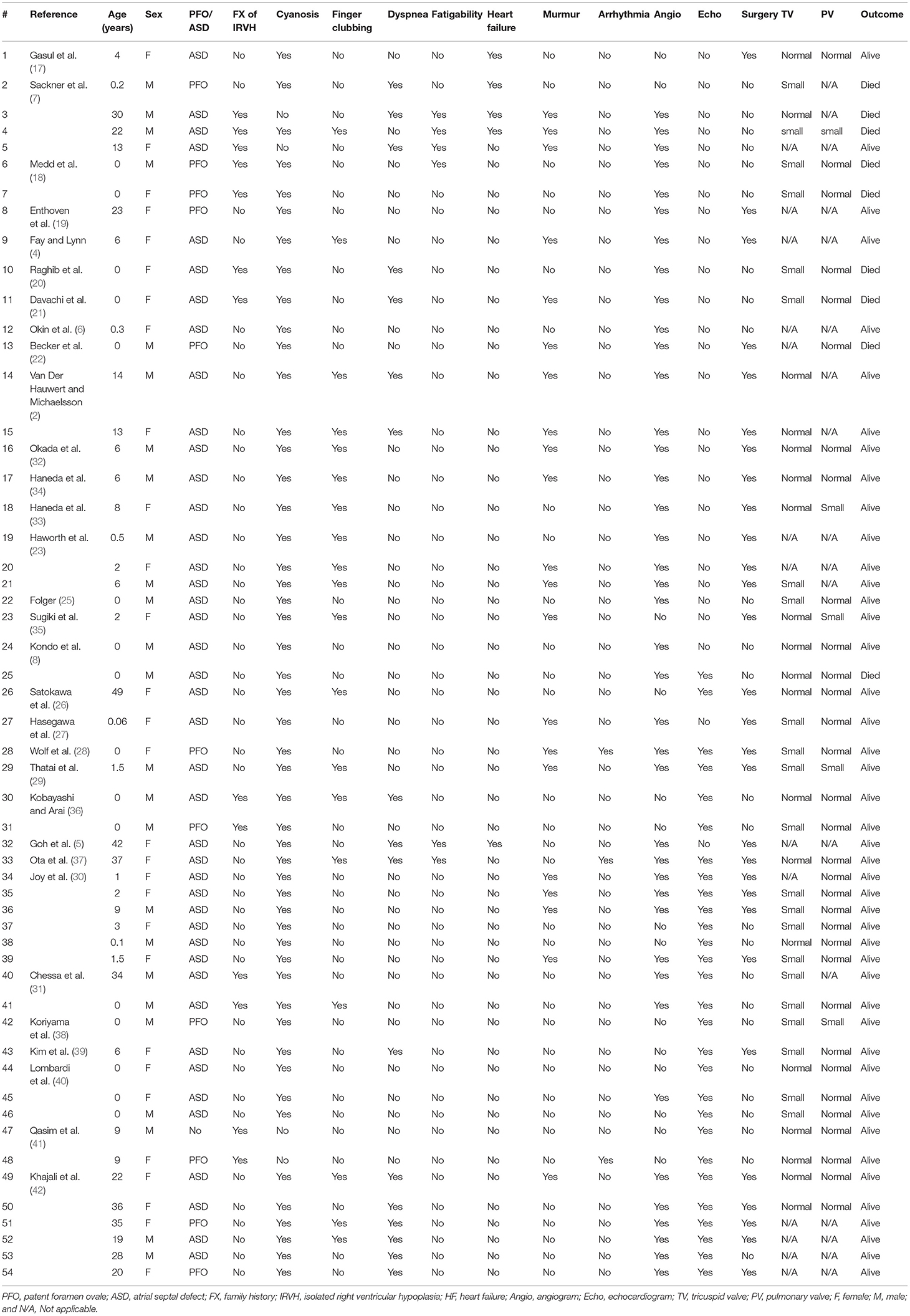
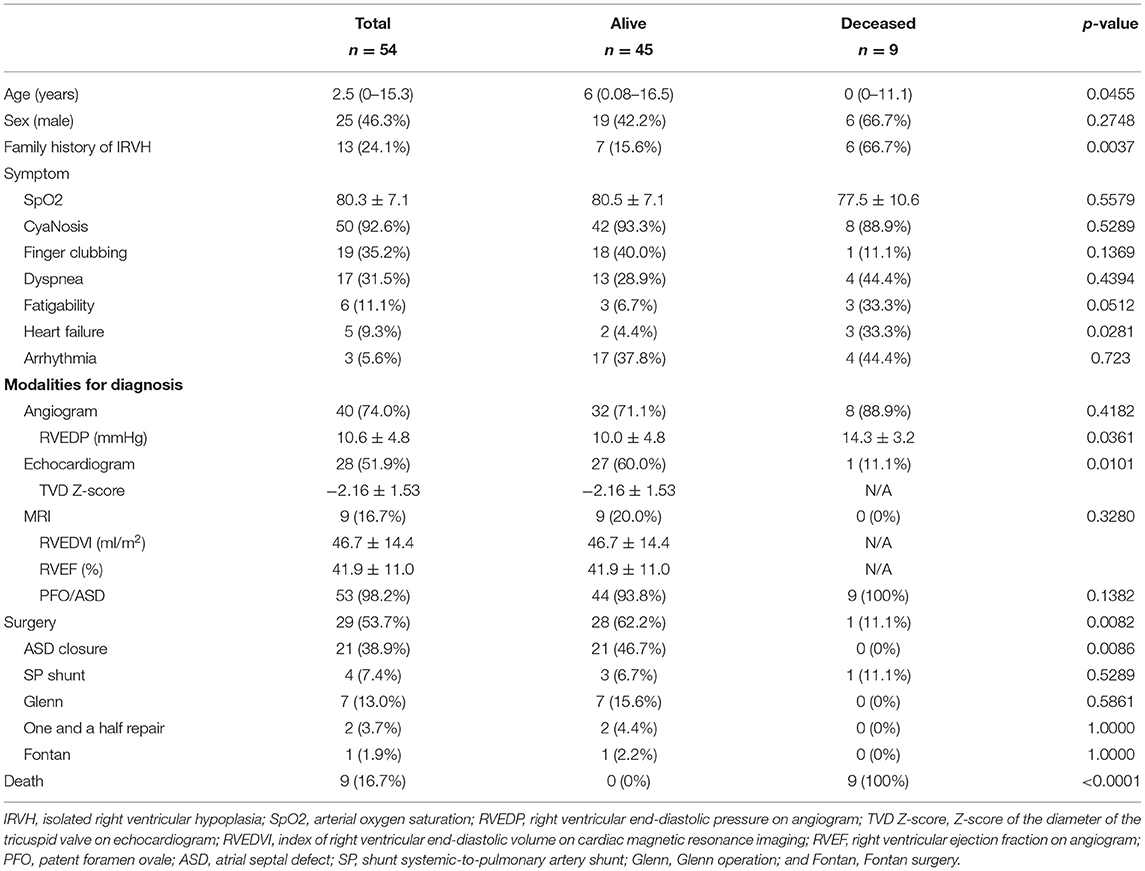
Data from 54 patients (25 males and 29 females) who had IRVH (Tables 1, 2) were obtained from the 31 included studies, all of which were case reports. The median age of the patients was 2.5 (0–15.3 years) years old. The follow-up period was 1.3 (0.3–4.3) years. Moreover, 13 (24.1%) patients reported a family history of IRVH. Furthermore, 50 (92.6%), 19 (35.2%), and 17 (31.5%) patients were diagnosed with cyanosis, finger clubbing, and dyspnea, respectively. Only three (5.6%) patients had arrhythmia.
Cardiovascular Characteristics
Of the patients, 40 (74.0%), 28 (51.9%), and nine (16.7%) were diagnosed by angiogram, echocardiogram, and magnetic resonance imaging (MRI), respectively. In addition, 11 (20.4%) and 42 (77.8%) patients had PFO and ASD, respectively. The Z-score of the diameter of the tricuspid valve on echocardiogram was−2.16 ± 1.53, concomitant with small right ventricular end-diastolic volume (RVEDV; Tables 2, 3). The right ventricular end-diastolic pressure (RVEDP) was lower in the survivors than that in the deceased (p = 0.0361).
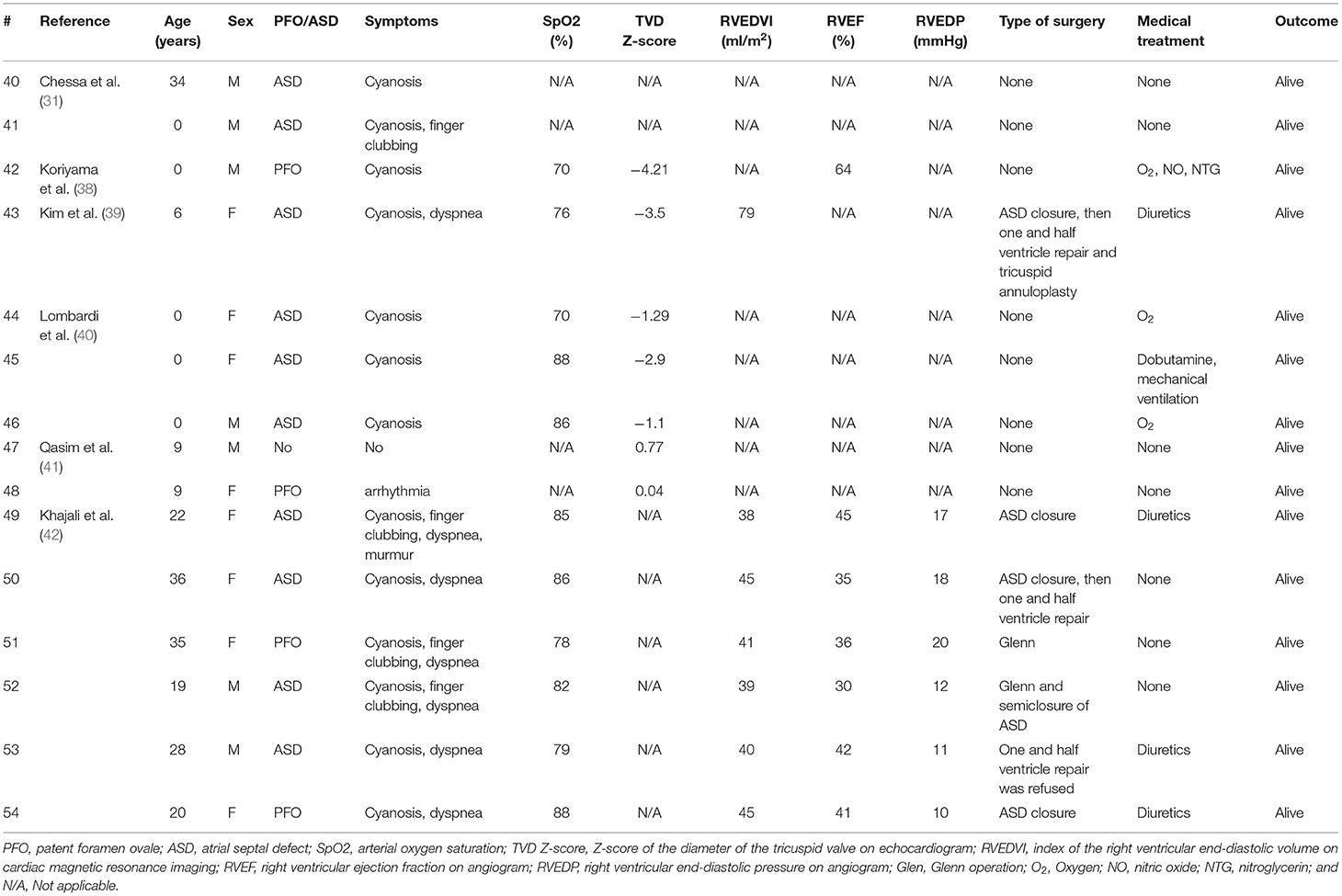
Table 3. Summary of surgical and medical management of previous studies on patients with isolated right ventricular hypoplasia (from 2000 to 2021).
Surgical Treatment
A total of 29 (53.7%) patients underwent surgery. Specifically, 21 (38.9%), 4 (7.4%), 7 (13.0%), 2 (3.7%), and 1 (5.9%) patient underwent ASD closure, systemic-to-pulmonary artery (SP) shunt placement, a Glenn operation, one and a half ventricular repair, and Fontan surgery (Tables 2, 3). Moreover, four patients underwent multiple surgeries; two patients had SP shunt twice and the other two patients had ASD closure and then one and a half ventricular repair. Arterial oxygen saturation was higher in patients who underwent ASD closure than that in patients who underwent Glenn operation or one and a half ventricular repair or the patient without surgery (Figure 2).
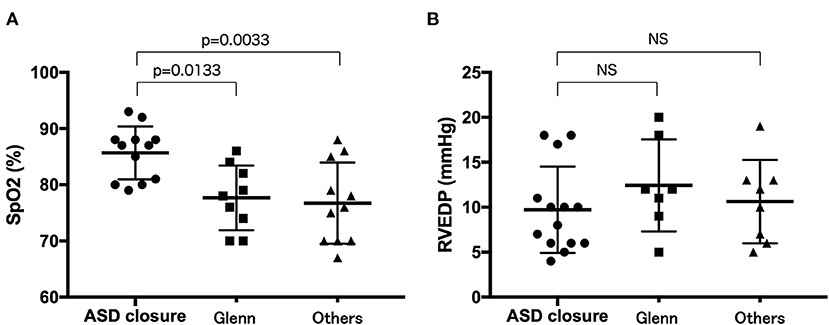
Figure 2. Comparison of arterial oxygen saturation (A) and right ventricular end-diastolic pressure (B) between the patients who underwent ASD closure, Glenn operation, or one and a half ventricular repair and those who did not underwent surgery. ASD closure patients who underwent ASD closure, Glenn patients who underwent Glenn operation or one and a half ventricular repair, others patients who did not undergo surgery.
Clinical Outcomes
Nine (16.7%) patients expired; seven and two patients were diagnosed at <1 year and in adulthood, respectively. The multivariable logistic regression analysis showed that <1 year at diagnosis, heart failure, and >12 mmHg of RVEDP were independent risk factors for death (p < 0.05, respectively; Table 4).
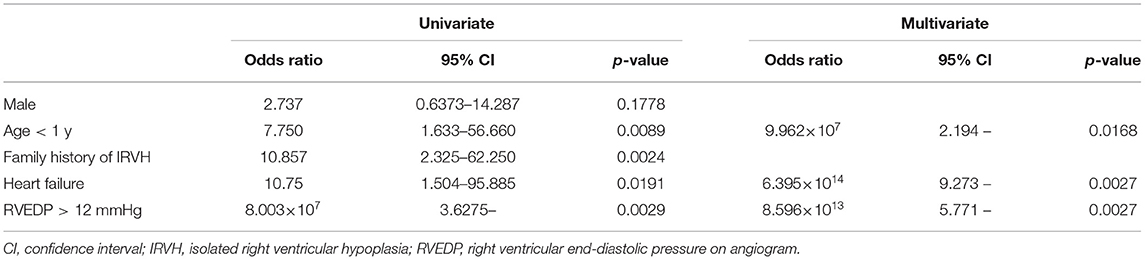
Table 4. Univariable and multivariable logistic regression analyses for independent predictors of mortality.
Discussion
This study revealed that IRVH was diagnosed early in children with cyanosis and was associated with high mortality between 1959 and 2000. It is believed that this is the first systematic review to evaluate the association between IRVH and surgery.
IRVH is characterized by trabecular musculature underdevelopment and a small RV cavity (2). An ASD or a PFO serves as an escape route causing a right-to-left shunt, resulting in cyanosis. This clinical symptom has a wide outcome spectrum, from mortality in early infancy to mild cyanosis. Congestive heart failure and deep cyanosis can appear during infancy in seven cases, whereas symptoms (e.g., mild cyanosis, dyspnea, and clubbed finger) may be found later in cases with less malformation. This may depend on hypoplasia degree and interatrial communication size.
From the systematic review and pooled analysis of the current study, IRVH was frequently observed in those patients diagnosed at <1 year. Thus, IRVH may be thought of as a primary developmental RV anomaly or that it may be due to a reduced tricuspid flow during the fetal stage (18). The resultant restrictive physiology reduces RV inflow via a detrimental feedback loop when the tricuspid size and RV compliance are decreased because of RV hypoplasia. In addition, the blood entering the RV is reduced further as the inflow of blood flowing from the right to the left atrium via a PFO or an ASD is increased.
Diagnostic tests are crucial to the differential diagnosis of IRVH from other diseases with cyanosis, and the diagnosis and evaluation of the syndrome severity were routinely performed by angiogram, echocardiography, and MRI. Cardiac catheterization can provide precise hemodynamics including RV filling and capacity quantification of the right-to-left shunts via ASD or PFO. MRI can provide comprehensive evaluations, and is widely used in the assessment of congenital heart defects. Moreover, MRI provides precise data on ventricular function and volume (43). Although the change of modalities may reflect the historical transition from angiography to echocardiography or MRI, combining multimodalities to assess IRVH hemodynamics and morphology is crucial to diagnose and make therapeutic management.
The surgical option may also vary according to the severity of RV hypoplasia from biventricular repair with ASD closure to univentricular repair with a Glenn operation and one and a half ventricular repair (2, 11, 23, 30, 33, 44). Although cyanosis was found in both the living and deceased patients, heart failure, reflecting on higher RVEDP, was observed more frequently in deceased patients, indicating that ASD closure could be undertaken in patients with mild IRVH. These data suggested that surgical options were limited for deceased patients in whom IRVH was more severe and symptomatic. Thus, right-to-left atrial communication is unnecessary for survival if RV hypoplasia is less severe, and such patients are expected to have a good prognosis. In contrast, the right-to-left atrial communication is necessary for survival if RV hypoplasia is more severe, and such patients are expected to have cyanosis and a poor prognosis, indicating Glenn operation or one and a half ventricular surgery. Thus, arterial oxygen saturation may be a predictive maker for deciding on surgery to assess the cyanosis degree.
Extrapolating from certain congenital heart anomalies with diastolic dysfunction of the right ventricle, such as severe pulmonary stenosis or atresia, certain mild to moderate forms of Ebstein's disease, in which the question arises of the feasibility of closure of the interatrial communication, in this type of pathophysiology of right to left interatrial shunt, a balloon occlusion test would be necessary before closing this communication. Percutaneous closure will follow in favorable cases.
Interestingly, the results of the current study showed that 24.1% of the patients had a family history of IRVH. Several reports have suggested that the mode of IRVH inheritance is an autosomal-dominant pattern (7, 18, 20, 21, 31, 36). Although genetic variants have not been reported, higher IRVH inheritance and positive family history is a risk factor for survival which may indicate that IRVH may be caused by a genetic disorder.
Study Limitations
Quantitative analyses were not performed in this systematic review due to the heterogeneity between studies and the limited amount of data in addition to the IRVH ambiguous definition. Any randomized controlled trials were not included because all the included studies were cohort studies and case series with small sample sizes. Therefore, we conducted a pooled analysis of these case report data to analyze factors related to life expectancy. Numerical data including RV size, tricuspid valve diameter, pulmonary valve diameter, or ASD size, could not be obtained from all the included studies. Therefore, the classification of cardiac phenotypes was subjective. This study considered >70 years, Treatments were developed that improved disease outcomes during the study period, which may have altered the results of the study. Several of the studies were retrospective and thus did no not perform long-term patient follow-up.
Conclusion
This systematic review and pooled analysis provided evidence to assess IRVH degree, and evaluate the clinical status and outcome of IRVH. Combining multiple modalities (e.g., cardiac MRI, echocardiography, and angiography) may be important in the diagnosis and treatment of each patient because the IRVH severity varies. However, the IRVH etiology has not yet been elucidated. The registry study we are conducting is ongoing. Further studies are warranted to reveal the IRVH etiology, including its genetic background and hemodynamic evaluation, which will lead to better IRVH management and treatment.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
KH was supported by grants from The Ministry of Education, Culture, Sports, Science and Technology in Japan (Grant-in-Aid for Scientific Research Nos. 18K07785 and 21K08124).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors wish to acknowledge to Hitoshi Moriuchi, Haruna Hirai and Eriko Masuda for their expert technical assistance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.794053/full#supplementary-material
Abbreviations
IRVH, Isolated right ventricular hypoplasia; HF, Heart failure; RV, Right ventricular; PV, Pulmonary valves; PFO, Patent foramen ovale; ASD, Atrium septum defect; TV, Tricuspid valve; RVEDV, Right ventricular end-diastolic volume; RVEDP, Right ventricular end-diastolic pressure.
References
1. Cooley RN, Sloan RD, Hanlon CR, Bahnson HT. Angiocardiography in congenital heart disease of cyanotic type. II observations on tricuspid stenosis or atresia with hypoplasia of the right ventricle. Radiology. (1950) 54:848–68. doi: 10.1148/54.6.848
2. Van der Hauwaert LG, Michaelsson M. Isolated right ventricular hypoplasia. Circulation. (1971) 44:466–74. doi: 10.1161/01.CIR.44.3.466
4. Fay JE, Lynn RB. Isolated right ventricular hypoplasia with atrial septal defect. Can Med Assoc J. (1963) 88:812–3.
5. Goh K, Sasajima T, Inaba M, Yamamoto H, Kawashima E, Kubo Y. Isolated right ventricular hypoplasia: intraoperative balloon occlusion test. Ann Thorac Surg. (1998) 65:551–3. doi: 10.1016/S0003-4975(97)01362-3
6. Okin JT, Vogel JH, Pryor R, Blount SG. Isolated right ventricular hypoplasia. Am J Cardiol. (1969) 24:135–40. doi: 10.1016/0002-9149(69)90060-5
7. Sackner MA, Robinson MJ, Jamison WL, Lewis DH. Isolated right ventricular hypoplasia with atrial septal defect or patent foramen ovale. Circulation. (1961) 24:1388–402. doi: 10.1161/01.CIR.24.6.1388
8. Kondo O, Ono Y, Arakaki Y, Takahashi O, Kamiya T. [Isolation right ventricular hypoplasia: report of two cases]. J Cardiol. (1989) 19:637–46.
9. Bondarev Iu I. Mal'sagov GU, Riumina EN. [Hypoplasia of the right ventricle with interatrial communication through the coronary sinus]. Kardiologiia. (1977) 17:76–9.
10. Buendia A, Munoz A, Attie F, Esquivel J, Kuri J, Munoz L, et al. [Isolated hypoplasia of the right ventricle]. Arch Inst Cardiol Mex. (1981) 51:471–9.
11. Cabrera A, Lekuona I, Galdeano JM, Lombardero JL, Pastor E, Vazquez C, et al. [Isolated hypoplasia of the right ventricle. study of 3 cases]. An Esp Pediatr. (1985) 23:281–6.
12. Branco LM, Goncalves JM, Velho HV, Ferreira MG, Oliveira JA, Agapito AF, et al. [Isolated hypoplasia of the right ventricle–apropos of a case]. Rev Port Cardiol. (1989) 8:791–4.
13. Amaral FT, Moreira-Neto FF, Sgarbieri RN, Carvalho SR, Haddad JL. [Congenital isolated hypoplasia of the right ventricle]. Arq Bras Cardiol. (1996) 66:277–9.
14. Hirono K, Miyao N, Yoshinaga M, Nishihara E, Yasuda K, Tateno S, et al. A significance of school screening electrocardiogram in the patients with ventricular noncompaction. Heart Vessels. (2020) 35:985–95. doi: 10.1007/s00380-020-01571-7
15. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. (2009) 6:e1000097. oi: 10.1371/journal.pmed.1000097
16. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
17. Gasul BM, Weinberg M, Luan LL, Fell EH, Bicoff J, Steiger Z. Superior vena cava-right main pulmonary artery anastomosis: surgical correction for patients with Ebstein's anomaly and for congenital hypoplastic right ventricle. J Am Med Assoc. (1959) 171:1797–803. doi: 10.1001/jama.1959.03010310029008
18. Medd WE, Neufeld HN, Weidman WH, Edwards JE. Isolated hypoplasia of the right ventricle and tricuspid valve in siblings. Br Heart J. (1961) 23:25–30. doi: 10.1136/hrt.23.1.25
19. Enthoven R, Dunst R, Benjamin R. Congenital hypoplasia of the right ventricule and tricuspid valve with survival into adult life. Am J Cardiol. (1963) 11:5. doi: 10.1016/0002-9149(63)90016-X
20. Raghib G, Amplatz K, Moller JH, Jue KL, Edwards JE. Hypoplasia of right ventricle and of tricuspid valve. Am Heart J. (1965) 70:7. doi: 10.1016/0002-8703(65)90337-6
21. Davachi F, McLean RH, Moller JH, Edwards JE. Hypoplasia of the right ventricle and tricuspid valve in siblings. J Pediatr. (1967) 71:869–74. doi: 10.1016/S0022-3476(67)80014-3
22. Becker AE, Becker MJ, Moller JH, Edwards JE. Hypoplasia of right ventricle and tricuspid valve in three siblings. Chest. (1971) 60:273–7. doi: 10.1378/chest.60.3.273
23. Haworth SG, Shinebourne EA, Miller GA. Right-to-left interatrial shunting with normal right ventricular pressure. A puzzling haemodynamic picture associated with some rare congenital malformations of the right ventricle and tricuspid valve. Br Heart J. (1975) 37:386–91. doi: 10.1136/hrt.37.4.386
24. Guerin F, Hazan E, Herreman F, Toussaint M. [Hypoplasia of the right ventricle with inter-atrial communication and without any other abnormality. apropos of a case treated surgically by closure of the inter-atrial communication]. Arch Mal Coeur Vaiss. (1977) 70:653–61.
25. Folger GM. Solitary hypoplasia of the right ventricle: a case report. Angiology. (1985) 36:646–9. doi: 10.1177/000331978503600909
26. Satokawa H, Iwaya F, Igari T, Hoshino S, Yamaguchi N, Maruyama Y, et al. case of right atrial thrombosis associated with isolated right ventricular hypoplasia. Fukushima J Med Sci. (1990) 36:91–5.
27. Hasegawa N, Sekiguchi A, Nagata N, Ookawa Y, Ito K, Miyazawa Y. [A case report: successful direct closure of atrial septal defect in isolated right ventricular hypoplasia]. Kyobu Geka. (1992) 45:179–82.
28. De Wolf D, Naeff MS, Losekoot G. Right ventricular hypoplasia: outcome after conservative perinatal management. Acta Cardiol. (1994) 49:267–73.
29. Thatai D, Kothari SS, Wasir HS. Right to left shunting in atrial septal defect due to isolated right ventricular hypoplasia. Indian Heart J. (1994) 46:177–8.
30. Joy MV, Venugopalan P, Sapru A, Subramanyan R. Isolated hypoplasia of right ventricle with atrial septal defect: a rare form of cyanotic heart disease. Indian Heart J. (1999) 51:440–3.
31. Chessa M, Redaelli S, Masszi G, Iascone M, Carminati M. Familial occurrence of isolated right ventricular hypoplasia. Am J Med Genet. (2000) 90:356–7. doi: 10.1002/(SICI)1096-8628(20000228)90:5<356::AID-AJMG2>3.0.CO;2-C
32. Okada Y, Horiuchi T, Ishitoya T, Ishizawa H. [Case of isolated right ventricular hypoplasia]. Kyobu Geka. (1972) 25:291–3.
33. Haneda K, Akino Y, Sato N, Horiuchi T. [A case report of isolated right ventricular hypoplasia: hemodynamic evaluation of 11 years following ASD closure]. Nihon Kyobu Geka Gakkai Zasshi. (1988) 36:2678–81.
34. Haneda K, Yamaki S, Kagawa K, Shibota M, Horiuchi T. Isolated right ventricular hypoplasia. Shinzo. (1974) 6:8.
35. Sugiki K, Izumiyama O, Tsukamoto M, Abe T, Komatsu S. [A successful surgical correction of isolated right ventricular hypoplasia with a large atrial septal defect after 3 conservative shunt operations]. Nihon Kyobu Geka Gakkai Zasshi. (1985) 33:2128–32.
36. Kobayashi T, Arai K. A familial case of isolated right ventricular hypoplasia; fetal echo cardiographic diagnosis. Nihon Syoni Junkanki Gakkai Zasshi. (1994) 10:6.
37. Ota H, Saijo Y, Saitou S, Yoshida A, Ido A, Ishii Y, et al. An adult case of isolated right ventricular hypoplasia. Shinzo. (1998) 30:6.
38. Koriyama T, Ehara E, Sugimito H, Ito Y, Shisida N, Miyagi N, et al. Syonika. Rinsho. (2000) 53:6.
39. Kim H, Park EA, Lee W, Chung JW, Park JH, Kim GB, et al. Magnetic resonance imaging findings of isolated right ventricular hypoplasia. Int J Cardiovasc Imaging. (2012) 28:149–52. doi: 10.1007/s10554-012-0162-x
40. Lombardi M, Tagliente MR, Pirolo T, Massari E, Vairo U. Transient and anatomic isolated right-ventricular hypoplasia. J Cardiovasc Med. (2016) 17:e257–63. doi: 10.2459/JCM.0b013e328364dc3b
41. Qasim A, Dasgupta S, Aly AM. Asymptomatic right ventricular hypoplasia in twin siblings: a normal variant or cause of early mortality? Case Rep Pediatr. (2019) 2019:6871340. doi: 10.1155/2019/6871340
42. Khajali Z, Arabian M, Aliramezany M. Best management in isolated right ventricular hypoplasia with septal defects in adults. J Cardiovasc Thorac Res. (2020) 12:237–43. doi: 10.34172/jcvtr.2020.36
43. Abouzeid CM, Shah T, Johri A, Weinsaft JW, Kim J. Multimodality imaging of the right ventricle. Curr Treat Options Cardiovasc Med. (2017) 19:82. doi: 10.1007/s11936-017-0584-9
Keywords: right ventricular hypoplasia, heart failure, cyanosis, patent foramen ovale, tricuspid valve
Citation: Hirono K, Origasa H, Tsuboi K, Takarada S, Oguri M, Okabe M, Miyao N, Nakaoka H, Ibuki K, Ozawa S and Ichida F (2022) Clinical Status and Outcome of Isolated Right Ventricular Hypoplasia: A Systematic Review and Pooled Analysis of Case Reports. Front. Pediatr. 10:794053. doi: 10.3389/fped.2022.794053
Received: 13 October 2021; Accepted: 14 March 2022;
Published: 21 April 2022.
Edited by:
Ruth Heying, University Hospital Leuven, BelgiumReviewed by:
Danny Eytan, Technion Israel Institute of Technology, IsraelZakhia Saliba, Hôtel-Dieu de France, Lebanon
Copyright © 2022 Hirono, Origasa, Tsuboi, Takarada, Oguri, Okabe, Miyao, Nakaoka, Ibuki, Ozawa and Ichida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keiichi Hirono, a2hpcm9ubzE5NzNAZ21haWwuY29t
 Keiichi Hirono
Keiichi Hirono Hideki Origasa2
Hideki Origasa2 Kaori Tsuboi
Kaori Tsuboi Masato Oguri
Masato Oguri Keijiro Ibuki
Keijiro Ibuki