- 1Department of Epidemiology and Health Statistics, Xiangya School of Public Health, Central South University, Changsha, China
- 2National Health Committee (NHC) Key Laboratory of Birth Defect for Research and Prevention, Hunan Provincial Maternal and Child Health Care Hospital, Changsha, China
- 3Hunan Provincial Key Laboratory of Clinical Epidemiology, Changsha, China
- 4Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
Objective: To estimate the association of selected maternal and fetal characteristics with the risk of perinatal mortality in South China.
Methods: A prospective cohort study was conducted from March 2013 to December 2019. The exposures of interest were maternal sociodemographic characteristics, lifestyle and habits during early pregnancy, and complications of pregnancy. Their effects on the development of perinatal death were analyzed in our study.
Results: A total of 44,048 eligible pregnant women were included in the analysis. Of these, 596 fetuses were perinatal deaths (perinatal mortality was 13.5 per 1,000 births). After adjustment, maternal obesity, being employed, history of gestational hypertension, taking antidepressants during early pregnancy, history of gestational diabetes mellitus, gestational diabetes mellitus, infertility drug treatment and assisted reproductive techniques, history of neonatal death, preterm birth, and congenital malformations all significantly increased the risk of perinatal death. Ethnic minority, income > 5,000, multiparous women, and cesarean section associated with reduced risk of perinatal death.
Conclusion: Some factors of maternal sociodemographic characteristics, abnormal pregnancy history, lifestyle and habits during early pregnancy, and complications of pregnancy were associated with the risk of perinatal death.
Introduction
Perinatal death, defined as a composite of stillbirths (fetal death at or after 28 weeks of gestation) and early neonatal deaths (neonatal death within 7 days of live birth), remains a major health problem globally (1, 2). It is estimated that there were 1.8 million early neonatal deaths and 2 million stillbirths in 2019 (3). The perinatal death rate has declined over the past 50 years (4). Although the stillbirth rate has been relatively stable, the rate of stillbirth is 10 times higher in developing countries compared to developed countries (5). Although neonatal deaths have been steadily decreasing, increased efforts to improve progress are still needed. It is estimated that 27.8 million neonatal deaths will occur from 2018 to 2030 if there are no decrements in neonatal mortality (6). Ninety-eight percent of globally reported perinatal deaths occur in developing countries (7), and there is a need to focus on reducing perinatal mortality in developing countries. The rate of stillbirth dropped by 4–6% from 2000 to 2015 in China. However, the rate of neonatal death is consistently higher than the rate of under-five death, and the decrement of stillbirth rate is slower than that of other adverse maternal and infant outcomes (8).
The effects of perinatal death are so devastating for mothers and their families, with long-term economic, psychological, and social consequences (9–11). Despite the slow progress in preventing perinatal deaths, ending preventable perinatal death remains a high concern for international public health (12). Numerous factors have been associated with perinatal death. Identifying risk factors and protective factors can facilitate the development of preventive strategies and improve clinical outcomes. Socioeconomic and demographic factors such as maternal age, education, and wealth are often associated with perinatal mortality (13). Some studies suggest parental health-related behaviors such as smoking and alcohol consumption are associated with stillbirths (14, 15). In addition, pregnancy complications such as gestational diabetes mellitus and gestational hypertension can increase the risk of perinatal death (16). Therefore, the use of antenatal interventions, modification of risk factors, and management of pregnancy complications can be taken into account to prevent perinatal death.
No comprehensive perspective investigation about the association of determinants with perinatal death in South China was conducted before. In order to recognize current determinants of perinatal death so as to guide future preventative care efforts, this study is based on a prospective cohort study to investigate the association of maternal and fetal characteristics with the risk of perinatal mortality and provide more evidence for protective factors and risk factors of perinatal death.
Materials and Methods
Recruitment of Study Participants
A prospective cohort study was conducted in Hunan provincial Maternal and Child Health Care Hospital in China. From March 13, 2013, to December 31, 2019, pregnant women who received their first antenatal care between 8 and 14 weeks of gestation were approached and invited to join the cohort. The study included pregnant women who met the inclusion criteria. The inclusion criteria for participation included being 18 years or older, intending to receive prenatal care and to deliver at the study hospital, and provided informed consent to be in the evaluation. Participants who met any of the following criteria were excluded from this study: (i) unable to cooperate with the investigation due to mental illness or extreme emotional instability; (ii) did not provide a blood sample or did not complete the questionnaire; (iii) women whose child had any known chromosomal abnormalities or syndromic CHD; and (iv) multiple pregnancies. Our study was approved by the ethics committee of the Xiangya School of Public Health of Central South University, and written informed consent was obtained from all mothers.
Data Collection
The trained investigators used a self-designed questionnaire to collect the following information through one-to-one interviews, including sociodemographic characteristics (i.e., maternal obesity, ethnicity, and occupation); maternal history (i.e., adverse pregnancy, gestational diabetes mellitus, cesarean section, and neonatal death), lifestyle and habits during early pregnancy (i.e., drinking, smoking, and taking antidepressants); complications of pregnancy (i.e., gestational diabetes mellitus and gestational hypertension); and folate supplementation before or during this pregnancy. All participants were followed up until 3 months after birth to collect perinatal survival status and pregnancy outcomes (i.e., cesarean section, perinatal death, preterm birth, and congenital malformations). Data on disease diagnosis were confirmed by medical records.
Definition and Assessment on Variables
The principal outcome of the study was perinatal death. We defined perinatal death as a death of offspring occurring at or after 28 weeks of gestation or up to 7 days after birth (17). The perinatal mortality rate was defined as the number of perinatal deaths per 1,000 births (including live births and stillbirths) in this study. Maternal education was categorized as ≤ 9 and > 9 years. Maternal ages were grouped as < 25, between 25 and 29, and ≥ 30 years. Residence was categorized as urban and rural. Ethnicity was categorized as Han nationality and other. Occupation was regrouped as unemployment and employment. Income was categorized as ≤ 5,000 and > 5,000 (RMB). Parity was defined as the number of deliveries a woman had, which was divided into nullipara (zero delivery) and multipara (one or more times deliveries). Mode of conception was categorized as spontaneous conception, spontaneous conception after infertility drug treatment (ovulation induction and simple drug therapy), and conception after assisted reproductive technology treatment [in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI)]. Obesity was defined as a body mass index (BMI) > 30 kg/m2. Preterm births were defined as infants born less than 37 weeks of gestation. Congenital malformation was defined as perinatal abnormalities (including live births and stillbirths), which were probably of prenatal origin, including genetic defects and chromosomal abnormalities. Results of other variables were divided into yes or no, with yes indicating the occurrence of these outcomes.
Statistical Analysis
Categorical variables were presented as rates or percentages. Generalized linear models of the Poisson family, with a log link, were utilized to calculate the relative risk (RR) and 95% CI for perinatal death in offspring associated with determinants. Unadjusted RR was calculated by univariate Poisson regression. Adjusted RR (aRR) was calculated by multivariate Poisson regression to control for potential confounders. Initially, all independent variables about perinatal mortality were included in the crude analyses. Only variables associated with perinatal death (p < 0.05) in the univariable analysis were entered into multivariate regression analyses. The database was developed using EpiData version 3.1 software. All analyses were performed using R software, version 3.6.1 (R Foundation for Statistical Computing).
Results
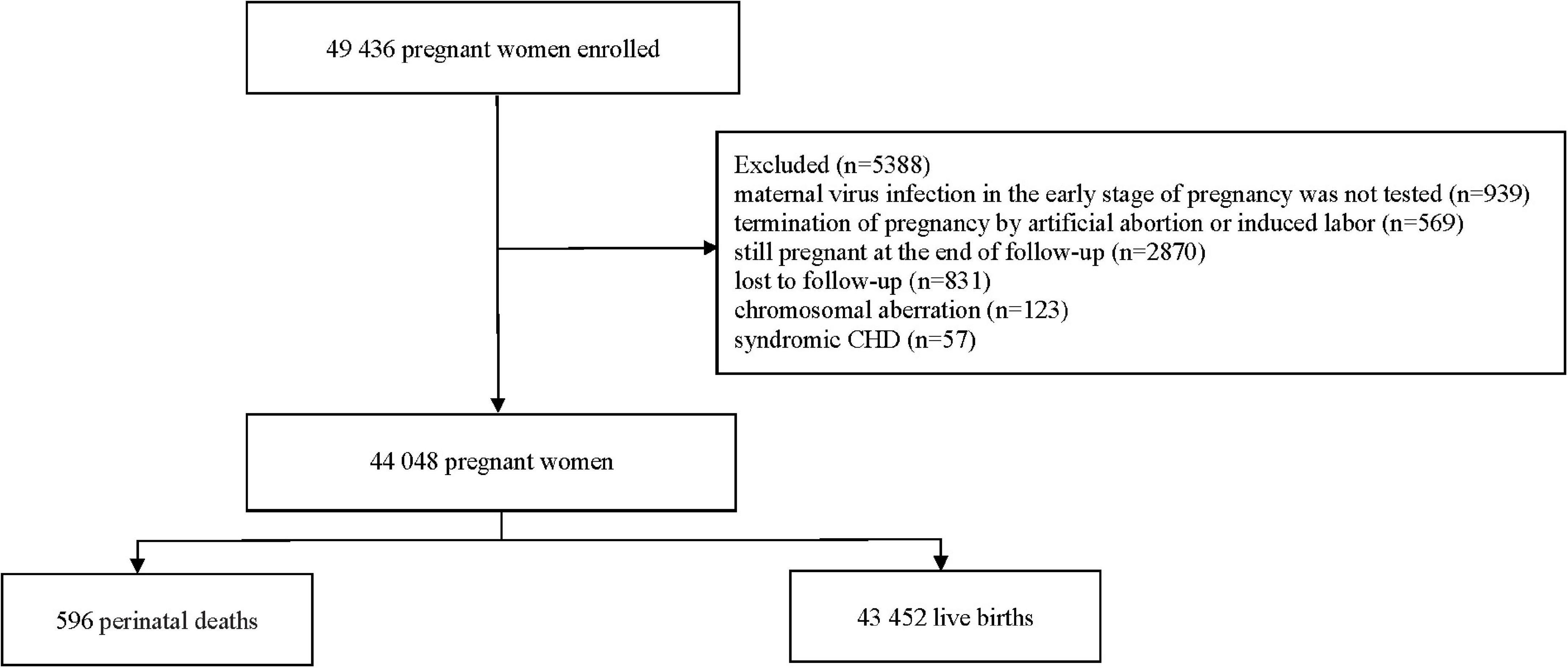
From March 13, 2013, to December 31, 2019, a total of 49,436 pregnant women with singleton pregnancies were registered and enrolled in the cohort during their first prenatal care in the early stage of pregnancy. According to the inclusion and exclusion criteria, the following pregnant women were excluded: (i) termination of pregnancy by artificial abortion or induced labor because of accidental pregnancy or ectopic pregnancy (n = 568; 1.1%); (ii) still pregnant at the end of follow-up (n = 2,870; 5.8%); (iii) lost to follow-up (n = 831; 1.7%); (iv) maternal virus infection in the early stage of pregnancy was not tested (n = 939; 1.9%); (v) their children were diagnosed with a chromosomal aberration (n = 123; 0.2%); and (vi) their children were diagnosed with syndromic CHD (n = 57; 0.1%). A total of 44,048 eligible pregnant women were included in the analysis (Figure 1). Of these, 43,452 (98.6%) were live births, and 596 (1.4%) were perinatal deaths. The perinatal mortality rate was 13.5 deaths per 1,000 births.
Univariate Analysis
The sociodemographic characteristics of the study participants are presented in Table 1. The unadjusted analysis showed that maternal education level ≤ 9 years, living in rural areas, maternal obesity, and being employed were factors associated with increased risk of perinatal death. Ethnic minority and income > 5,000 were associated with decreased risk of perinatal death.
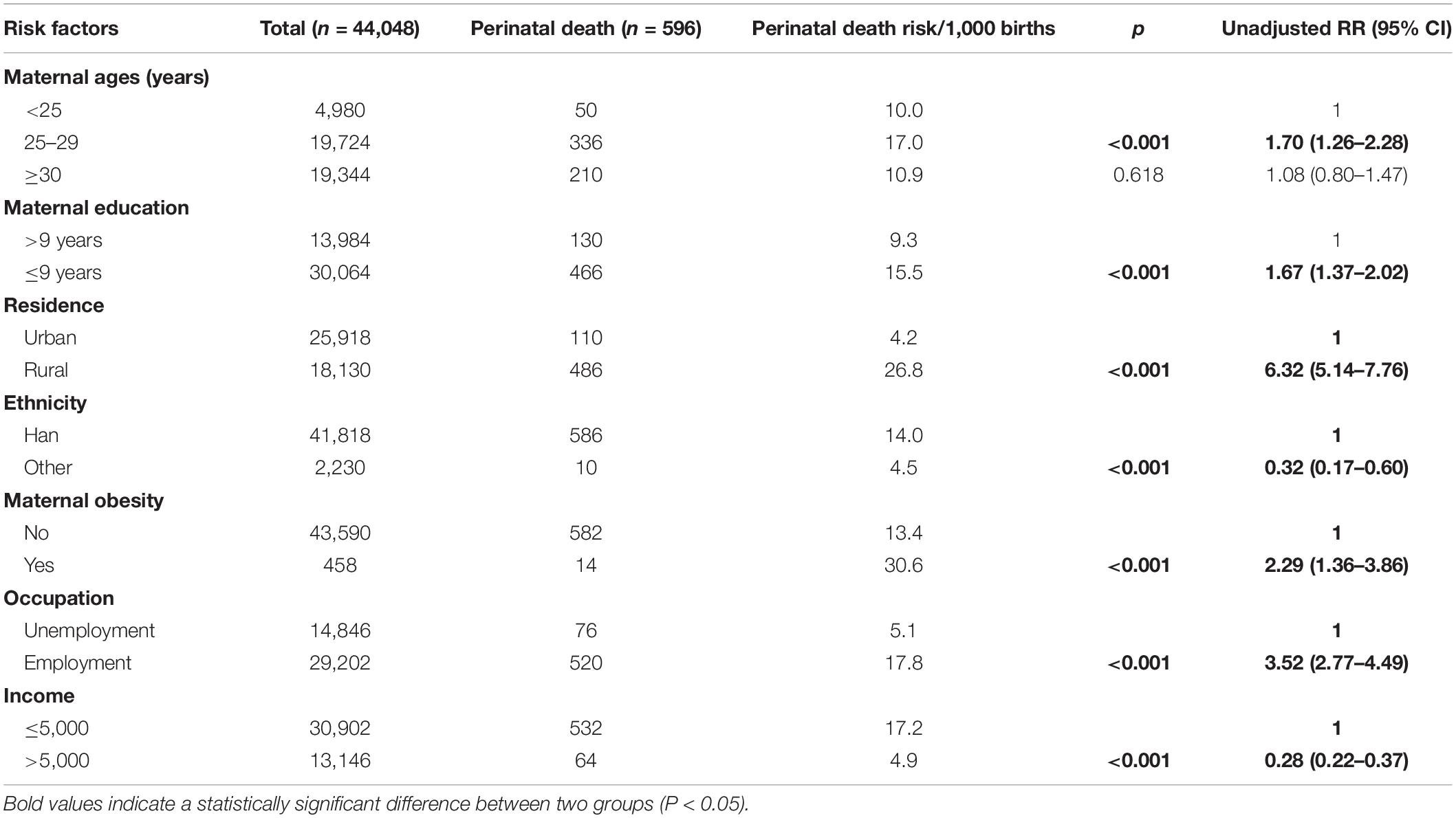
Table 1. Sociodemographic characteristics and unadjusted relative risk (RR) for perinatal death among 44,048 pregnant women.
The findings of the maternal history associated with perinatal death are shown in Table 2. The unadjusted analysis showed that history of gestational hypertension, history of neonatal death, history of gestational diabetes mellitus, infertility drug treatment, and assisted reproductive techniques were associated with increased risk of perinatal death. Multiparous women and a history of cesarean section were associated with decreased risk of perinatal death.
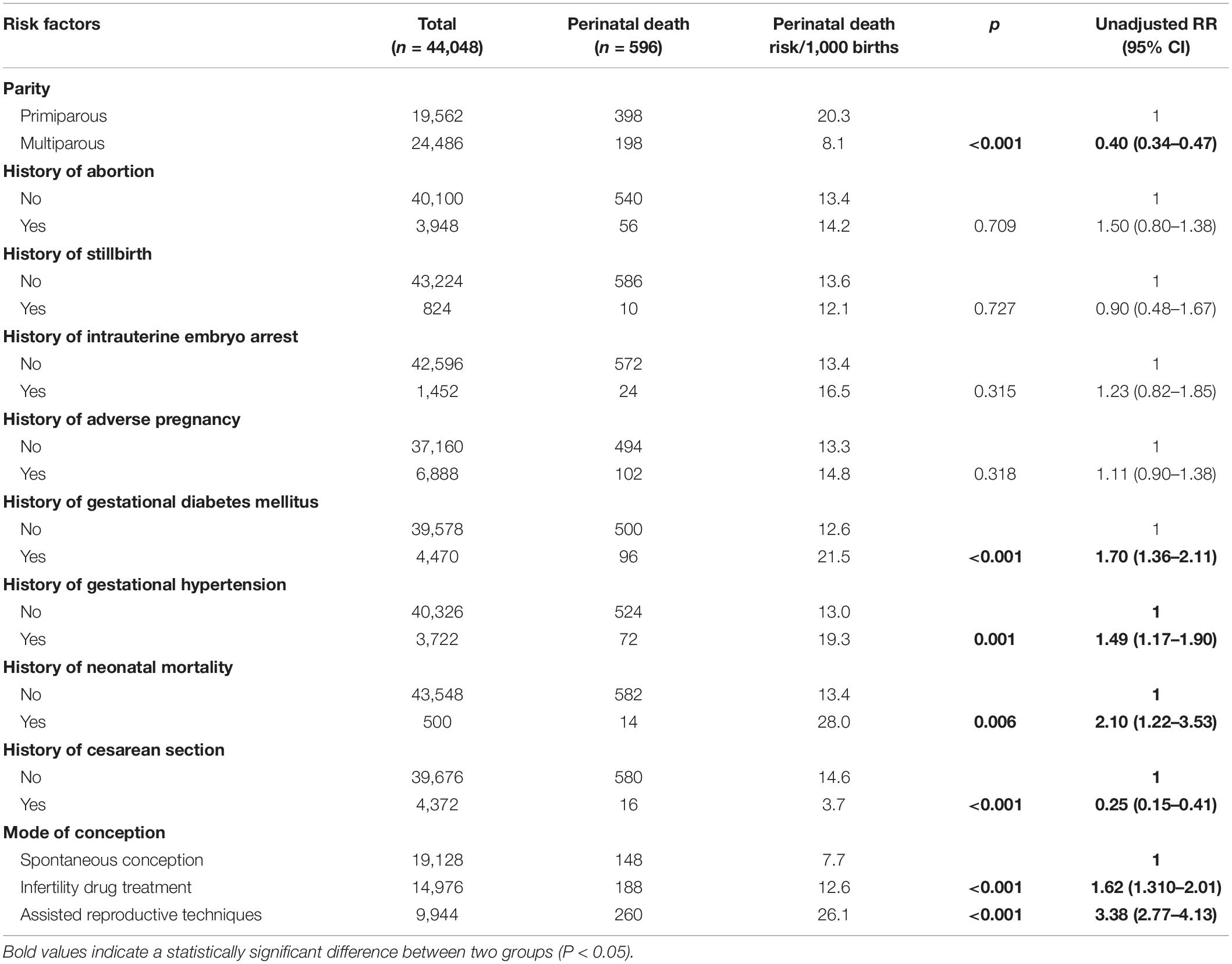
Table 2. Maternal history and unadjusted relative risk (RR) for perinatal death among 44,048 pregnant women.
The unadjusted analysis on the pregnancy-related factors associated with perinatal death (Table 3) showed that taking antidepressants during early pregnancy was the factor associated with increased risk of perinatal death.
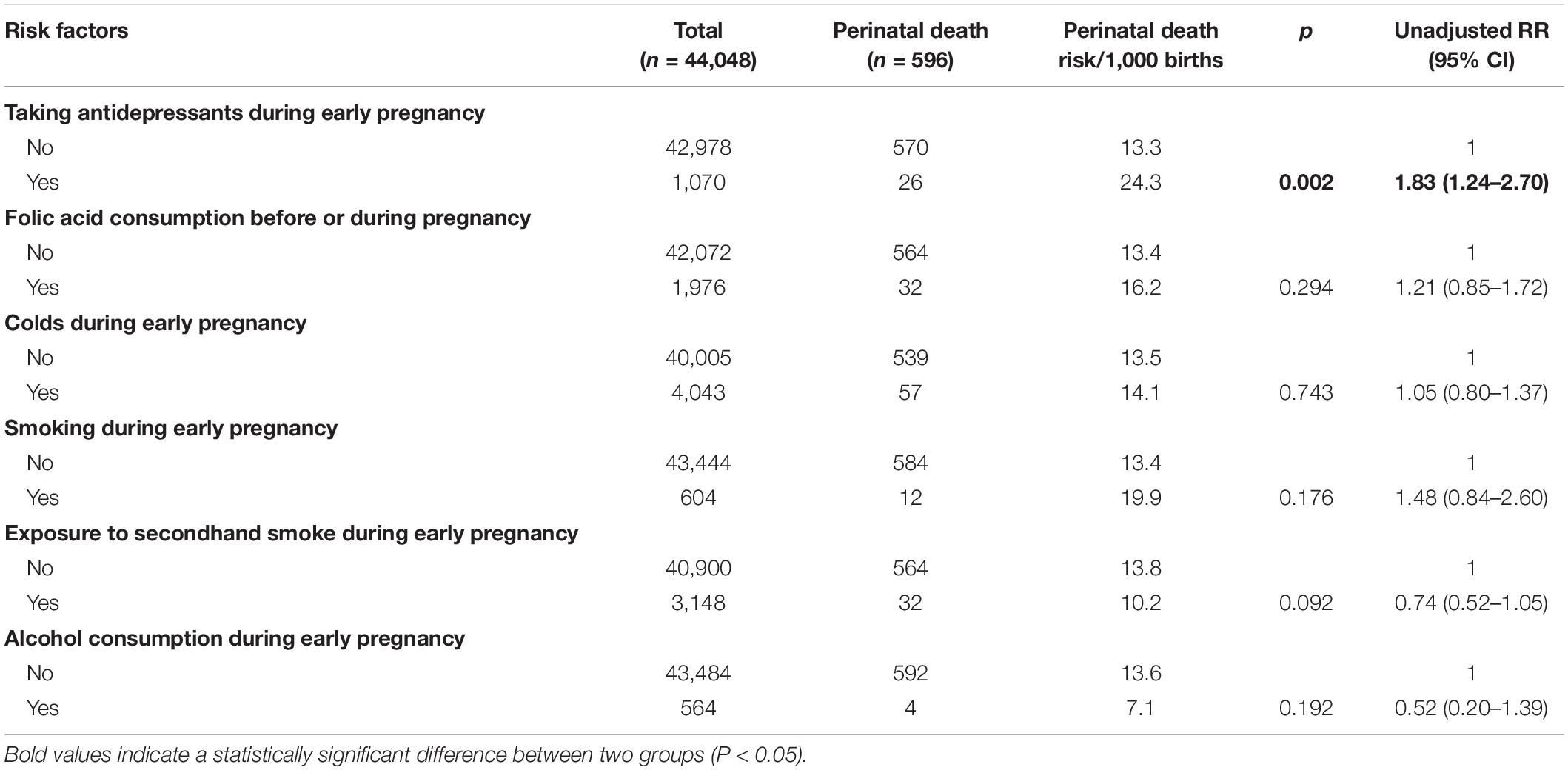
Table 3. Lifestyle and habits during early pregnancy and unadjusted relative risk (RR) for perinatal death among 44,048 pregnant women.
The unadjusted analysis on the complications and outcomes of pregnancy-associated with perinatal death (Table 4) found that gestational diabetes mellitus, preterm birth, and congenital malformations were factors associated with increased risk of perinatal death. Cesarean section was associated with decreased risk of perinatal death.
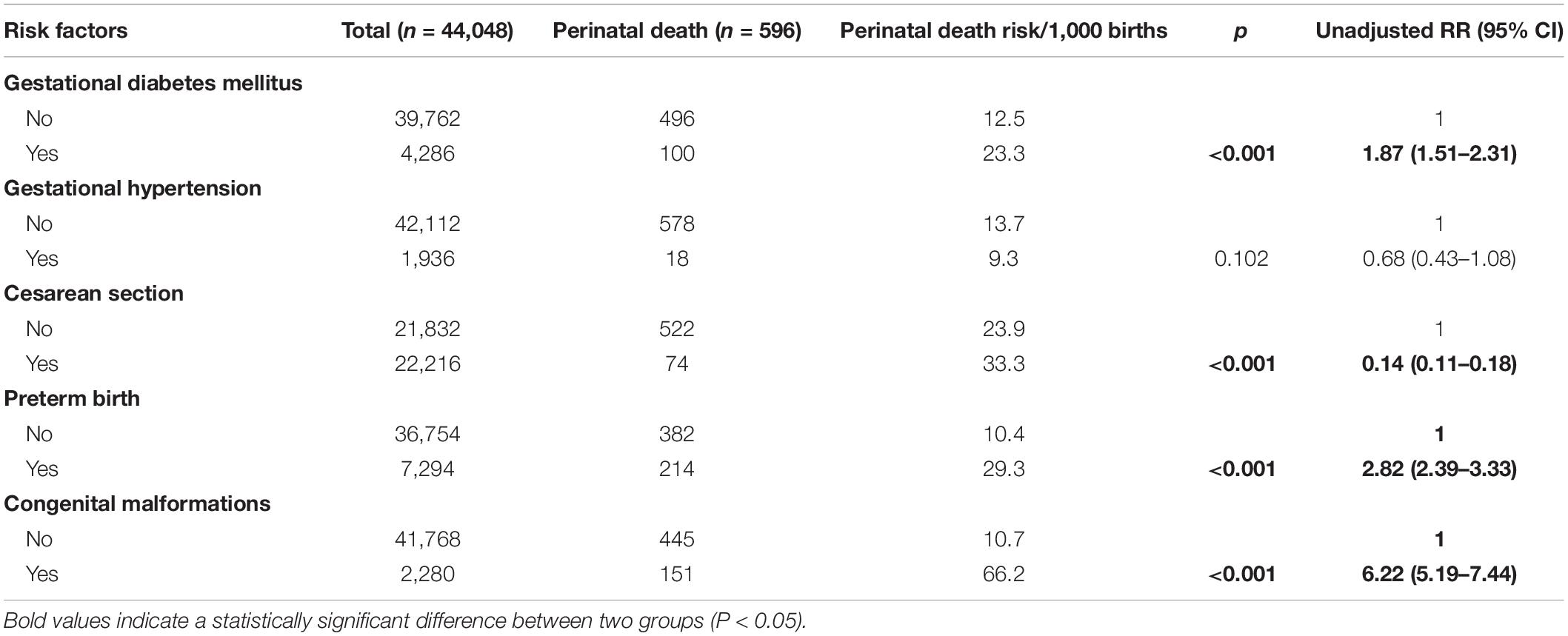
Table 4. Complications and outcomes of pregnancy and unadjusted relative risk (RR) for perinatal death among 44,048 pregnant women.
Multivariable Analysis
After adjustment, the results of the multivariate Poisson regression model (Figure 2) showed that maternal obesity (aRR = 3.91, 95% CI: 2.24–6.85), being employed (aRR = 5.22, 95% CI: 4.13–6.59), history of gestational diabetes mellitus (aRR = 1.13, 95% CI: 0.92–1.40), history of gestational hypertension (aRR = 1.72, 95% CI: 1.29–2.30), history of neonatal death (aRR = 2.98, 95% CI: 1.75–5.07), taking antidepressants during early pregnancy (aRR = 1.51, 95% CI: 1.14–1.94), gestational diabetes mellitus (aRR = 1.66, 95% CI: 1.42–1.94), infertility drug treatment (aRR = 1.73, 95% CI: 1.40–2.13) and assisted reproductive techniques (aRR = 4.64, 95% CI: 3.77–5.71), preterm birth (aRR = 3.25, 95% CI: 2.67–3.95), and congenital malformations (aRR = 2.07, 95% CI: 1.75–2.46) were still associated with an increased risk of perinatal death. And ethnic minority (aRR = 0.28, 95% CI: 0.13–0.57), income > 5,000 (aRR = 0.35, 95% CI: 0.26–0.45), multiparous women (aRR = 0.44, 95% CI: 0.37–0.52), and cesarean section (aRR = 0.07, 95% CI: 0.05–0.10) were still associated with a decreased risk of perinatal death.
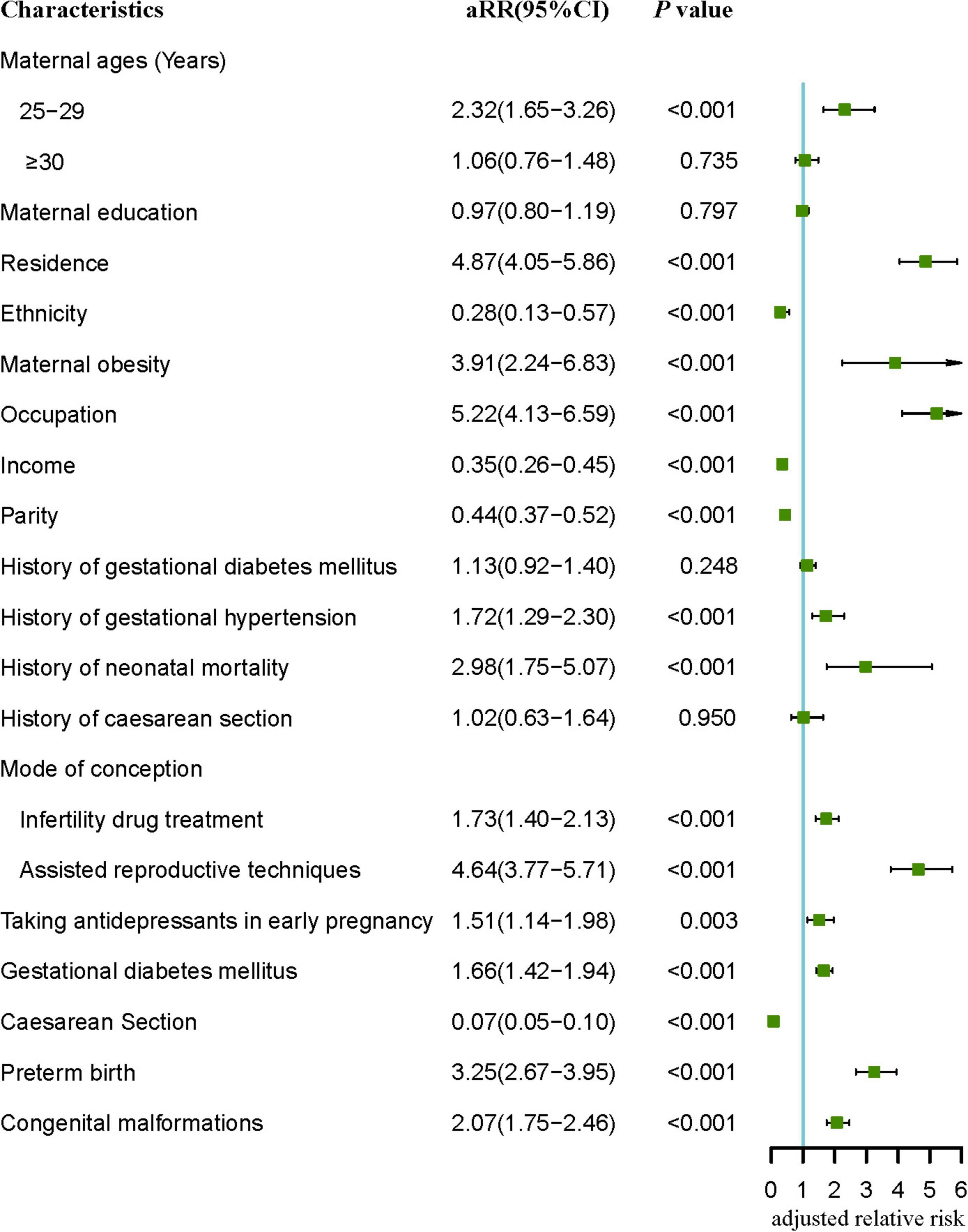
Figure 2. Multivariate analysis of significant (p < 0.05) risk factors in Tables 1–4. aRR, adjusted relative risk.
Discussion
In this prospective cohort study, we exhaustively explored the association of maternal and fetal characteristics with perinatal death. Independent risk factors for perinatal death were maternal obesity, being employed, history of gestational hypertension, taking antidepressants during early pregnancy, history of gestational diabetes mellitus, gestational diabetes mellitus, infertility drug treatment and assisted reproductive techniques, history of neonatal death, preterm birth, and congenital malformations. Ethnic minority, income > 5,000, multiparous women, and cesarean section were the protective factors of perinatal death. The perinatal death rate in the present research was 13.5 per 1,000 births, which was higher than in developed countries such as the United States (6.0/1,000 births) (18) and Ireland (5.4/1,000 births) (19). But the perinatal mortality in this study was much lower than the 41.6 per 1,000 births reported in northern Tanzania (20) and 75.3 per 1,000 births reported in Eastern Sudan (21). These differences in the perinatal mortality were likely attributable to differences in population health, lifestyle and habits, and availability of prenatal and obstetric emergency care between developed and developing countries. The specific reasons for differences in perinatal mortality between developed and developing countries also require more studies.
Our study found that after controlling for other factors, the risk of perinatal death in the offspring of mothers aged 25–29 was higher than that of mothers aged < 25, and advanced maternal age was not associated with perinatal death. Usynina et al. (22) also found that there was no association of advanced maternal age with perinatal death, because the association between maternal age and adverse pregnancy outcomes might be affected by age-related confounders or intermediates (23). A case–control study indicated that the offspring of mothers with only primary education or no schooling had a 5.4-fold higher risk of perinatal death than educated mothers (24). This finding is in contradiction with a prospective study that suggested that the offspring of women who completed secondary education or above had a higher risk of perinatal death (25). In our study, maternal education of less than 9 years elevated the incidence of perinatal death in the univariate analysis. But it lost statistical significance after adjustment. The previous study found that perinatal mortality was higher in rural areas compared to urban areas (26). We found the same result that can be due to the remoteness and the short availability of the health service in rural areas. In addition, urban residents possessed better wealth and better medical care services for their newborns (27). Maternal employment might also have a negative impact on fetuses and newborns because mothers who are employed might not have enough time to rest and care for their babies. Our study suggested that maternal employment may be one of the risk factors of perinatal death, consistent with previous studies (28). Prior literature indicated that wealthy families were more likely to receive higher cash incomes than poor ones, thus enabling them to have access to a quality diet and better medical care (27). Our study found that income > 5,000 was a protective factor for perinatal death. The RR of perinatal mortality was 3.91 times in babies born to obese mothers compared with non-obese mothers. A number of studies had revealed similar results in both developed and developing countries (29–31). Obesity might affect placental function (32) or lead to other maternal obesity-related pregnancy complications (33). Obesity was still the dominant and modifiable risk factor for perinatal mortality. According to reports, effective weight loss during pregnancy can decrease the occurrence of gestational diabetes mellitus, which had a significant impact on the gestational age at birth and the rate of neonatal death (34).
The offspring of multiparous women had a lower risk of perinatal death as compared to those of nulliparous women. Our research was consistent with the studies conducted in Bangladesh, Uganda, and Burkina Faso (13, 35, 36). Neonatal asphyxia and perinatal death easily occurred in the offspring of nulliparous women (37), which might be because nulliparous women were prone to delivery complications, such as obstruction (38). One of the vital factors of perinatal death in this survey was the history of neonatal death. A study conducted in Missouri (United States) revealed that women with a history of neonatal death had an increased risk of subsequent stillbirths (39) because women with a history of neonatal death might suffer from anatomical problems associated with the pelvis, the uterus, or any parts of the birth canal, resulting in complications and perinatal mortality. This also demonstrated the significance of special investigation and care during the pregnancy and childbirth period to women who had a history of neonatal death in order to avoid subsequent adverse pregnancy outcomes. Elevated evidence verified that IVF/ICSI significantly increased the risk of adverse pregnancy outcomes in offspring as compared with spontaneous conception (40–42). Our study found that compared with that of spontaneous conception, the RR of perinatal mortality of IVF/ICSI and infertility drug treatment was 4.642 and 1.726, respectively. This might be due to the increased risk of systemic complications in pregnant women through assisted reproductive techniques, and the operation in the treatment may produce certain stimulation to the uterus. The use of ovulation induction drugs resulted in the body’s estrogen levels greatly exceeding the normal physiological level and increased the risk of perinatal death (43, 44). Antidepressants might have negative effects on people such as memory difficulties, irritability, reasoning loss, and coordination disorders (45). Our study found that taking antidepressants during early pregnancy increased the risk of perinatal mortality. As far as we know, this was the first evidence that the associations of taking antidepressants during early pregnancy with perinatal mortality were discovered through a prospective cohort study. However, the specific mechanism and whether there was a quantitative relationship between the two needs further research in the future.
A study of gestational hypertension suggested that pregnant women with gestational hypertension had a remarkably elevated risk of perinatal death as compared with pregnant women without gestational hypertension (46). However, our study found that gestational hypertension did not correlate with perinatal death, and a history of gestational hypertension was a risk factor for perinatal mortality. There had been controversy over whether maternal gestational diabetes had adverse effects on the perinatal mortality of offspring (16, 47, 48). Theoretically, the high maternal glucose levels in pregnant women with diabetes contributed to high fetal glucose levels. Fetal hyperglycemia consumed the circulating oxygen, thus leading to hypoxia and acidosis, and the maternal oxyhemoglobin reduced the release of oxygen to tissues, thus resulting in fetal chronic hypoxia (49). Our study prospectively confirmed that mothers with gestational diabetes mellitus had higher perinatal mortality. Interestingly, Feig et al. (50) revealed that women with gestational diabetes mellitus had a lower risk of perinatal death than non-diabetic women. They argued that stricter regulation in pregnancies of women with gestational diabetes mellitus contributed to the low perinatal death in offspring. This provided evidence and ideas for us to manage and control the perinatal mortality of pregnancies with gestational diabetes mellitus.
Although the individual research had found that cesarean section did not affect perinatal mortality compared with vaginal delivery (51), most studies showed the rate of perinatal death was significantly higher among infants who had undergone cesarean section (52, 53). The reason was that the indication for cesarean section was usually an emergency, so severe obstetric problems or an abnormal delivery resulted in an increased risk of fetal death (54). Our study found that cesarean section was a protective factor for perinatal mortality in accordance with the results of a recent retrospective cohort study (20). This might be related to the planned cesarean section in China, which was not carried out in an emergency condition. On the other hand, the provider’s good skills and timeliness of cesarean section were still associated with the decrease of perinatal mortality through cesarean section. Preterm birth might exacerbate the risk of perinatal mortality because premature infants had insufficient anatomical and physical development of all systems. More seriously, premature infants with respiratory distress syndrome, immature pulmonary development, and susceptibility to infections caused by an underdeveloped immune system were prone to death (55). Our survey suggested that the RR of perinatal mortality in preterm infants was 3.25 times higher than that in non-preterm infants.
This study utilized a large sample of pregnant women and is a prospective cohort study. This gave us a powerful ability to detect the association between interesting exposure and perinatal death. Many findings were discovered in this study in accordance with the previous studies, which provide further confidence for our analysis. However, some limitations should be considered.
First, the study population might conceal their risk behaviors, such as history of smoking and history of drinking, thus underestimating the association between these behaviors and perinatal adverse outcomes. Second, the data of this study were mainly from the same hospital, and the sample source may be concentrated on a certain type of population, which may affect the representativeness of the sample and lead to selection bias. Third, although adjusting for a variety of potential confounding factors, we still cannot completely exclude the involvement of the possibility of residual confounding, because these factors investigated in this research did not account for all the determinants of perinatal death. Fourth, our study was limited by our inability to distinguish antepartum from intrapartum deaths and the likely misclassification of some early neonatal deaths as stillbirths, so the perinatal deaths were not separated into stillbirths and early neonatal deaths to analysis in our research. In addition, the causes of perinatal death were not involved in our study.
Conclusion
The present study is the first to comprehensively evaluate the association of maternal and fetal characteristics with the risk of perinatal death, which suggests that some factors such as maternal sociodemographic characteristics, abnormal pregnancy history, lifestyle and habits during early pregnancy, and complications of pregnancy are significantly associated with perinatal death. To identify some modifiable risk factors and to give effective reduction and control are an important link to reducing perinatal mortality. However, the limitations in this study should be carefully taken into account. In the future, more specific studies are required to refine and confirm our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Xiangya School of Public Health of Central South University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YpL: study design, statistical analysis, and manuscript writing. JQ and TY: manuscript revision and review. YpL, QL, TW, LC, SZ, YhL, JD, JL, MS, JW, XS, and JS: data collection. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Project Funded by National Natural Science Foundation Program of China (82073653 and 81803313), the Hunan Provincial Key Research and Development Program (2018SK2063 and 2018SK2062), the Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), the China Postdoctoral Science Foundation (2020M682644), and Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank the editors and reviewers for their suggestions and all colleagues working in the Maternal and Child Health Promotion and Birth Defect Prevention Group.
References
1. Saleem S, McClure EM, Goudar SS, Patel A, Esamai F, Garces A, et al. A prospective study of maternal, fetal and neonatal deaths in low- and middle-income countries. Bull World Health Organ. (2014) 92:605–12. doi: 10.2471/BLT.13.127464
2. Lancet T. Ending Preventable Stillbirths: An Executive Summary for The Lancet’s Series. Honolulu, HI: HNN Team (2016).
3. Arach A, Tumwine JK, Nakasujja N, Ndeezi G, Kiguli J, Mukunya D, et al. Perinatal death in Northern Uganda: incidence and risk factors in a community-based prospective cohort study. Glob Health Action. (2021) 14:1859823. doi: 10.1080/16549716.2020.1859823
4. Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. (2005) 193:1923–35. doi: 10.1016/j.ajog.2005.03.074
5. McClure EM, Wright LL, Goldenberg RL, Goudar SS, Parida SN, Jehan I, et al. The global network: a prospective study of stillbirths in developing countries. Am J Obstet Gynecol. (2007) 197:241–7. doi: 10.1016/j.ajog.2007.07.004
6. Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. (2019) 7:e710–20. doi: 10.1016/S2214-109X(19)30163-9
7. Zupan J. Perinatal mortality in developing countries. N Engl J Med. (2005) 352:2047–8. doi: 10.1056/NEJMp058032
8. Chen D, Cui S, Liu C, Qi H, Zhong N. Stillbirth in China. Lancet. (2016) 387:1995–6. doi: 10.1016/S0140-6736(16)30461-5
9. Heazell A, Siassakos D, Blencowe H, Burden C, Bhutta ZA, Cacciatore J, et al. Stillbirths: economic and psychosocial consequences. Lancet. (2016) 387:604–16. doi: 10.1016/S0140-6736(15)00836-3
10. Gausia K, Moran AC, Ali M, Ryder D, Fisher C, Koblinsky M. Psychological and social consequences among mothers suffering from perinatal loss: perspective from a low income country. BMC Public Health. (2011) 11:451. doi: 10.1186/1471-2458-11-451
11. Arach A, Nakasujja N, Nankabirwa V, Ndeezi G, Kiguli J, Mukunya D, et al. Perinatal death triples the prevalence of postpartum depression among women in Northern Uganda: a community-based cross-sectional study. PLoS One. (2020) 15:e240409. doi: 10.1371/journal.pone.0240409
12. Allanson E, Tunçalp Ö, Gardosi J, Pattinson RC, Erwich JJ, Flenady VJ, et al. Classifying the causes of perinatal death. Bull World Health Organ. (2016) 94:79. doi: 10.2471/BLT.15.168047
13. Nankabirwa V, Tumwine JK, Tylleskär T, Nankunda J, Sommerfelt H. Perinatal mortality in eastern Uganda: a community based prospective cohort study. PLoS One. (2011) 6:e19674. doi: 10.1371/journal.pone.0019674
14. Aliyu MH, Salihu HM, Wilson RE, Kirby RS. Prenatal smoking and risk of intrapartum stillbirth. Arch Environ Occup Health. (2007) 62:87–92. doi: 10.3200/AEOH.62.2.87-92
15. Aliyu MH, Wilson RE, Zoorob R, Chakrabarty S, Alio AP, Kirby RS, et al. Alcohol consumption during pregnancy and the risk of early stillbirth among singletons. Alcohol. (2008) 42:369–74. doi: 10.1016/j.alcohol.2008.04.003
16. Wendland EM, Duncan BB, Mengue SS, Schmidt MI. Lesser than diabetes hyperglycemia in pregnancy is related to perinatal mortality: a cohort study in Brazil. BMC Pregnancy Childbirth. (2011) 11:92. doi: 10.1186/1471-2393-11-92
17. Zupan J, ÅHman E, Ebrary I, World Health Organization. Neonatal and Perinatal Mortality: Country, Regional and Global Estimates 2004. Geneva: WHO Press (2007).
18. Gregory E, Drake P, Martin JA. Lack of change in perinatal mortality in the United States, 2014-2016. NCHS Data Brief. (2018) 316:1–8.
19. Unterscheider J, O’Donoghue K, Daly S, Geary MP, Kennelly MM, McAuliffe FM, et al. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth. (2014) 14:63. doi: 10.1186/1471-2393-14-63
20. Mboya IB, Mahande MJ, Obure J, Mwambi HG. Predictors of perinatal death in the presence of missing data: a birth registry-based study in northern Tanzania. PLoS One. (2020) 15:e231636. doi: 10.1371/journal.pone.0231636
21. Ali AA, Elgessim ME, Taha E, Adam GK. Factors associated with perinatal mortality in Kassala, Eastern Sudan: a community-based study 2010-2011. J Trop Pediatr. (2014) 60:79–82. doi: 10.1093/tropej/fmt075
22. Usynina AA, Grjibovski AM, Krettek A, Odland JØ, Kudryavtsev AV, Anda EE. Risk factors for perinatal mortality in Murmansk county, Russia: a registry-based study. Glob Health Action. (2017) 10:1270536. doi: 10.1080/16549716.2017.1270536
23. Kenny LC, Lavender T, McNamee R, O’Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. (2013) 8:e56583. doi: 10.1371/journal.pone.0056583
24. Tachiweyika E, Gombe N, Shambira G, Chadambuka A, Mufuta T, Zizhou S. Determinants of perinatal mortality in Marondera district, Mashonaland East Province of Zimbabwe, 2009: a case control study. Pan Afr Med J. (2011) 8:7. doi: 10.4314/pamj.v8i1.71054
25. Bari W, Chowdhury RI, Islam MA, Chakraborty N, Akhter HA. The differentials and determinants of perinatal mortality in rural Bangladesh. Eur J Contracept Reprod Health Care. (2002) 7:216–22. doi: 10.1080/ejc.7.4.216.222
26. Ezeh OK, Uche-Nwachi EO, Abada UD, Agho KE. Community-and proximate-level factors associated with perinatal mortality in Nigeria: evidence from a nationwide household survey. BMC Public Health. (2019) 19:811. doi: 10.1186/s12889-019-7151-0
27. Woldeamanuel BT, Gelebo KK. Statistical analysis of socioeconomic and demographic correlates of perinatal mortality in Tigray region, Ethiopia: a cross sectional study. BMC Public Health. (2019) 19:1301. doi: 10.1186/s12889-019-7642-z
28. Hossain MB, Kanti MS, Mohsin M, Rahaman KM. Trends and determinants of perinatal mortality in Bangladesh. PLoS One. (2019) 14:e221503. doi: 10.1371/journal.pone.0221503
29. Adewuyi EO, Zhao Y, Lamichhane R. Risk factors for infant mortality in rural and urban Nigeria: evidence from the national household survey. Scand J Public Health. (2017) 45:543–54. doi: 10.1177/1403494817696599
30. Cresswell JA, Campbell OM, De Silva MJ, Filippi V. Effect of maternal obesity on neonatal death in sub-Saharan Africa: multivariable analysis of 27 national datasets. Lancet. (2012) 380:1325–30. doi: 10.1016/S0140-6736(12)60869-1
31. Lindam A, Johansson S, Stephansson O, Wikström AK, Cnattingius S. High maternal body mass index in early pregnancy and risks of stillbirth and infant mortality-a population-based sibling study in Sweden. Am J Epidemiol. (2016) 184:98–105. doi: 10.1093/aje/kww046
32. Kristensen J, Vestergaard M, Wisborg K, Kesmodel U, Secher NJ. Pre-pregnancy weight and the risk of stillbirth and neonatal death. BJOG. (2005) 112:403–8. doi: 10.1111/j.1471-0528.2005.00437.x
33. Chu SY, Kim SY, Lau J, Schmid CH, Dietz PM, Callaghan WM, et al. Maternal obesity and risk of stillbirth: a metaanalysis. Am J Obstet Gynecol. (2007) 197:223–8. doi: 10.1016/j.ajog.2007.03.027
34. Cnattingius S, Villamor E. Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet. (2016) 387:558–65. doi: 10.1016/S0140-6736(15)00990-3
35. Sikder SS, Labrique AB, Shamim AA, Ali H, Mehra S, Wu L, et al. Risk factors for reported obstetric complications and near misses in rural northwest Bangladesh: analysis from a prospective cohort study. BMC Pregnancy Childbirth. (2014) 14:347. doi: 10.1186/1471-2393-14-347
36. Diallo AH, Meda N, Zabsonré E, Sommerfelt H, Cousens S, Tylleskär T. Perinatal mortality in rural Burkina Faso: a prospective community-based cohort study. BMC Pregnancy Childbirth. (2010) 10:45. doi: 10.1186/1471-2393-10-45
37. Ghimire PR, Agho KE, Akombi BJ, Wali N, Dibley M, Raynes-Greenow C, et al. Perinatal mortality in South Asia: systematic review of observational studies. Int J Environ Res Public Health. (2018) 15:1428. doi: 10.3390/ijerph15071428
38. Kabakyenga JK, Östergren PO, Turyakira E, Mukasa PK, Pettersson KO. Individual and health facility factors and the risk for obstructed labour and its adverse outcomes in south-western Uganda. BMC Pregnancy Childbirth. (2011) 11:73. doi: 10.1186/1471-2393-11-73
39. August EM, Salihu HM, Weldeselasse H, Biroscak BJ, Mbah AK, Alio AP. Infant mortality and subsequent risk of stillbirth: a retrospective cohort study. BJOG. (2011) 118:1636–45. doi: 10.1111/j.1471-0528.2011.03137.x
40. Liang Y, Chen L, Yu H, Wang H, Li Q, Yu R, et al. Which type of congenital malformations is significantly increased in singleton pregnancies following after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Oncotarget. (2018) 9:4267–78. doi: 10.18632/oncotarget.23689
41. Marino JL, Moore VM, Willson KJ, Rumbold A, Whitrow MJ, Giles LC, et al. Perinatal outcomes by mode of assisted conception and sub-fertility in an Australian data linkage cohort. PLoS One. (2014) 9:e80398. doi: 10.1371/journal.pone.0080398
42. Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: a controlled national cohort study. Hum Reprod. (2013) 28:230–40. doi: 10.1093/humrep/des377
43. Xu XK, Wang YA, Li Z, Lui K, Sullivan EA. Risk factors associated with preterm birth among singletons following assisted reproductive technology in Australia 2007-2009–a population-based retrospective study. BMC Pregnancy Childbirth. (2014) 14:406. doi: 10.1186/s12884-014-0406-y
44. Tepper NK, Farr SL, Cohen BB, Nannini A, Zhang Z, Anderson JE, et al. Singleton preterm birth: risk factors and association with assisted reproductive technology. Matern Child Health J. (2012) 16:807–13. doi: 10.1007/s10995-011-0787-8
45. Fitton CA, Steiner M, Aucott L, Pell JP, Mackay DF, Fleming M, et al. In utero exposure to antidepressant medication and neonatal and child outcomes: a systematic review. Acta Psychiatr Scand. (2020) 141:21–33. doi: 10.1111/acps.13120
46. Adu-Bonsaffoh K, Ntumy MY, Obed SA, Seffah JD. Perinatal outcomes of hypertensive disorders in pregnancy at a tertiary hospital in Ghana. BMC Pregnancy Childbirth. (2017) 17:388. doi: 10.1186/s12884-017-1575-2
47. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
48. Ramtoola S, Home P, Damry H, Husnoo A, Ah-Kion S. Gestational impaired glucose tolerance does not increase perinatal mortality in a developing country: cohort study. BMJ. (2001) 322:1025–6. doi: 10.1136/bmj.322.7293.1025
49. Miodovnik M, Lavin JP, Harrington DJ, Leung LS, Seeds AE, Clark KE. Effect of maternal ketoacidemia on the pregnant ewe and the fetus. Am J Obstet Gynecol. (1982) 144:585–93. doi: 10.1016/0002-9378(82)90232-0
50. Feig DS, Hwee J, Shah BR, Booth GL, Bierman AS, Lipscombe LL. Trends in incidence of diabetes in pregnancy and serious perinatal outcomes: a large, population-based study in Ontario, Canada, 1996-2010. Diabetes Care. (2014) 37:1590–6. doi: 10.2337/dc13-2717
51. Zhao Y, Zhang J, Zamora J, Vogel JP, Souza JP, Jayaratne K, et al. Increases in caesarean delivery rates and change of perinatal outcomes in low- and middle-income countries: a hospital-level analysis of two WHO surveys. Paediatr Perinat Epidemiol. (2017) 31:251–62. doi: 10.1111/ppe.12363
52. Fenton PM, Whitty CJ, Reynolds F. Caesarean section in Malawi: prospective study of early maternal and perinatal mortality. BMJ. (2003) 327:587. doi: 10.1136/bmj.327.7415.587
53. Gray R, Quigley MA, Hockley C, Kurinczuk JJ, Goldacre M, Brocklehurst P. Caesarean delivery and risk of stillbirth in subsequent pregnancy: a retrospective cohort study in an English population. BJOG. (2007) 114:264–70. doi: 10.1111/j.1471-0528.2006.01249.x
54. Villar J, Valladares E, Wojdyla D, Zavaleta N, Carroli G, Velazco A, et al. Caesarean delivery rates and pregnancy outcomes: the 2005 WHO global survey on maternal and perinatal health in Latin America. Lancet. (2006) 367:1819–29. doi: 10.1016/S0140-6736(06)68704-7
Keywords: perinatal death, perinatal mortality, prospective cohort study, Poisson regression, determinants
Citation: Liu Y, Li Q, Wang T, Zhang S, Chen L, Li Y, Diao J, Li J, Song X, Sun M, Wei J, Shu J, Yang T and Qin J (2022) Determinants for Perinatal Mortality in South China: A Prospective Cohort Study. Front. Pediatr. 10:756444. doi: 10.3389/fped.2022.756444
Received: 13 December 2021; Accepted: 08 February 2022;
Published: 18 March 2022.
Edited by:
Dora Il’yasova, Duke University, United StatesReviewed by:
Jan Jaap H. M. Erwich, University Medical Center Groningen, NetherlandsDrucilla Jane Roberts, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2022 Liu, Li, Wang, Zhang, Chen, Li, Diao, Li, Song, Sun, Wei, Shu, Yang and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tubao Yang, MTA2NDk2MDY2OUBxcS5jb20=; Jiabi Qin, cWluamlhYmkxMjNAMTYzLmNvbQ==
 Yiping Liu1
Yiping Liu1 Qiongxuan Li
Qiongxuan Li Xinli Song
Xinli Song Jiabi Qin
Jiabi Qin