- 1Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology and Research (A*STAR), Singapore, Singapore
- 2Department of Biostatistics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 3Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 4Khoo Teck Puat-National University Children's Medical Institute, National University Hospital, National University Health System, Singapore, Singapore
- 5Human Potential Translational Research Programme, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 6Department of Paediatrics, KK Women's and Children's Hospital, Singapore, Singapore
- 7Department of Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore, Singapore
- 8Folkhälsan Research Center, Helsinki, Finland
- 9Department of General Practice and Primary Health Care, University of Helsinki, Helsinki, Finland
- 10MRC Lifecourse Epidemiology Unit and NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom
- 11Liggins Institute, University of Auckland, Auckland, New Zealand
- 12Department of Reproductive Medicine, KK Women's and Children's Hospital, Singapore, Singapore
- 13Academic Program in Obstetrics and Gynaecology, Duke-NUS Medical School, Singapore, Singapore
- 14Department of Psychiatry, Douglas Mental Health University Institute, McGill University, Montreal, QC, Canada
- 15Sackler Program for Epigenetics and Psychobiology, McGill University, Montreal, QC, Canada
- 16Ludmer Centre for Neuroinformatics and Mental Health, McGill University, Montréal, QC, Canada
Background: Increasing evidence suggests that maternal distress is a risk factor for development of respiratory infections and allergic diseases in the offspring. We aim to evaluate the link between maternal distress during critical periods in early life, namely the preconception, pregnancy and postnatal periods, and development of respiratory infections and allergic diseases in the offspring from the Singapore PREconception Study of long Term maternal and child Outcomes (S-PRESTO) cohort.
Methods: Maternal perceived distress was evaluated using validated questionnaires including Beck Depression Inventory-II (BDI-II) administered during three time periods: preconception (three months apart at four timepoints), pregnancy (during each trimester) and postnatal (3 and 6 months post-delivery). Child eczema, rhinitis and wheeze outcomes were evaluated using a modified ISAAC questionnaire at ages 3, 6, 12, and 18 months. Child allergic sensitization was determined by skin prick testing at 18 months.
Results: Among 332 mother-child pairs studied, higher maternal distress during preconception and pregnancy increased the risks of wheeze development in the first 18 months; for example, preconception and pregnancy BDI-II scores ≥20 were associated with increased risks of wheeze by 18 months [adjusted risk ratios 3.2 (95%CI 1.1–9.4) and 2.5 (1.0–5.9), respectively]. Emotional and practical support from family during preconception decreased the risks of offspring wheeze. No associations were observed between maternal distress and offspring eczema, rhinitis and allergic sensitization.
Conclusion: Maternal distress during critical early life periods was associated with offspring wheeze in the first 18 months of life. Supporting maternal mental health even before pregnancy could reduce the risk of offspring wheeze.
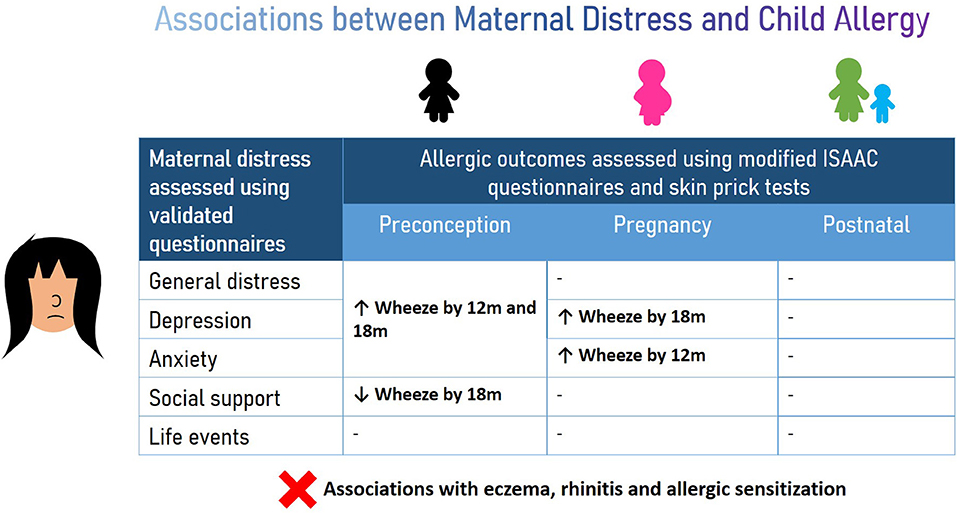
Graphical Abstract. Maternal distress during preconception and pregnancy periods increased the risk of wheeze development in children while social support decreased the risk. No association was observed between maternal distress experienced during all three window periods and eczema, rhinitis and allergic sensitization in the offspring.
Introduction
Allergy and respiratory infections are global health issues (1, 2) and impact the quality of life as well as school performance of children. The rapid increase in prevalence of allergic diseases and respiratory infections is postulated to be due to environmental and lifestyle factors such as psychosocial distress, which is defined as an emotional state of discomfort resulting from exposure to stress (3). Findings from epidemiological studies strongly suggest that maternal health during preconception and over the course of pregnancy and postnatal development influence child's health. The influence of early life environment on child's health forms the basis for the Developmental Origins of Health and Disease (DOHaD) paradigm which hypothesizes that early environmental stimuli during preconception, pregnancy and early life may influence fetal and neonatal immune development and cause development of diseases including eczema, asthma, allergic rhinitis and allergic sensitization during early life (4–6).
Increasing evidence suggests that maternal distress is a risk factor for development of allergic and respiratory diseases in the offspring. In a meta-analysis of 30 studies and a cross-sectional study involving 3,758 Italian mother-child pairs, prenatal maternal distress was associated with increased risk of development of eczema, rhinitis, wheeze, and asthma in the offspring (7, 8). Prenatal maternal anxiety, depression and distress were also associated with higher risk of eczema in two Korean cohorts of children at 4 and 5 years of age (9). The Generation R study from the Netherlands reported that mothers with higher distress levels had an increased risk of having offspring who wheezed at 1–4 years of age (10). In China, schoolchildren had increased risk of rhinitis if their mothers experienced symptoms of depression during and after pregnancy (11). Furthermore, the prevalence of maternal distress has increased in recent years; prenatal depression was twice as common in a cohort of young mothers as compared to their mothers, while severe postnatal depression increased by 34% over a five-year period in a US study (12, 13).
Extensive research over the past years showed that maternal distress can influence the offspring's immune system by regulating the hypothalamic-pituitary-adrenal (HPA) axis that plays a pivotal role in regulating adaptive immunological responses to stressors (Figure 1). High maternal distress promotes cortisol production and secretion, downregulates expression of 11β-hydroxysteroid dehydrogenase 2 in the placenta and consequently exposes the fetus to excessive cortisol levels (14–16). Elevated cortisol exposure is linked to dysregulated HPA axis function in infants, which can aggravate allergic inflammation (17, 18) and favor a T-helper 2 (Th2) immune response by inhibiting interleukin-12, a Th1 cytokine (19, 20). Studies have also reported prenatal maternal distress to be linked to higher respiratory infection rates/risk in the offspring possibly due to dysregulated HPA axis and poorer maternal dietary and lifestyle habits (21, 22).
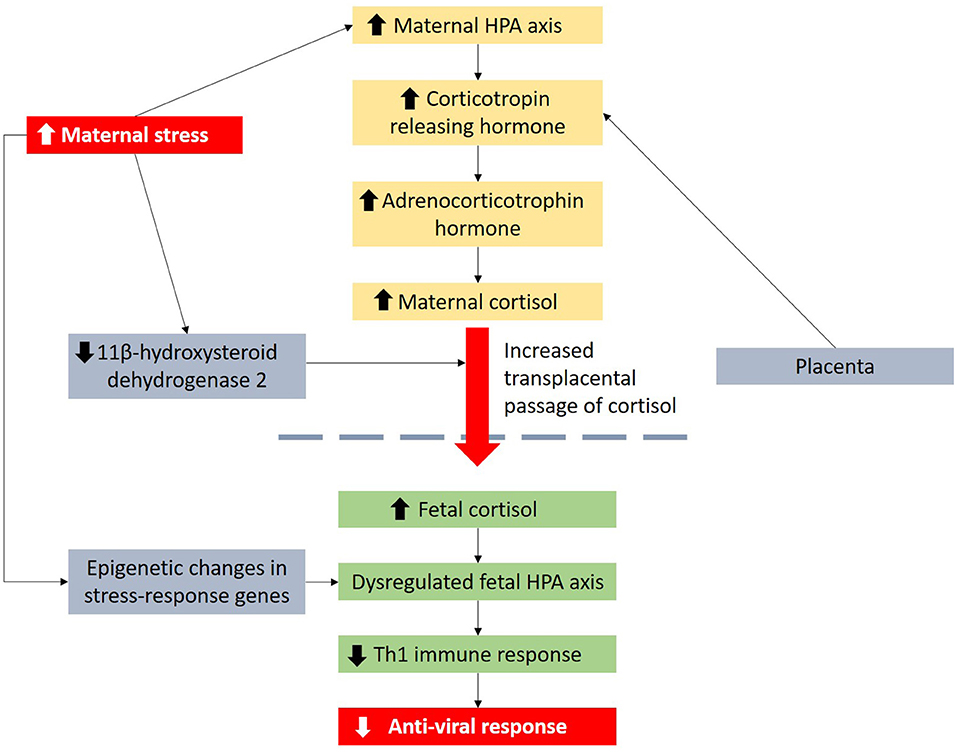
Figure 1. Maternal distress is linked to viral infection in children through dysregulation of the HPA axis and influence of Th1 immune response. Maternal distress can also lead to epigenetic changes in stress-response genes.
While several studies have focused on prenatal maternal distress (23), very few studies have explored the association between maternal distress during preconception and allergy as well as respiratory infections in the offspring. Among 3,008 mother-child pairs in the Southampton Women's Survey, a positive association was found between preconception maternal distress and development of eczema in infants at 12 months (24). A Swedish study of 3.2 million mother-child pairs showed that the offspring of mothers who experienced severe life events up to 6 months before and during pregnancy had increased risk of hospitalization due to asthma and other related diagnoses including bronchiolitis, eczema and respiratory infections especially in the first 2 years of life (25). Our focus on early life starting periconceptually and across critical development periods allows us to examine the earliest possible developmental influences independent of numerous confounders that emerge subsequently. This will enable the identification of earliest risk factors where interventions may be more effective.
To the best of our knowledge, there are no studies that have evaluated the impact of maternal distress during all three critical time periods, namely preconception, pregnancy and postnatal, and the development of allergic diseases and respiratory infections in the offspring. Hence, we aimed to evaluate this relationship in the Singapore PREconception Study of long Term maternal and child Outcomes (S-PRESTO) cohort.
Materials and Methods
S-PRESTO Study Design and Definition of Allergic Outcomes
The S-PRESTO study is a prospective cohort study which recruited women aged 18–45 years old who planned to conceive and deliver in Singapore, out of which 373 infants were born. The detailed methodology was described by Loo et al. (26). Trained interviewers gathered information on demographic characteristics, family history of allergy, socioeconomic data, and lifestyle factors. The ISAAC modified questionnaire was used to evaluate offspring eczema, wheeze, and rhinitis symptoms at ages 3, 6, 12, and 18 months. Eczema was determined as maternally reported doctor diagnosis of eczema. Wheeze with use of nebulizer/inhaler was defined by positive responses to the questions: “Has your child ever wheezed?” and “Has your child ever been prescribed with nebulizer/inhaler treatment?”. Rhinitis was defined as a positive response to the question “Has your child had running nose, blocked or congested nose, snoring or noisy breathing during sleep or when awake that has lasted for 2 or more weeks duration?”. Cumulative eczema, wheeze with the use of nebulizer/inhaler or rhinitis by 6, 12, and 18 months were classified as “yes” when a subject answered “yes” by the time point and “no” if the subject answered “no” at all time points. Ethical approval was obtained from the SingHealth Centralised Institutional Review Board (reference 2014/692/D). This study has been registered at ClinicalTrials.gov (NCT 03531658). Written informed consent was provided by the participants.
Allergen Sensitization
Skin prick testing (SPT) was performed at 18 months for the major relevant allergens cow's milk, whole egg, peanut, soy, wheat, shrimp, crab, and house dust mites Dermatophagoides pteronyssinus (Der p), Dermatophagoides farina (Derp f ) and Blomia tropicalis (Blo t). The infant was classified as having positive SPT if any of the SPT to the allergens was positive (minimum wheal size of 3 mm) and negative if all of the SPT to the allergens were negative.
Distress Assessment
Maternal perceived distress was assessed using a battery of validated questionnaires assessing symptoms of depression [Edinburgh Postnatal Depression Scale (EPDS) and Beck Depression Inventory-II (BDI-II)], anxiety [State-Trait Anxiety Inventory (STAI) and Pregnancy Anxiety Questionnaire (PAQ)], facets of social support [Multidimensional Scale of Perceived Social Support (MSPSS)], life events [Life Experiences Survey (LES)] and levels of general perceived stress [General Health Questionnaire (GHQ), Pregnancy Experience Scale (PES) and Perceived Stress Scale (PSS)]. Depression refers to prolonged feelings of loss of interest, sadness and hopelessness (27). Anxiety refers to feelings of uneasiness or apprehension due to anticipation of future negative events (28). The MSPSS evaluates perceived support from spouse, family and friends in terms of ability to share joys and sorrows, obtain comfort, share problems and help in decision-making and solving problems (29). The questionnaires were administered at different time points from preconception to postnatal: at each trimester during pregnancy and at two time points during the postnatal period. The maximum distress during preconception, pregnancy and postnatal were computed from each of the questionnaires.
Statistical Analysis
All analyses were performed using SPSS for Windows version 26.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was set at two-sided p < 0.05. Descriptive statistics for numerical variables were presented as mean (SD) when normality and homogeneity assumptions were satisfied, otherwise median (IQR) were presented and n (%) for categorical variables. Predictors for offspring allergic outcomes by ages 6, 12, and 18 months and SPT at month 18 were assessed using modified Poisson regression for prospective studies with binary outcomes (30–33), adjusting for demographic and relevant covariates period of maximum distress (if distress accessed at several time points), ethnicity, maternal age at birth, length of education, parity, smoking during pregnancy, maternal history of allergy, infant sex and gestational age at birth as assessed from literature review (7, 34). Smoking during pregnancy was not adjusted for in the postnatal period. Type 1 error for multiple comparisons were adjusted using Benjamini–Hochberg procedure with false discovery rate at 0.45.
Results
Study Population Characteristics
In this study, 332 mother-child pairs with data on both maternal distress and child respiratory infections and allergic outcomes were included. The mothers' mean age at delivery was 31.6 years (SD 3.2, Table 1). The majority of mothers were of Chinese ethnicity [254 (76.5%)], had at least 12 years of education [309 (93.1%)], had a history of allergy [235 (70.8%)], were nulliparous [203 (61.3%)] and did not smoke during pregnancy [310 (99.4%)]. Of the 332 infants, 182 (55.0%) were boys. There were 51 (17.1%), 73 (25.7%) and 87 (31.5%) infants who developed eczema by ages 6, 12, and 18 months, respectively. There were 106 (34.5%), 142 (48.0%) and 159 (55.2%) infants who developed rhinitis by ages 6, 12, and 18 months, respectively and 10 (3.3%), 30 (10.8%) and 33 (12.8%) wheezed and used nebulizer by ages 6, 12, and 18 months respectively. At age 18 months, 40 (16.5%) had a positive SPT.
Association Between Maternal Distress and Allergic Outcomes in the Offspring
General Distress
Univariate associations are presented in Supplementary Tables 1–4. In multivariate analyses, higher preconception GHQ scores were associated with increased risk of wheeze by 12 and 18 months after adjusting for demographic and relevant covariates (AdjRR 1.2, 95% CI 1.1–1.4 and AdjRR 1.2, 95% CI 1.1–1.3, respectively, Table 2). Higher preconception PSS scores were associated with increased risk of wheeze by 12 and 18 months (AdjRR 1.1, 95% CI 1.0–1.2 and AdjRR 1.1, 95% CI 1.0–1.2, respectively). There were no associations between GHQ and Perceived Stress Scale scores and PES Hassles/Uplifts frequency and intensity ratios with child eczema, rhinitis and allergic sensitization outcomes (Supplementary Tables 5–7).
Depression
Higher preconception BDI scores were associated with increased risk of wheeze by 12 and 18 months (AdjRR 1.07, 95% CI 1.01–1.13 and AdjRR 1.06, 95% CI 1.00–1.12, respectively, Table 2). Further analysis with BDI categories showed that preconception BDI scores ≥ 20 increased the risk of wheeze by 12 months (AdjRR 3.5, 95% CI 1.2–10.9). Preconception and pregnancy BDI scores ≥ 20 were associated with increased risk of wheeze by 18 months (AdjRR 3.2, 95% CI 1.1–9.4, respectively, AdjRR 2.5, 95% CI 1.0–5.9, respectively).
Higher preconception EPDS scores were associated with increased risk of wheeze by 12 and 18 months (AdjRR 1.1, 95% CI 1.0–1.3 and AdjRR 1.1, 95% CI 1.0–1.2, respectively).
There were no associations between BDI and EPDS scores and child eczema, rhinitis and allergic sensitization outcomes (Supplementary Tables 5–7).
Anxiety
Higher preconception STAI trait scores were associated with increased risk of wheeze by 12 and 18 months, respectively (AdjRR 1.06, 95% CI 1.00–1.13 and AdjRR 1.07, 95% CI 1.01–1.13, respectively, Table 2). Higher pregnancy STAI state scores were associated with increased risk of wheeze by 12 months (AdjRR 1.04, 95% CI 1.00–1.08).
There were no associations between STAI trait and state scores and Pregnancy Anxiety Questionnaire scores and child eczema, rhinitis and allergic sensitization outcomes (Supplementary Tables 5–7).
Social Support
Higher preconception MSPSS emotional support from family scores and practical support from family scores were associated with a lower risk of wheeze by age 18 months (AdjRR 0.58, 95% CI 0.38–0.89) and AdjRR 0.66, 95% CI 0.43–0.99, respectively, Table 2).
There were no associations between MSPSS emotional and physical support scores and child eczema, rhinitis and allergic sensitization outcomes (Supplementary Tables 5–7).
Life Events
There were no associations between positive and negative LES scores and child eczema, rhinitis, wheeze with the use of nebuliser and allergic sensitization outcomes (Supplementary Tables 5–7).
Discussion
In this study, we examined aspects of maternal distress during preconception, pregnancy and postnatal periods using a battery of validated questionnaires to assess maternal distress and their associations with eczema, rhinitis, wheeze, and allergic sensitization outcomes in the offspring in early life.
We observed associations of higher maternal distress during preconception and pregnancy with higher risks of wheeze development by ages 12 and 18 months, while social support decreased the risk. Supportive evidence is provided by the GUSTO cohort from Singapore which reported significant associations between maternal depression during pregnancy and child wheeze by age 1 year (35) and a meta-analysis which reported a 56% higher risk of wheeze in offspring whose mothers experienced prenatal psychological distress levels (36), suggesting that control of maternal distress through social support can reduce offspring wheeze risk.
Wheezing illnesses are mainly caused by respiratory viruses, and not by allergy, in young children (37). Supporting evidence of the role of viruses in the etiology of wheeze has been provided by a number of studies. The COAST study in the US identified 90% of wheezing in children up to 3 years of age to be associated with viral etiology (37). A US study of children who visited the emergency department for wheezing reported that respiratory viruses were detected in 82% of wheezing infants younger than age 2 years (38). We postulate that the associations between maternal distress and wheeze may be due to lower anti-viral responses in the offspring (Figure 1). Hyper-reactivity of the HPA axis to stress is linked to enhanced production of glucocorticoids which inhibit Th1 responses that are essential in anti-viral responses (39, 40). Maternal distress during preconception and pregnancy can also result in persisting and epigenetic changes in genes involved in stress responses (41, 42) which may be passed to the offspring. For example, murine models showed that maternal preconception distress resulted in increased expression of corticotropin releasing factor type 1, a protein key in stress responses, in mature oocytes and offspring brain (43). Higher cord blood Nuclear Receptor Subfamily 3 Group C Member 1 (NR3C1) CpG3 methylation is also linked to higher maternal depression and anxiety during third trimester of pregnancy and increased infant salivary cortisol stress responses at 3 months of age, suggesting increased HPA stress response in infants (44).
Supporting evidence of the link between maternal distress and lower immunity in the offspring is also provided by a number of studies; Rusconi et al. reported that higher maternal GHQ scores i.e. poorer mental health during and after pregnancy increased the risk of wheezing as well as respiratory and gastroenteric infections in the offspring at 1–2 years (45). In another cohort of more than 1.6 million Danish children, maternal stressful events up to 11 months before pregnancy were linked to higher risk of infectious disease hospitalization in the offspring (46).
In this study, we did not observe any associations between maternal distress experienced preconception, or during the pregnancy or postnatal periods and eczema, rhinitis and allergic sensitization in the offspring. Existing studies have yielded conflicting results on the association between maternal distress and these allergic outcomes (47). In support of our findings, the Ulm SPATZ Health Study reported that mothers belonging to the highest quartile in relation to prenatal distress, anxiety and depression did not observe more parental report of child eczema diagnosis by 2 years (48). The GUSTO cohort reported non-significant associations between maternal depression and anxiety during pregnancy as assessed by EPDS and STAI, respectively, with child eczema by age 1 year (35). The LISA Study did not observe significant associations between maternal distress during pregnancy and child eczema in the first 6 years of life (49). Similarly, the ALSPAC study reported no associations between maternal anxiety at 18 and 32 weeks of pregnancy and child allergic sensitization at 7.5 years (34). The Western Australian Pregnancy Cohort also did not observe significant associations between maternal distress during pregnancy and child rhinitis at ages 6 and 14 years (50). Contrary to our observations, the UK Southampton Women's Survey observed that preconception distress as assessed by the Short Form (36) Health Survey was linked to higher risk of eczema development in the offspring at 12 months (24). The China National Birth Cohort Study also reported an association between maternal distress during pregnancy and infant eczema development at 6 months (51). Another study of 24200 mother-child pairs in Taiwan reported that postpartum depression at 6 months was associated with an increased risk of child eczema at 3 years (52). The Viadana study reported that maternal stressful life events during pregnancy increased the risk of allergic rhinitis in children aged ~8.5 years (8). Possible explanations for these discrepancies include the use of different types of distress assessments methods. For example, the Ulm SPATZ study used Trier Inventory of Chronic Stress, Pregnancy Related Anxiety Questionnaire and Hospital Anxiety and Depression Scale while the UK Southampton Women's Survey used the Short Form (36) Health Survey. Moreover, although rhinitis can also be viral-induced (53), our study did not differentiate between allergic and infectious rhinitis which might have reduced the strength of associations between maternal distress and rhinitis. Taken together, our observations suggest that maternal distress may result in specific lower anti-viral immune responses to respiratory viruses in the offspring rather than allergic disease development.
The strengths of this study include the comprehensive assessment of maternal distress at multiple time points via a battery of validated questionnaires from preconception to pregnancy and after birth. The specific design of this preconceptional study can offer new insights into the earliest precursors and risk factors of child's health in an Asian population. A limitation of our study is the modest sample size. However, we have increased the reliability of our results using robust statistical methods. Although we used questionnaires to gather information on allergic disease diagnosis and maternal mental health, these questionnaires had also been used by numerous studies in the field (54–71). This limitation is also mitigated by regular follow-ups to reduce recall bias. We also did not assess physiological responses to maternal distress in both mothers and offspring and this should be evaluated in future research.
In conclusion, maternal distress during critical early life periods was associated with an increased risk of wheeze development in children in the first 18 months of life. This study highlights the importance of supporting maternal mental health, even before pregnancy, to improve offspring's health.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Ethics Statement
The studies involving human participants were reviewed and approved by SingHealth Centralised Institutional Review Board (reference 2014/692/D). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
HL and QY analyzed the data and wrote the manuscript. MK provided intellectual input and wrote the manuscript. YHC provided statistical advice and intellectual input. ET, AG, OT, JE, KG, PG, YSC, JC, HV, BL, LS and MM contributed to the study design and provided intellectual input. EL conceptualized the study design, contributed to the analysis and wrote the manuscript. All authors critically reviewed the manuscript.
Funding
This work is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme, administered by the Singapore Ministry of Health's National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science and Technology. KG is supported by the UK Medical Research Council (MC_UU_12011/4), the National Institute for Health Research [NIHR Senior Investigator (NF-SI-0515-10042) and NIHR Southampton Biomedical Research Centre (IS-BRC-1215-20004)], the European Union (Erasmus+ Programme ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP) and the British Heart Foundation (RG/15/17/3174).
Conflict of Interest
KG has received reimbursement for speaking at conferences sponsored by Nestle. KG and YC are part of an academic consortium that has received research funding from Abbot Nutrition, Nestle and Danone.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the S-PRESTO study group and all clinical and home-visit staff involved. The voluntary participation of all participants is greatly appreciated. The S-PRESTO study group includes includes Airu Chia, Anna Magdalena Fogel, AG, Anne Hin Yee Chu, Anne Rifkin-Graboi, Anqi Qiu, BL, Bobby Kyungbeom Cheon, Candida Vaz, Christiani Jeyakumar Henry, Ciaran Gerard Forde, Claudia Chi, Dawn Xin Ping Koh, Desiree Y. Phua, Doris Ngiuk Lan Loh, Elaine Phaik Ling Quah, Elizabeth Huiwen Tham, Evelyn Chung Ning Law, Faidon Magkos, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Hannah Ee Juen Yong, Helen Yu Chen, Heng Hao Tan, Hong Pan, Hugo P S van Bever, Hui Min Tan, Izzuddin Bin Mohd Aris, Jeannie Tay, JC, Jia Xu, Joanne Su-Yin Yoong, JE, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Jonathan Yinhao Huang, Jun Shi Lai, Karen Mei Ling Tan, KG, Kenneth Yung Chiang Kwek, Keri McCrickerd, Kothandaraman Narasimhan, Kok Wee Chong, Kuan Jin Lee, Li Chen, Lieng Hsi Ling, Ling-Wei Chen, Lourdes Mary Daniel, LS, Marielle V. Fortier, Mary Foong-Fong Chong, Mei Chien Chua, Melvin Khee-Shing Leow, Michelle Zhi Ling Kee, Min Gong, Mya Thway Tint, Navin Michael, Ngee Lek, OT, Priti Mishra, Queenie Ling Jun Li, Sambasivam Sendhil Velan, Seng Bin Ang, Shirong Cai, Si Hui Goh, Sok Bee Lim, Stella Tsotsi, Stephen Chin-Ying Hsu, Sue-Anne Ee Shiow Toh, Suresh Anand Sadananthan, Teng Hong Tan, Tong Wei Yew, Varsha Gupta, Victor Samuel Rajadurai, Wee Meng Han, Wei Wei Pang, Wen Lun Yuan, Yanan Zhu, Yin Bun Cheung, Yiong Huak Chan and Zai Ru Cheng.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.749323/full#supplementary-material
Abbreviations
BDI-II, Beck Depression Inventory-II; Blo t, Blomia tropicalis; Der f , Dermatophagoides farina; Der p, Dermatophagoides pteronyssinus; EPDS, Edinburgh Postnatal Depression Scale; GHQ, General Health Questionnaire; HPA, Hypothalamic-pituitary-adrenal; LES, Life Experiences Survey; MSPSS, Multidimensional Scale of Perceived Social Support; PAQ, Pregnancy Anxiety Questionnaire; PES, General Health Questionnaire; PSS, Perceived Stress Scale; S-PRESTO, Singapore PREconception Study of long Term maternal and child Outcomes; SPT, Skin prick testing; STAI, State-Trait Anxiety Inventory.
References
2. Zar HJ, Ferkol TW. The global burden of respiratory disease—Impact on child health. Pediatr Pulmonol. (2014) 49:430–4. doi: 10.1002/ppul.23030
3. Ridner SH. Psychological distress: concept analysis. J Adv Nurs. (2004) 45:536–45. doi: 10.1046/j.1365-2648.2003.02938.x
4. Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. (2007) 27:363–88. doi: 10.1146/annurev.nutr.27.061406.093705
5. Larsen AD, Schlünssen V, Christensen BH, Bonde JP, Obel C, Thulstrup AM, et al. Exposure to psychosocial job strain during pregnancy and odds of asthma and atopic dermatitis among 7-year old children-a prospective cohort study. Scand J Work Environ Health. (2014) 40:639–48. doi: 10.5271/sjweh.3452
6. Mathilda Chiu Y-H, Coull BA, Cohen S, Wooley A, Wright RJ. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. Am J Respir Crit Care Med. (2012) 186:147–54. doi: 10.1164/rccm.201201-0162OC
7. Flanigan C, Sheikh A, DunnGalvin A, Brew BK, Almqvist C, Nwaru BI. Prenatal maternal psychosocial stress and offspring's asthma and allergic disease: a systematic review and meta-analysis. Clin Exp Allergy. (2018) 48:403–14. doi: 10.1111/cea.13091
8. de Marco R, Pesce G, Girardi P, Marchetti P, Rava M, Ricci P, et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatr Allergy Immunol. (2012) 23:724–9. doi: 10.1111/j.1399-3038.2012.01346.x
9. Chang HY, Suh DI, Yang S-I, Kang MJ, Lee SY, Lee E, et al. Prenatal maternal distress affects atopic dermatitis in offspring mediated by oxidative stress. J Allergy Clin Immunol. (2016) 138:468–75. e465. doi: 10.1016/j.jaci.2016.01.020
10. Guxens M, Sonnenschein–van der Voort AM, Tiemeier H, Hofman A, Sunyer J, de Jongste JC, et al. Parental psychological distress during pregnancy and wheezing in preschool children: the Generation R Study. J Allergy Clin Immunol. (2014) 133:59–67. e12. doi: 10.1016/j.jaci.2013.04.044
11. Li Y, Jiang Y, Li S, Shen X, Liu J, Jiang F. Pre-and postnatal risk factors in relation to allergic rhinitis in school-aged children in China. PLoS ONE. (2015) 10:e0114022. doi: 10.1371/journal.pone.0114022
12. Pearson RM, Carnegie RE, Cree C, Rollings C, Rena-Jones L, Evans J, et al. Prevalence of Prenatal Depression Symptoms Among 2 Generations of Pregnant Mothers: The Avon Longitudinal Study of Parents and Children. JAMA Netw Open. (2018) 1:e180725–e180725. doi: 10.1001/jamanetworkopen.2018.0725
13. França UL, McManus ML. Frequency, trends, and antecedents of severe maternal depression after three million US births. PLOS ONE. (2018) 13:e0192854. doi: 10.1371/journal.pone.0192854
14. Fan F, Zou Y, Zhang Y, Ma X, Zhang J, Liu C, et al. The relationship between maternal anxiety and cortisol during pregnancy and birth weight of chinese neonates. BMC Pregnancy Childbirth. (2018) 18:265. doi: 10.1186/s12884-018-1798-x
15. Duthie L, Reynolds RM. Changes in the Maternal Hypothalamic-Pituitary-Adrenal Axis in Pregnancy and Postpartum: Influences on Maternal and Fetal Outcomes. Neuroendocrinology. (2013) 98:106–15. doi: 10.1159/000354702
16. O'Donnell KJ, Jensen AB, Freeman L, Khalife N, O'Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology. (2012) 37:818–26. doi: 10.1016/j.psyneuen.2011.09.014
17. McGowan PO, Matthews SG. Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology. (2018) 159:69–82. doi: 10.1210/en.2017-00896
18. Buske-Kirschbaum A, Geiben A, Höllig H, Morschhäuser E, Hellhammer D. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. J Clin Endocrinol Metab. (2002) 87:4245–51. doi: 10.1210/jc.2001-010872
19. Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. (1999) 10:359–68. doi: 10.1016/S1043-2760(99)00188-5
20. Von Hertzen LC. Maternal stress and T-cell differentiation of the developing immune system: possible implications for the development of asthma and atopy. J Allergy Clin Immunol. (2002) 109:923–8. doi: 10.1067/mai.2002.124776
21. Phelan AL, DiBenedetto MR, Paul IM, Zhu J, Kjerulff KH. Psychosocial Stress During First Pregnancy Predicts Infant Health Outcomes in the First Postnatal Year. Matern Child Health J. (2015) 19:2587–97. doi: 10.1007/s10995-015-1777-z
22. Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal Prenatal Anxiety and Stress Predict Infant Illnesses and Health Complaints. Pediatrics. (2010) 126:e401. doi: 10.1542/peds.2009-3226
23. Andersson N, Hansen M, Larsen A, Hougaard K, Kolstad H, Schlünssen V. Prenatal maternal stress and atopic diseases in the child: a systematic review of observational human studies. Allergy. (2016) 71:15–26. doi: 10.1111/all.12762
24. El-Heis S, Crozier SR, Healy E, Robinson SM, Harvey NC, Cooper C, et al. Maternal stress and psychological distress preconception: association with offspring atopic eczema at age 12 months. Clin Exp Allergy. (2017) 47:760–9. doi: 10.1111/cea.12910
25. Khashan AS, Wicks S, Dalman C, Henriksen TB Li J, Mortensen PB, et al. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosom Med. (2012) 74:635–41. doi: 10.1097/PSY.0b013e31825ac5e7
26. Loo EXL, Soh S-E, Loy SL, Ng S, Tint MT, Chan SY, et al. Cohort profile: Singapore Preconception Study of Long-Term Maternal and Child Outcomes (S-PRESTO). Eur J Epidemiol. (2020).
28. Craske MG, Rauch SL, Ursano R, Prenoveau J, Pine DS, Zinbarg RE. What is an anxiety disorder? Depress Anxiety. (2009) 26:1066–85. doi: 10.1002/da.20633
29. Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support. J Pers Assess. (1988) 52:30–41. doi: 10.1207/s15327752jpa5201_2
30. Zou G A. Modified Poisson Regression Approach to Prospective Studies with Binary Data. Am J Epidemiol. (2004) 159:702–6. doi: 10.1093/aje/kwh090
31. Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. (1987) 125:761–8. doi: 10.1093/oxfordjournals.aje.a114593
32. Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. J Clin Epidemiol. (1994) 47:881–9. doi: 10.1016/0895-4356(94)90191-0
33. Nurminen M. To use or not to use the odds ratio in epidemiologic analyses? Eur J Epidemiol. (1995) 11:365–71. doi: 10.1007/BF01721219
34. Cookson H, Granell R, Joinson C, Ben-Shlomo Y, Henderson AJ. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. (2009) 123:847–53.e11. doi: 10.1016/j.jaci.2009.01.042
35. Cheng TS, Chen H, Lee T, Teoh OH, Shek LP, Lee BW, et al. An independent association of prenatal depression with wheezing and anxiety with rhinitis in infancy. Pediatr Allergy Immunol. (2015) 26:765–71. doi: 10.1111/pai.12453
36. van de Loo KFE, van Gelder MMHJ, Roukema J, Roeleveld N, Merkus PJFM, Verhaak CM. Prenatal maternal psychological stress and childhood asthma and wheezing: a meta-analysis. Eur Respir J. (2016) 47:133. doi: 10.1183/13993003.00299-2015
37. Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing Rhinovirus Illnesses in Early Life Predict Asthma Development in High-Risk Children. Am J Respir Crit Care Med. (2008) 178:667–72. doi: 10.1164/rccm.200802-309OC
38. Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, et al. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. Am J Respir Crit Care Med. (1999) 159:785–90. doi: 10.1164/ajrccm.159.3.9801052
39. Buske-Kirschbaum A. Cortisol responses to stress in allergic children: interaction with the immune response. Neuroimmunomodulation. (2009) 16:325–32. doi: 10.1159/000216190
40. Komastu T, Ireland DDC, Reiss CS. IL-12 and Viral Infections. Cytokine Growth Factor Rev. (1998) 9:277–85. doi: 10.1016/S1359-6101(98)00017-3
41. McCormick CM, Robarts D, Kopeikina K, Kelsey JE. Long-lasting, sex- and age-specific effects of social stressors on corticosterone responses to restraint and on locomotor responses to psychostimulants in rats. Horm Behav. (2005) 48:64–74. doi: 10.1016/j.yhbeh.2005.01.008
42. Tyrka AR, Price LH, Marsit C, Walters OC, Carpenter LL. Childhood Adversity and Epigenetic Modulation of the Leukocyte Glucocorticoid Receptor: Preliminary Findings in Healthy Adults. PLoS ONE. (2012) 7:e30148. doi: 10.1371/journal.pone.0030148
43. Zaidan H, Leshem M, Gaisler-Salomon I. Prereproductive stress to female rats alters corticotropin releasing factor type 1 expression in ova and behavior and brain corticotropin releasing factor type 1 expression in offspring. Biol Psychiatry. (2013) 74:680–7. doi: 10.1016/j.biopsych.2013.04.014
44. Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. (2008) 3:97–106. doi: 10.4161/epi.3.2.6034
45. Rusconi F, Gagliardi L, Gori E, Porta D, Popovic M, Asta F, et al. Perinatal maternal mental health is associated with both infections and wheezing in early childhood. Pediatr Allergy Immunol. (2019) 30:732–8. doi: 10.1111/pai.13103
46. Nielsen NM, Hansen AV, Simonsen J, Hviid A. Prenatal stress and risk of infectious diseases in offspring. Am J Epidemiol. (2011) 173:990–7. doi: 10.1093/aje/kwq492
47. Chan CWH, Law BMH, Liu Y-H, Ambrocio ARB, Au N, Jiang M, et al. The association between maternal stress and childhood eczema: a systematic review. Int J Environ Res Public Health. (2018) 15:395. doi: 10.3390/ijerph15030395
48. Braig S, Weiss JM, Stalder T, Kirschbaum C, Rothenbacher D, Genuneit J. Maternal prenatal stress and child atopic dermatitis up to age 2 years: The Ulm SPATZ health study. Pediatr Allergy Immunol. (2017) 28:144–51. doi: 10.1111/pai.12680
49. Sausenthaler S, Rzehak P, Chen C, Arck P, Bockelbrink A, Schäfer T, et al. Stress-related maternal factors during pregnancy in relation to childhood eczema: results from the LISA Study. J Investig Allergol Clin Immunol. (2009) 19:481.
50. Hartwig IRV, Sly PD, Schmidt LA, van Lieshout RJ, Bienenstock J, Holt PG, et al. Prenatal adverse life events increase the risk for atopic diseases in children, which is enhanced in the absence of a maternal atopic predisposition. J Allergy Clin Immunol. (2014) 134:160–9.e167. doi: 10.1016/j.jaci.2014.01.033
51. Shen Q, Zhang Q, Zhao J, Huang Z, Wang X, Ni M, et al. Association between maternal perceived stress in all trimesters of pregnancy and infant atopic dermatitis: a prospective birth cohort study. Front Pediatr. (2020) 8:730. doi: 10.3389/fped.2020.526994
52. Wang IJ, Wen HJ, Chiang TL, Lin SJ, Guo YL. Maternal psychologic problems increased the risk of childhood atopic dermatitis. Pediatr Allergy Immunol. (2016) 27:169–76. doi: 10.1111/pai.12518
53. Regan EN. Diagnosing Rhinitis: Viral and Allergic Characteristics. Nurse Pract. (2008) 33:20–6. doi: 10.1097/01.NPR.0000335565.56896.d9
54. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. (1995) 8:483. doi: 10.1183/09031936.95.08030483
55. Keil T, Kulig M, Simpson A, Custovic A, Wickman M, Kull I, et al. European birth cohort studies on asthma and atopic diseases: II. Comparison of outcomes and exposures—a GA2LEN initiative. Allergy. (2006) 61:1104–11. doi: 10.1111/j.1398-9995.2006.01167.x
56. Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. (2020) 371:m4022. doi: 10.1136/bmj.m4022
57. Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the Validity of the Beck Depression Inventory. Psychopathology. (1998) 31:160–8. doi: 10.1159/000066239
58. Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Braz J Psychiatry. (2013) 35:416–31. doi: 10.1590/1516-4446-2012-1048
59. Julian LJ. Measures of anxiety: State-Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale-Anxiety (HADS-A). Arthritis Care Res. (2011) 63:S467–S72. doi: 10.1002/acr.20561
60. Rini CK, Dunkel-Schetter C, Wadhwa PD, Sandman CA. Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol. (1999) 18:333–45. doi: 10.1037/0278-6133.18.4.333
61. Lin B, Kaliush PR, Conradt E, Terrell S, Neff D, Allen AK, et al. Intergenerational transmission of emotion dysregulation: Part I. Psychopathology, self-injury, and parasympathetic responsivity among pregnant women. Dev Psychopathol. (2019) 31:817–31. doi: 10.1017/S0954579419000336
62. Mahrer NE, Ramos IF, Guardino C, Davis EP, Ramey SL, Shalowitz M, et al. Pregnancy anxiety in expectant mothers predicts offspring negative affect: The moderating role of acculturation. Early Hum Dev. (2020) 141:104932. doi: 10.1016/j.earlhumdev.2019.104932
63. Zimet GD, Powell SS, Farley GK, Werkman S, Berkoff KA. Psychometric characteristics of the multidimensional scale of perceived social support. J Pers Assess. (1990) 55:610–7. doi: 10.1207/s15327752jpa5503&4_17
64. Dambi JM, Corten L, Chiwaridzo M, Jack H, Mlambo T, Jelsma J, et al. systematic review of the psychometric properties of the cross-cultural translations and adaptations of the Multidimensional Perceived Social Support Scale (MSPSS). Health Qual Life Outcomes. (2018) 16:80. doi: 10.1186/s12955-018-0912-0
65. Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the life experiences survey. J Consult Clin Psychol. (1978) 46:932–46. doi: 10.1037/0022-006X.46.5.932
66. Frewen P, Zhu J, Lanius R. Lifetime traumatic stressors and adverse childhood experiences uniquely predict concurrent PTSD, complex PTSD, and dissociative subtype of PTSD symptoms whereas recent adult non-traumatic stressors do not: results from an online survey study. Eur J Psychotraumatol. (2019) 10:1606625. doi: 10.1080/20008198.2019.1606625
67. Archea C, Yen IH, Chen H, Eisner MD, Katz PP, Masharani U, et al. Negative life events and quality of life in adults with asthma. Thorax. (2007) 62:139. doi: 10.1136/thx.2006.065730
68. Ip WY, Martin C. Psychometric properties of the 12-item General Health Questionnaire (GHQ-12) in Chinese women during pregnancy and in the postnatal period. Psychol Health Med. (2006) 11:60–9. doi: 10.1080/13548500500155750
69. Dipietro JA, Christensen AL, Costigan KA. The pregnancy experience scale–brief version. J Psychosom Obstet Gynecol. (2008) 29:262–7. doi: 10.1080/01674820802546220
70. Lee E-H. Review of the psychometric evidence of the perceived stress scale. Asian Nurs Res. (2012) 6:121–7. doi: 10.1016/j.anr.2012.08.004
Keywords: maternal distress, wheeze, rhinitis, eczema, allergic sensitization, preconception, pregnancy, postnatal
Citation: Lau HX, Kee MZL, Yap QV, Tham EH, Chan YH, Goh AEN, Teoh OH, Eriksson JG, Godfrey KM, Gluckman PD, Chong YS, Chan JKY, Van Bever H, Lee BW, Shek LP-c, Meaney MJ and Loo EXL (2022) Associations Between Maternal Distress During Early Life Periods and Offspring Respiratory Infections and Allergic Outcomes. Front. Pediatr. 10:749323. doi: 10.3389/fped.2022.749323
Received: 29 July 2021; Accepted: 16 February 2022;
Published: 30 March 2022.
Edited by:
Raz Gross, Sheba Medical Center, IsraelReviewed by:
Joanna Jerzyńska, Medical University of Lodz, PolandIvana I. Kavecan, University of Novi Sad, Serbia
Copyright © 2022 Lau, Kee, Yap, Tham, Chan, Goh, Teoh, Eriksson, Godfrey, Gluckman, Chong, Chan, Van Bever, Lee, Shek, Meaney and Loo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Evelyn Xiu Ling Loo, ZXZlbHluX2xvb0BzaWNzLmEtc3Rhci5lZHUuc2c=
 Hui Xing Lau
Hui Xing Lau Michelle Zhi Ling Kee
Michelle Zhi Ling Kee Qai Ven Yap
Qai Ven Yap Elizabeth Huiwen Tham
Elizabeth Huiwen Tham Yiong Huak Chan2
Yiong Huak Chan2 Keith M. Godfrey
Keith M. Godfrey Peter D. Gluckman
Peter D. Gluckman Hugo Van Bever
Hugo Van Bever Bee Wah Lee
Bee Wah Lee Michael J. Meaney
Michael J. Meaney Evelyn Xiu Ling Loo
Evelyn Xiu Ling Loo