
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 21 April 2022
Sec. Pediatric Critical Care
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.707136
 Xiu-Bing Gong1†
Xiu-Bing Gong1† Rui-Hua Feng2†
Rui-Hua Feng2† Hong-Mei Dong3
Hong-Mei Dong3 Wen-Hua Liu4
Wen-Hua Liu4 Ya-Nan Gu4
Ya-Nan Gu4 Xiang-Yue Jiang5
Xiang-Yue Jiang5 Ye-Hao Lou6
Ye-Hao Lou6 Jun Xu7*
Jun Xu7* Qing-Li Dou1,4*
Qing-Li Dou1,4*
Background: Preclinical and clinical evidence suggests that hyperbaric oxygen therapy (HBOT) may benefit newborns. The effectiveness of HBOT for neonatal hypoxic-ischemic encephalopathy (HIE) remains controversial. We conducted a meta-analysis to evaluate the efficacy and prognosis of HBOT in neonates with HIE.
Methods: A systematic search of eight databases was performed for available articles published between January 1, 2015, and September 30, 2020, to identify randomized controlled clinical trials (RCTs) on HBOT for neonatal HIE. Methodological quality assessment was performed by applying the simple procedure detailed by the Cochrane collaboration. Afterward, quality assessment and data analysis were performed using Revman 5.3 software. STATA 15 software was used to detect publication bias as well as for sensitivity analysis.
Results: A total of 46 clinical RCTs were selected for the study and included 4,199 patients with neonatal HIE. The results indicated that HBOT significantly improved the total efficiency (TEF) of treatment for neonatal HIE patients [odds ratio (OR) = 4.61, 95% confidence interval (CI) (3.70, 5.75), P < 0.00001] and reduced the risk of sequelae (OR = 0.23, 95% CI (0.16, 0.33), P < 0.00001) and the neonatal behavioral neurological assessment (NBNA) scores [mean difference (MD) = 4.51, 95%CI (3.83,5.19, P < 0.00001)].
Conclusion: In light of the effectiveness of HBOT neonatal HIE, this meta-analysis suggested that HBOT can be a potential therapy for the treatment of neonatal HIE. Due to the heterogeneity of studies protocol and patient selection being only from China, more research is needed before this therapy can be widely implemented in the clinic.
Protocol Registration: PROSPERO (ID: CRD42020210639). Available online at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020210639.
Neonatal hypoxic-ischemic encephalopathy (HIE) is a clinical syndrome caused by long-term cerebral hypoxia and ischemia in premature or full-term infants before or after birth due to placenta loss, umbilical cord prolapse, and uterine rupture (1). Neonatal acute brain injury (2) is characterized by long-term neurological dysfunction due to perinatal hypoxic ischemia, leading to a high incidence of sequelae and mortality (3). Studies have shown that neonates who died of hypoxic-ischemic encephalopathy (HIE) accounted for up to 23% of neonatal deaths (4–6). In developed countries, the incidence is approximately 1.5 cases per 1,000 live births; however, a higher incidence of 10–20 cases per 1,000 live births is observed in lower- and middle-income countries (7).
Neonates with HIE experience seizure activity, cranial nerve dysfunction (e.g., weak or absent suck reflex), impaired motor ability, and altered mental state. Neonatal HIE is categorized as mild, moderate, or severe based on the symptoms using a modified scoring system (8, 9). Hence, it is essential first to understand the HIE stage and use the proper treatment to ensure a successful treatment outcome. Neuroprotective drugs such as melatonin, allopurinol, topiramate, erythropoietin, and mild hypothermia are effective treatment measures (10). At present, the gold standard treatment for neonates with moderate to severe HIE includes the application of moderate hypothermia (HT; a decrease in temperature by 2–5°C), where the body temperature is maintained at 33.5°C for up to 72 h within the first 6 h of life (11). The goal of hypothermia is to slow cerebral metabolism, reduce reperfusion injury, and prevent neuronal apoptosis. Hypothermia therapy does not increase damage to other tissues and organs and has matured to treat cerebral hemorrhage, cerebral ischemia, and cerebral hypoxia (12). However, inducing hypothermia for a longer period and applying deeper cooling has not shown any additional benefits.
Similarly, for patients with severe HIE, minimal hypothermia is not appropriate to treat neurodevelopment disabilities. In a recent review, the authors concluded that hypothermia is effective only for a small number of neonates; however, it is still considered one of the most significant recent innovations for the treatment of HIE (13). Hence, we can conclude that the effect of conventional treatment is limited mainly because HIE is not a single disease entity but a condition with diverse causes that manifest as signs of brain injury, and this disease possesses multifactorial etiopathogenesis. Consequently, there are numerous contraindications, multiple side effects, and unclear pharmacological mechanisms for the available drugs (10).
In recent years, researchers have suggested that appropriate treatments can improve newborn HIE. Hyperbaric oxygen therapy (HBOT) has a particularly good effect on hypoxic diseases, can improve the survival rate of neonatal HIE, and has the potential to improve neurological dysfunction, reducing the incidence of sequelae (14). HBOT is achieved when a patient inhales 100% oxygen inside a hyperbaric chamber pressurized to a value greater than 1 atmosphere (atm). HBOT can reduce the tissue damage from brain edema caused by ischemia and hypoxia by improving tissue oxygenation, increasing the cerebral blood flow, promoting vascular repair, enhancing angiogenesis, and decreasing inflammation, thereby promoting neurological recovery through the regeneration of the axial white matter and improving patient prognosis and survival (15, 16). These chronological events can gradually improve stunted areas of the brain and the metabolic function through the activation of neuroplasticity (16). The efficiency of HBOT was examined in a recent randomized control trial (RCT) on stroke patients. The researchers observed enhanced perfusion in the regions with low living cell activity, among which the diencephalon was a major area. As cortico-thalamic projections regulate the network function, the researchers hypothesized that improved diencephalon perfusion with HBOT may contribute to the recovery of consciousness (17). Zhou et al. (18) confirmed that HBOT could reduce the mortality and disability rate of neonatal HIE. In this study, neonates with HIE were treated with HBOT at 1.4 ATA, 1.5 ATA, and 1.6 ATA of pressure. Serum superoxide dismutase (SOD), nitric oxide synthase (NOS), malondialdehyde (MDA), and nitric oxide (NO) levels were measured on Days 1 and 7 after HBOT. The authors observed an elevation in the serum SOD and a reduction in NOS, MDA, and NO levels after HBOT, confirming that as the hyperbaric oxygen pressure increased, the antioxidant capacity was enhanced. HBOT has been identified as an effective treatment for ischemic injuries in the central nervous system. Several animal studies have also confirmed that HBOT can alleviate hypoxic-ischemic brain injury and significantly improve neurological function deficits in neonatal rats (19–21). In an in vivo rat model of permanent middle cerebral artery occlusion (MCAO), it was found that HBOT lowered the infarct volume and improved the neurological scores in the injured rats (22). Although some studies have reported that HBOT is effective for neonatal HIE, the findings lack further confirmation with large sample sizes, multi-center studies, and randomized clinical studies. Therefore, this meta-analysis aimed to comprehensively analyze previous clinical RCTs involving HBOT for neonatal HIE to facilitate more reliable, clinical, evidence-based medicine.
This systematic review was conducted and reported according to the instructions provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (23).
Several articles were found during an initial literature search with no restrictions on the year, but only studies published from 2003 to 2020 were considered. We extensively searched various types of published literature using several databases. RCTs on HBOT for neonatal HIE were retrieved from eight databases: PubMed, EMBASE, Cochrane Library, Web of Science, Chinese Biomedical (CBM), Chinese Science and Technology Periodical Database (VIP), WanFang, and China National Knowledge Infrastructure (CNKI). The electronic search strategy for HBOT on neonatal HIE was “neonate” or “neonatal infant” and “hyperbaric oxygenation therapy” or “oxygen therapies, hyperbaric” and “hypoxic-ischemic encephalopathy” and “RCTs.” There was no language limit. The references for the retrieved articles were examined to identify relevant studies. The Cochrane Collaboration database was also searched, and a “cited reference search” was performed to identify articles citing the retrieved studies. The author data were reviewed for relevant articles.
We included RCTs that reported HBOT for neonatal HIE. It was mandatory to follow each step of the protocol meticulously. If there were any deviations from the study protocol, the committee would not approve the study. The reviewers insisted on checking the primary safety and efficacy endpoints of the treatment strategy.
(i) Clinical RCTs conducted with and without a blinding strategy were included in the study literature search.
(ii) Children who met the HIE diagnostic criteria confirmed with obstetric history, neurological symptoms of the newborn, computed tomography, ultrasound, or magnetic resonance imaging (MRI) were included in the study. Children were excluded from the study if they had any other encephalopathies.
(iii) The experimental group was treated with HBOT in addition to conventional treatment. The conventional treatment (CT) group was treated with medication, oxygen inhalation, spasm relief measures, and the correction of water and electrolyte imbalance.
(i) Non-RCTs were not considered for the study
(ii) Absence of a control group in the study
(iii) Experiments on animals (in vivo)
(iv) Repeated publication of data, a compilation of letters, case reports, reviews, and systematic evaluations
(v) Literature consisting of incomplete data
Research studies, review articles, other systematic review articles, and articles without information on HBOT for neonates with HIE were excluded. Study reports were thoroughly screened again and rechecked by two independent reviewers to determine whether the articles matched the inclusion and exclusion criteria. Two reviewers were paired up based on their educational background to ensure the pair contained at least one person with clinical expertise and one person with research experience. The two reviewers independently reviewed the same research articles that met the criteria and retrieved the articles for a full review independently. The following data were extracted: (i) General trial characteristics (first author’s surname, publication date, and study time); (ii) Baseline characteristics of the patient and clinical data (number and age of patients per group); (iii) Intervention measures (duration and dose of HBOT). If any discrepancies regarding the eligibility criteria of the studies were found, they were brought to the notice of the full study team for a final decision. Additional articles were also reviewed based on the reference lists, and appropriate information was obtained. Again, the reviewers cross-checked all the articles, obtained a final list of references, and discussed the data with a third reviewer for the meta-analysis.
The quality of the included articles was evaluated by two of the authors independently. Disagreements were resolved through discussion or consultation with a third author. The quality of the included studies was assessed based on the Cochrane Reviewers’ Handbook 5.1.0 RCT risk assessment tool (24). The following seven items were evaluated: (i) The random sequence generation method; (ii) The allocation hiding mechanism; (iii) Whether the operators/patients were blinded; (iv) Whether the evaluator of the results was blinded; (v) Whether the data were complete; (vi) Whether there was selective reporting; (vii) Other bias risks.
Outcome indicators for our study are the following:
(i) Total efficiency (TEF) (25): The efficacy was evaluated according to the children’s clinical symptoms, signs, and craniocerebral computed tomography manifestations. Significantly effective: the child becomes awake after treatment, the pre-halogen tension, muscle tension, and breathing conditions return to normal, the pupils are of equal size, and there is no symptom of twitching, and the child can have grasping and hugging reflexes after guidance. The brain CT results showed no abnormalities. Invalid: After the treatment, the symptoms and signs of the child are still abnormal, or the brain CT results suggest an abnormal condition.
(ii) Risk of sequelae: Sequelae can reflect the prognosis of the treatment, including hydrocephalus, epilepsy, cerebral palsy, linguistic intelligence, and physical development.
(iii) Neonatal behavioral, neurological assessment (NBNA) (26): The NBNA score has a total of 20 items in 5 parts, including four active muscle tension, four passive muscle tension, four original reflexes, four behavioral abilities, and four general evaluations. Each item is worth 4 points, 40 points in total, < 35 Score means abnormal, ≥ 35 means normal. The higher the score, the better the behavioral, neurological ability of the child.
The Reviewer Manager 5.3.5 software was used to conduct statistical analysis on the extracted data. Dichotomous data were expressed as odds ratio (OR) and 95% confidence interval (CI), whereas the continuous data were presented as mean difference (MD) with 95%CI. The data heterogeneity was associated with a combination of the fixed-effects (FE) and random-effects (RE) models, and the chi-square test was used to determine the heterogeneity. A fixed-effects and a random-effects model were used to merge the data according to heterogeneity, which was determined using the chi-square test. The I2 statistic, an I2 < 25%, indicates that heterogeneity may not be important, a value between 25 and 50% represents moderate inconsistency, and I2 > 50% suggests severe heterogeneity. We defined P ≥ 0.1 and I2 < 50 as an indicator that the results have a good agreement and that the fixed-effects model (FEM) may be set, while I2 > 50% was defined as an indicator of striking heterogeneity between the data (27). Otherwise, the RE model was employed to pool the results to minimize potential clinical heterogeneity. STATA 15.1 was used for sensitivity analysis to detect the possible sources of significant heterogeneity. Publication bias was evaluated using Egger’s test. A P-value < 0.05 suggested that there was publication bias.
We found similar studies close to our research topic during the search, but we focused only on our selected MESH terms and retrieved the full-text articles relevant to our study. We also identified additional studies from the reference lists during the literature search, and 1,601 eligible studies were finally retrieved from the eight databases used. A total of 46 clinical RCTs that matched the inclusion/exclusion criteria were included (28–73). All of the trials were performed in China. The characteristics of each article’s literature were identified and are shown in Figure 1. Additional information is presented in Table 1, with detailed information on each of the studies included.
We found 46 studies eligible for the meta-analysis. Among these, 18 trials (28–45) were performed using random number tables, lottery grouping, and envelope randomization to generate random sequences. Therefore, we concluded that these studies were low-risk. Four trials (48–51) were judged to be high risk due to using non-standard randomization methods based on the treatment regimen. The remaining 24 studies did not provide detailed information about the randomization methods used for the study. Most studies did not provide specific allocation concealment, operator/patient blindness, result evaluator blindness, and random sequence generation. All studies provided complete data on the outcomes, and the risks were found low. The results of the bias assessments are summarized in Figures 2, 3.
Thirty-eight studies (28, 30–34, 36–43, 47–51) compared the effect of conventional treatment and HBOT on the TEF of neonatal HIE patients. There were 3,368 patients with neonatal HIE, specifically 1,579 patients in the HBOT group and 1,242 in the conventional treatment group. The results of the heterogeneity test indicated that there was heterogeneity between the studies (P = 0.09; I2 = 24%), which disappeared (P = 0.99; I2 = 0%) after the study by Ni (56) was removed. The effect sizes were combined with the fixed-effects model, and the results showed that the differences between the two groups were statistically significant [OR = 4.61, 95% CI (3.70.5.75), P < 0.00001]. It shows that the effect of HBOT neonatal HIE is significantly better than conventional treatment. The subgroup analysis showed no statistical difference (P = 0.79, I2 = 0%) (Figure 4).
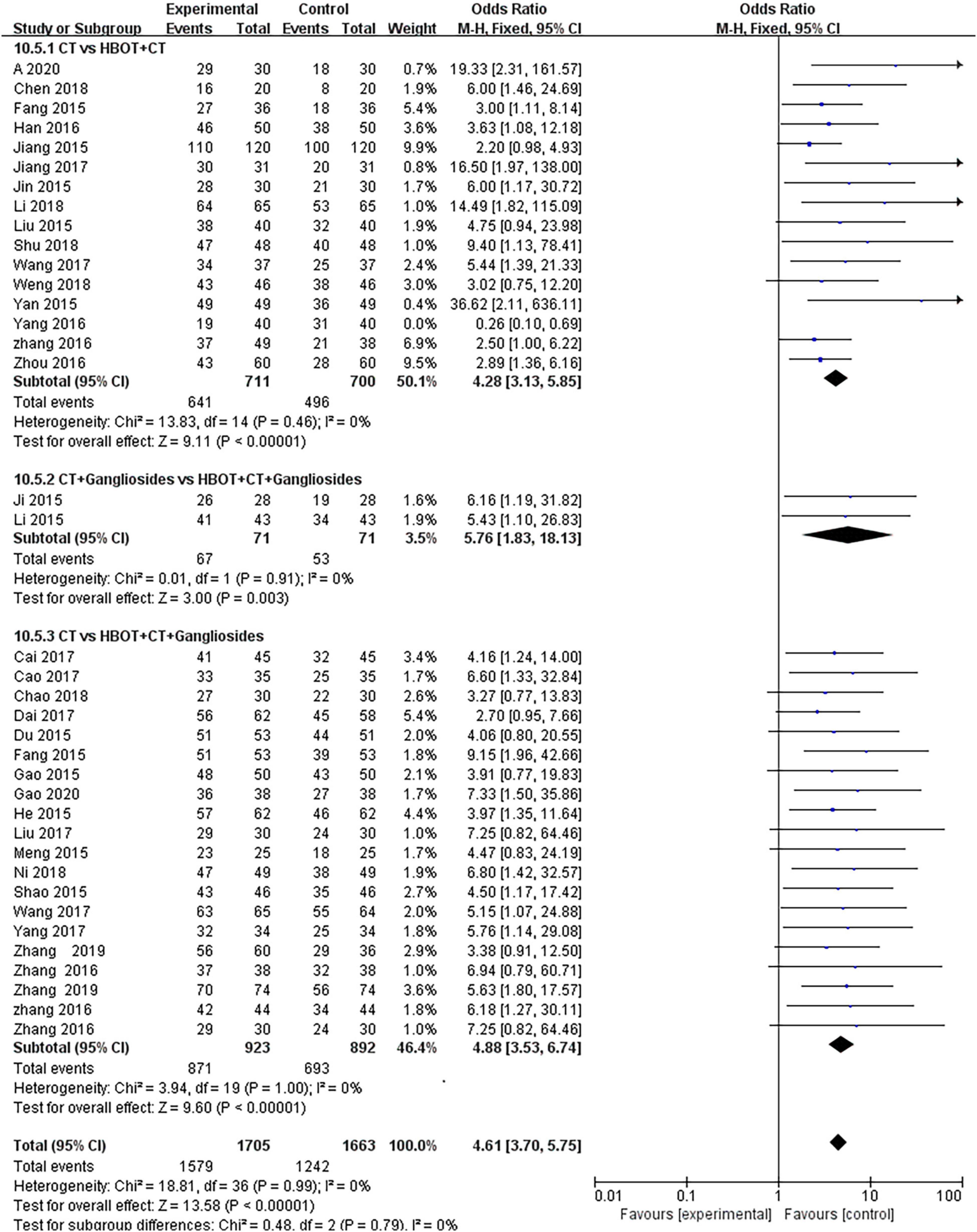
Figure 4. Subgroup analysis of TEF: (1) CT vs. HBOT + CT; (2) CT + Gangliosides vs. HBOT + CT + Gangliosides; (3) CT vs. HBOT + CT + Gangliosides.
Seven studies (32, 34, 39, 50, 54, 55, 69) compared the effects of conventional treatment and HBOT with the risk of sequelae in neonates with HIE. Long-term follow-up was conducted in two trials (33, 35), for 1 year and 30 months, respectively. Follow-up durations were not specified for the other included trials. Seven hundred fifty-eight neonates with HIE were enrolled, specifically 391 patients in the HBOT group and 367 in the conventional treatment group. The heterogeneity test results showed no heterogeneity between the studies (P = 0.69; I2 = 0%); therefore, the effect sizes were combined with the fixed-effects model. The results showed that there was a statistically significant difference between the two groups [OR = 0.23, 95% CI (0.16, 0.33), P < 0.00001]. This indicated that HBOT was superior to the conventional treatment in reducing the risk of sequelae in neonatal HIE patients. No statistical differences were observed in the subgroup analysis (P = 0.80, I2 = 0%) (Figure 5).
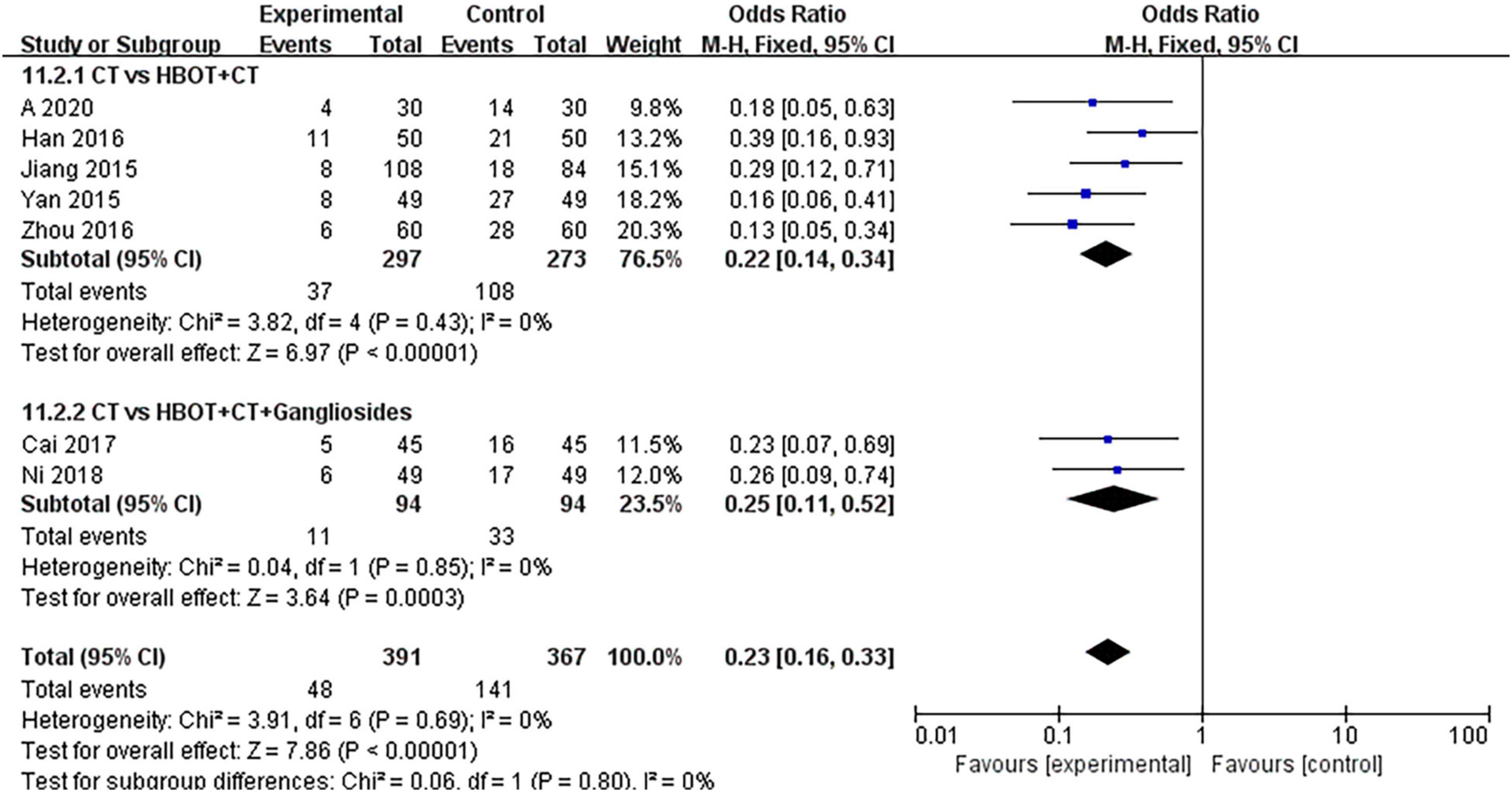
Figure 5. Subgroup analysis of risk of sequelae: (1) CT vs. HBOT + CT; (2) CT vs. HBOT + CT + Gangliosides.
Eighteen studies (19–24, 28–33, 37, 42, 44, 45, 47, 57, 61, 64, 66, 68–72, 74) compared the effects of conventional treatment and HBOT on the NBNA scores of patients with neonatal HIE. There were 1,570 cases of neonatal HIE, with 880 cases in the HBOT group and 876 cases in the conventional treatment group. The results of the heterogeneity test showed that there was heterogeneity between the studies (P = 0.02; I = 44%), but no heterogeneity was found after the removal of the study by Zhang (72) (P = 0.55; I2 = 0%). The differences between the two groups were statistically significant [MD = 4.51, 95% CI (3.83, 5.19), P < 0.00001]. This indicated that HBOT was superior to conventional therapy in improving the NBNA scores of neonates with HIE. No statistically significant differences were observed in the subgroup analysis (P = 0.22, I2 = 34.1%) (Figure 6).
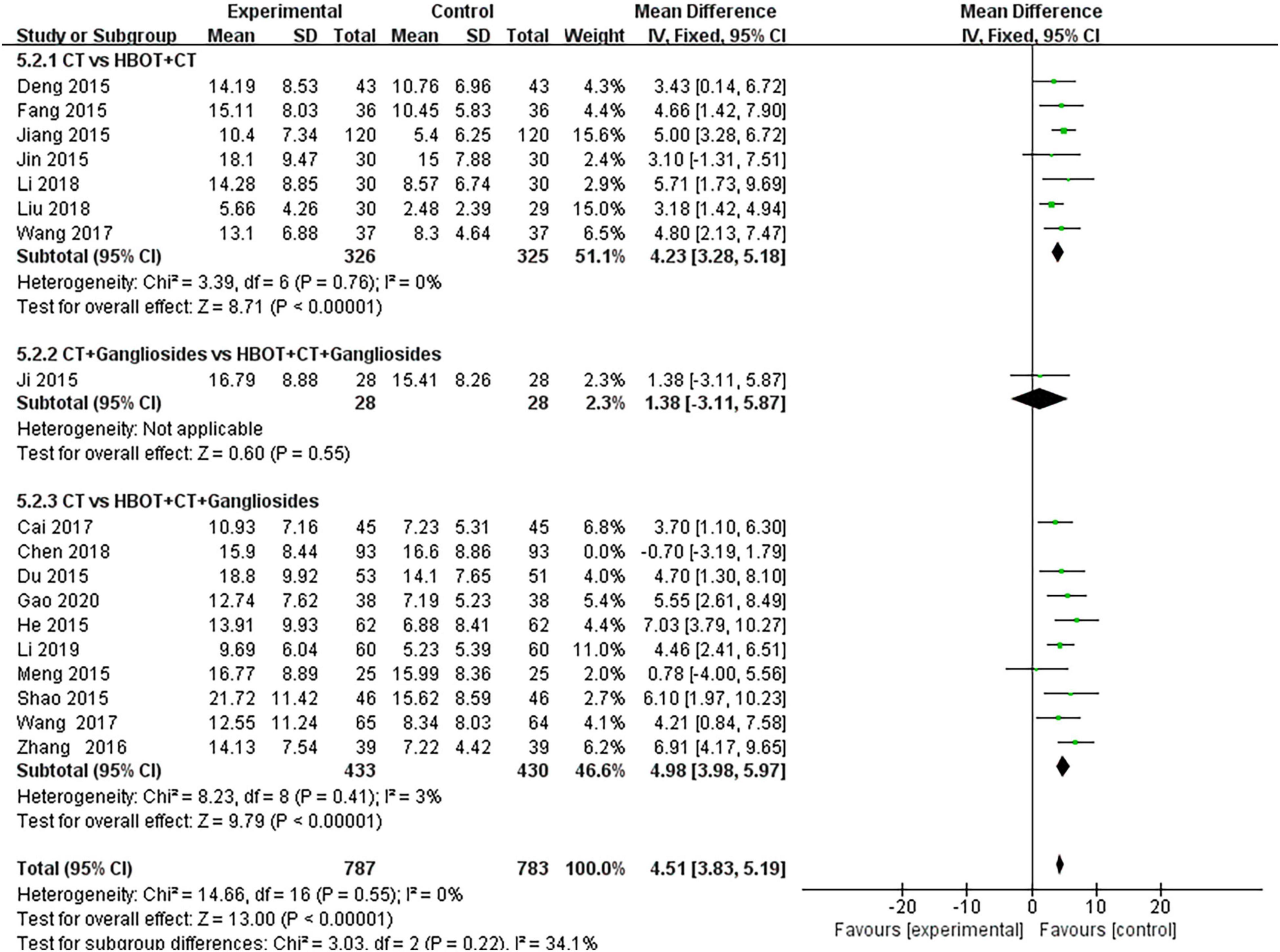
Figure 6. Subgroup analysis of NBNA score: (1) CT vs. HBOT + CT; (2) CT + Gangliosides vs. HBOT + CT + Gangliosides; (3) CT vs. HBOT + CT + Gangliosides.
For the 46 reference articles (28–73) evaluated in this study, I2 = 22% < 25% after the heterogeneity test and P = 0.1 for the Q test, suggesting a minor possibility of heterogeneity among the reference articles. A fixed-effects model was used for meta-analysis. To ensure the accuracy and stability of the study, sensitivity analysis was performed.
STATA15 was used for sensitivity analysis of the key outcome indicators, specifically TEF, the risk of sequelae, and the NBNA score. The results showed that the elimination of each outcome and individual studies did not significantly alter the meta-analysis results, indicating that the study had good stability; hence, the study results were considered reliable (Figure 7).
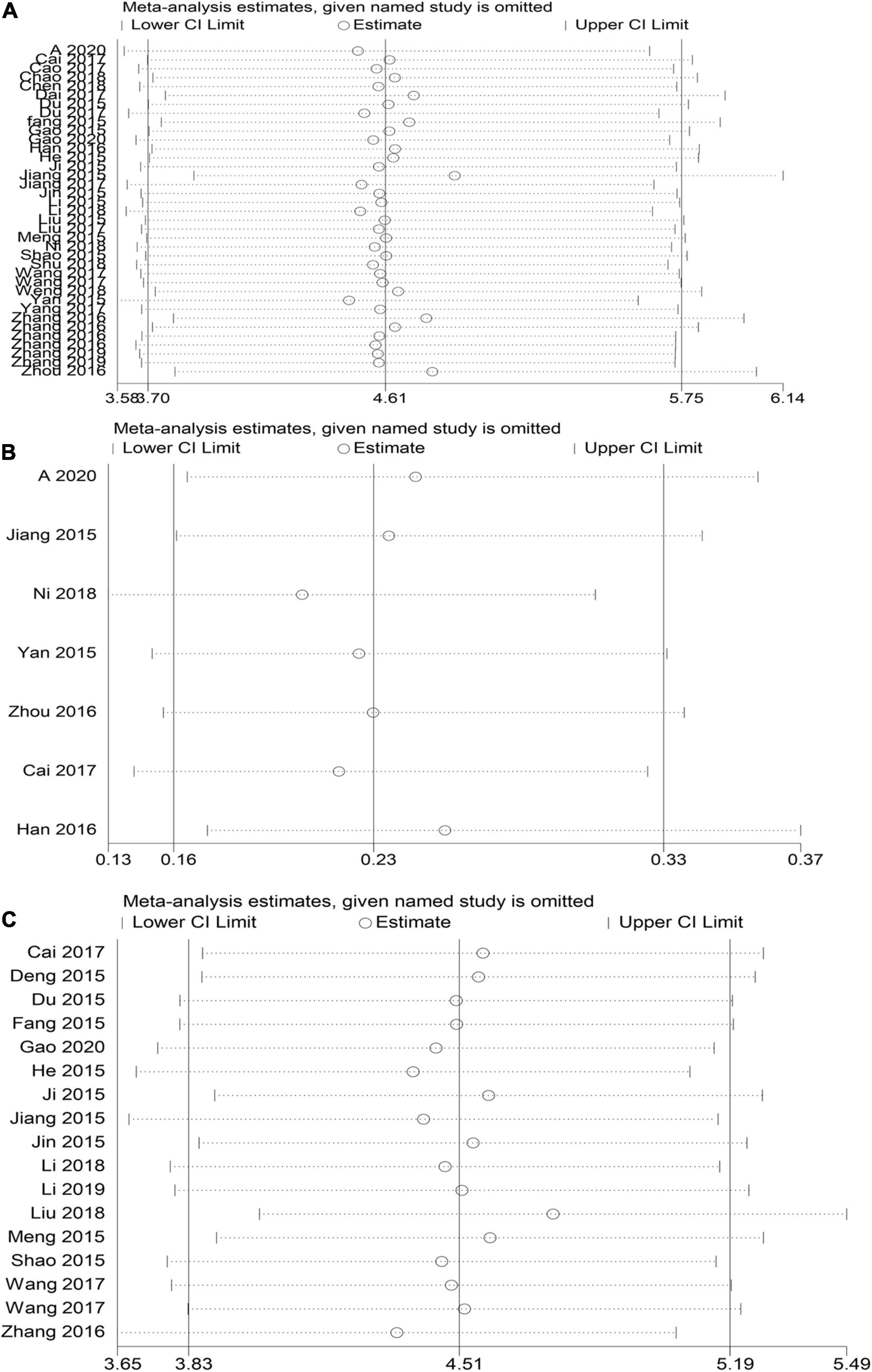
Figure 7. HBOT and conventional treatment of neonates with HIE sensitivity analysis curve. (A) TEF, (B) risk of sequelae, (C) NBNA score).
STATA15 was used to test the publication bias for the major outcome indicators. If the preliminary results were likely biased, i.e., P < 0.05, significant publication bias was evaluated with the shear complement method. According to Egger’s inspection results, there was no publication bias in the risk of sequelae [P > |t| = 0.264, 95% CI (−2.23, 0.77)] and the NBNA score [P > |t| = 0.422, 95% CI (−2.14, 4.86)] in the two groups before and after the treatment. There was significant publication bias [P > |t| = 0.0001, 95% CI (9.85, 18.32)] (Figure 8) in the TEF. The TRIM and padding method was used to evaluate the reliability of the results affected by the significant publication bias. The OR and 95% CI after dressing and filling [OR = 3.47, 95% CI (3.27, 3.67), P < 0.00001] were consistent with the previous results [OR = 4.61, 95% CI (3.70, 5.75), P < 0.00001], indicating that the results were reliable (Figure 9). The possibility of publication bias is mainly based on small studies’ (over-) presence with (very) high effect sizes. Small studies have large standard errors, and only those with very high effect sizes, thus overcoming their standard error, are deemed to get published.
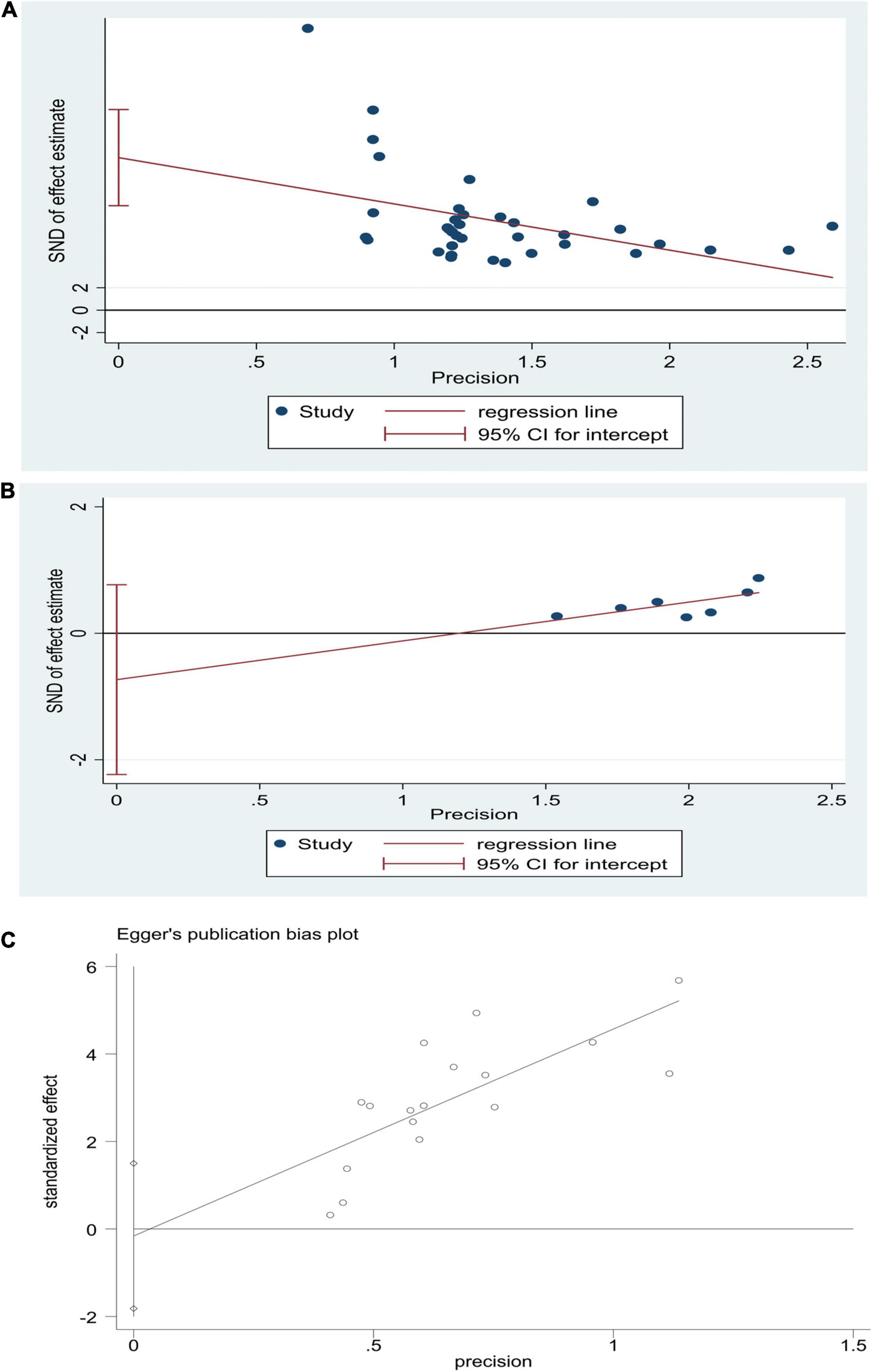
Figure 8. Egger’s publication bias graphs for HBOT and conventional treatment of neonatal HIE. (A) TEF, (B) risk of sequelae, (C) NBNA score.
Neonatal HIE is a clinical syndrome caused by brain injury during the perinatal period, in the weeks leading up to birth, or during labor and delivery. This is caused by partial or complete hypoxia in infants during perinatal asphyxia, resulting in the reduction or suspension of cerebral blood flow, which is the main cause of hypoxia (75, 76). Early diagnosis and timely intervention are of great significance to improve the prognosis of neonatal HIE and reduce the mortality of children. The diagnostic methods of neonatal HIE mainly include ultrasound, computed tomography, MRI, etc. Among them, MRI has high spatial resolution and soft-tissue resolution, judging intracranial lesions and reducing radiation damage. It is the current neonatal ischemic deficiency. The best way to check oxygen encephalopathy (77). In some instances, the appropriate diagnosis is essential within 24 h to reduce the mortality rate. According to research, to date, there is no specific therapy for neonatal HIE treatment. The existing treatment methods mainly include support for symptomatic treatment, mild hypothermia treatment, EPO, xenon, stem cell therapy, etc. (14, 17, 78, 79). However, current treatments focused on reducing the occurrence of sequelae, reducing mortality and neuroprotection are unsatisfactory. Therefore, finding clinically effective complementary therapies with lower adverse events can improve the efficacy of conventional treatments in the treatment of neonatal HIE.
Studies have shown that hypoxia is the main pathological factor leading to brain nerve cell function damage. The use of HBOT has gradually increased as it is a non-invasive method that involves inhaling pure oxygen in a pressurized chamber with high levels of atmospheric pressure. In HBOT of patients with brain injury, tracheal intubation is an effective way of inhaling oxygen, which continuously injects hyperbaric oxygen into the patient’s body. It can increase blood oxygen content, increase blood oxygen partial pressure, increase oxygen diffusion distance, and by doing so, it corrects the hypoxic state of brain tissue (80). Furthermore, HBOT activates the lungs by providing oxygen to other systemic organs, minimizing secondary brain injury events like rampant inflammation, apoptosis initiation, and oxidative stress (81). The partial pressure of oxygen in the alveoli induced by pressurized oxygen increases the oxygen level of brain tissue, reduces the energy failure of brain tissue, inhibits cellular apoptosis, and reduces brain damage, which can minimize brain nerve damage from HIE (14, 81). An earlier animal study showed that HBOT can enhance the proliferation of neural stem cells in the subventricular region of HIE neonatal rats and has the therapeutic potential to promote nervous system recovery after brain injury (82). At the cellular level, hyperbaric oxygen preconditioning can increase the expression of Nrf2 and the activity of downstream proteins to reduce hypoxic-ischemic brain injury, which significantly reduces the infarct area, neuronal injury, and cell apoptosis (21). Liu et al. (83) and Zhang et al. (84) published systematic evaluations of neonatal HIE treated with HBOT in China. Both studies showed that HBOT reduced the mortality rate and neurological sequelae in neonatal HIE. However, due to the lack of inadequate information in the literature, the results are likely to be biased; hence, the evidence may be unreliable, and further investigation is required. The studies also did not provide details of the treatment strategy or protocol information, and therefore, subgroup analysis could not be performed according to the HBOT protocol. Hence, this meta-analysis aimed to evaluate the efficacy and prognosis of HBOT for HIE quantitatively. The results obtained suggest that HBOT for HIE is more effective than the routine, conventional treatment and can effectively improve the neurological function of HIE children and efficiently reduce the risk of neurological sequelae. Our results were in agreement with the meta-analysis by Zhang and Liu. We also described detailed strategies and schemes for HBOT in neonatal HIE for the first time by providing more reliable clinical evidence-based HBOT for neonatal HIE in clinical practice.
This study evaluated evidence from 46 RCTs, and a total of 4,199 neonatal HIE patients were randomized to receive HBOT or conventional treatment from 2015 to 2020. The results of the study are as follows: (1) TEF of the HBOT group is significantly better than that of the CT group; (2) the HBOT group is significantly better than the CT group in reducing the incidence of sequelae in children; (3) the NABA score of the HBOT group was significantly higher than that of the CT group. So the benefits of HBOT are obvious. Although this study proved that HIE in neonates with HBOT is effective, the included studies have not reported on mortality, so the safety of hyperbaric oxygen therapy in neonates with HIE has aroused everyone’s attention. A meta-analysis of 51 animal studies confirmed that the HBOT group exhibited a 32% reduction in the cerebral infarction area compared to the control group. Significant improvements were observed in neurological function [95% CI (28–37%), P < 0.00001] and the mortality rate decreased by 8.3% (85). In addition, as early as 2016, a meta-analysis by Wang F showed that HBOT plays an important role in traumatic brain injury and can significantly improve the Glasgow coma scale (GCS) and Glasgow outcome score (GOS) for patients as well as reduce disability and mortality (86). However, prolonged hyperbaric oxygen exposure may lead to oxygen poisoning, causing damage to multiple organs of the body. When the partial pressure of oxygen is > 3 ATA, oxygen poisoning may occur. The severity of oxygen poisoning is closely related to the length of exposure (87). However, as long as the pressure and time of hyperbaric oxygen treatment are strictly controlled, the probability of oxygen poisoning is extremely small. Studies have shown that HBOT pressures of 1.4, 1.5, and 1.6 ATA are safe and effective for neonatal HIE (18, 88). Clinically, the following methods can be used to prevent the occurrence of oxygen poisoning: (1) Strictly follow the treatment strategy for treatment and do not increase the treatment pressure arbitrarily. (2) Strictly control the treatment time, adopt the intermittent oxygen inhalation method of inhaling oxygen, inhaling air at rest, and inhaling oxygen again (87).
To determine the best treatment scheme for HBOT neonates with HIE, we conducted a subgroup analysis of the total effective rate according to the daily oxygen uptake, treatment duration, and pressure with HBOT. The results showed that the therapeutic effect for the group with daily oxygen intake for 30–40 min was higher than that for the group with daily oxygen intake for 50–60 min and 70–90 min. This suggests that daily oxygen intake for 30–40 min provides the maximum therapeutic effect for patients with neonatal HIE. Subgroup analysis of the treatment duration showed that the therapeutic effect with a treatment duration of over 30 days was higher than that with a treatment duration of 1–10 days and 10–30 days, suggesting that HBOT over 30 days provides the maximum therapeutic effect for patients with neonatal HIE. The pressure subgroup analysis for HBOT showed that pressure between 0.04 and 0.08 mpa had the best therapeutic effect on neonatal HIE patients. Therefore, based on the results of subgroup analysis findings of HBOT for neonatal HIE patients, treatment could be as follows: daily oxygen uptake for 30–40 min at a pressure of 0.04–0.08 mpa, with the treatment lasting for more than 30 days (Table 2). However, due to the heterogeneity of studies protocol, more research is needed before this potential therapy for HIE can be widely used. Furthermore, because the patients selected for the study are mainly from China, the conclusions of this meta-analysis may not apply to other ethnic groups.
This study had the following limitations: (i) We searched only English and Chinese databases. (ii) Some of the included studies did not specify the blinding method used, which may have impacted the objectivity of the neonatal HIE results, leading to measurement bias. (iii) The quality of some included studies was low. Most of the articles did not describe the randomization method used or the concealment of randomly assigned schemes; hence, there may have been a selection bias. (iv) Most of the selected studies in this study had a small sample size and a low design quality, which may have affected the efficacy of HBOT. (v) We have not included any other standard of care such as moderate hypothermia as we believe that these are not in a competitive relationship with HBOT, and they play different roles through different pathways at different stages of the disease and together promote the recovery of the disease (89). However, although the deficiencies listed above may affect the quality of the evidence, the included studies were highly comparable, and the articles were selected with relatively strict inclusion criteria. Therefore, this study highlights the shortcomings of existing studies and can indicate a direction for future studies. The present study has value for clinical research and application and can provide reliable evidence for the effectiveness and prognosis of HBOT for neonatal HIE.
This meta-analysis showed that the addition of HBOT to the standard and conventional treatment of neonatal HIE significantly improved the children’s NBNA score and clinical efficacy and reduced the risk of sequelae. In light of the effectiveness of HBOT, it holds promise as a potential complementary treatment for neonatal HIE. However, due to the heterogeneity of studies protocol, more research is needed to understand its potential as a therapy for HIE. It is worth noting that because the patients selected for the study are mainly from China, the conclusions of this meta-analysis may not apply to other ethnic groups.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JX, Q-LD, R-HF, and X-BG had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. X-BG and JX contributed to design, statistical analysis, supervision, and manuscript drafting. H-MD and Q-LD contributed to the conception and design, data analysis, funding, supervision, and critical revision of the manuscript. Y-NG, R-HF, and W-HL participated in the acquisition and analysis of data, statistical analysis, and manuscript revision. X-YJ and Y-HL contributed to the analysis and interpretation of the data and revision of the manuscript. All authors read and approved the final manuscript.
This work was supported by the Construction of Key Medical Disciplines in Shenzhen City, Guangdong Province (No. 2020-6), support for Applied Basic Research Fund of the Qinghai Science and Technology Department (No. 2018-ZJ-705), and support for All Military Logistics Projects (No. CWH17JOO6). The funding bodies had no role in the study’s design, the collection, analysis, or interpretation of the data, or writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Glass HC. Hypoxic-ischemic encephalopathy and other neonatal encephalopathies. Continuum (Minneap Minn). (2018) 24:57–71. doi: 10.1212/con.0000000000000557
2. Li B, Concepcion K, Meng X, Zhang L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol. (2017) 159:50–68. doi: 10.1016/j.pneurobio.2017.10.006
3. Rocha-Ferreira E, Hristova M. Plasticity in the neonatal brain following hypoxic-ischaemic injury. Neural Plast. (2016) 2016:4901014. doi: 10.1155/2016/4901014
4. Schump EA. Neonatal encephalopathy: current management and future trends. Crit Care Nurs Clin North Am. (2018) 30:509–21. doi: 10.1016/j.cnc.2018.07.007
5. Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. (2010) 86:329–38. doi: 10.1016/j.earlhumdev.2010.05.010
6. Juul SE, Ferriero DM. Pharmacologic neuroprotective strategies in neonatal brain injury. Clin Perinatol. (2014) 41:119–31. doi: 10.1016/j.clp.2013.09.004
7. Greco P, Nencini G, Piva I, Scioscia M, Volta CA, Spadaro S, et al. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol Belg. (2020) 120:277–88. doi: 10.1007/s13760-020-01308-3
8. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. (1976) 33:696–705. doi: 10.1001/archneur.1976.00500100030012
9. Thompson CM, Puterman AS, Linley LL, Hann FM, van der Elst CW, Molteno CD, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. (1997) 86:757–61. doi: 10.1111/j.1651-2227.1997.tb08581.x
10. Yıldız EP, Ekici B, Tatlı B. Neonatal hypoxic ischemic encephalopathy: an update on disease pathogenesis and treatment. Expert Rev Neurother. (2017) 17:449–59. doi: 10.1080/14737175.2017.1259567
11. Oorschot DE, Sizemore RJ, Amer AR. Treatment of neonatal hypoxic-ischemic encephalopathy with erythropoietin alone, and erythropoietin combined with hypothermia: history, current status, and future research. Int J Mol Sci. (2020) 21:1487. doi: 10.3390/ijms21041487
12. Nabetani M, Shintaku H, Hamazaki T. Future perspectives of cell therapy for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. (2018) 83:356–63. doi: 10.1038/pr.2017.260
13. Martinello K, Hart AR, Yap S, Mitra S, Robertson NJ. Management and investigation of neonatal encephalopathy: 2017 update. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F346–58. doi: 10.1136/archdischild-2015-309639
14. Hentia C, Rizzato A, Camporesi E, Yang Z. An overview of protective strategies against ischemia/reperfusion injury: the role of hyperbaric oxygen preconditioning. Brain Behav. (2018) 8:e00959. doi: 10.1002/brb3.959
15. McMonnies CW. Hyperbaric oxygen therapy and the possibility of ocular complications or contraindications. Clin Exp Optom. (2015) 98:122–5. doi: 10.1111/cxo.12203
16. Chen CY, Wu RW, Tsai NW, Lee MS, Lin WC, Hsu MC, et al. Increased circulating endothelial progenitor cells and improved short-term outcomes in acute non-cardioembolic stroke after hyperbaric oxygen therapy. J Transl Med. (2018) 16:255. doi: 10.1186/s12967-018-1629-x
17. Sankaran R, Radhakrishnan K, Sundaram KR. Hyperbaric oxygen therapy in patients with hypoxic ischemic encephalopathy. Neurol India. (2019) 67:728–31. doi: 10.4103/0028-3886.263236
18. Zhou BY, Lu GJ, Huang YQ, Ye ZZ, Han YK. Efficacy of hyperbaric oxygen therapy under different pressures on neonatal hypoxic-ischemic encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi. (2008) 10: 133–5.
19. Zhu M, Lu M, Li QJ, Zhang Z, Wu ZZ, Li J, et al. Hyperbaric oxygen suppresses hypoxic-ischemic brain damage in newborn rats. J Child Neurol. (2015) 30:75–82. doi: 10.1177/0883073814530500
20. Wei L, Ren Q, Zhang Y, Wang J. Effects of hyperbaric oxygen and nerve growth factor on the long-term neural behavior of neonatal rats with hypoxic ischemic brain damage. Acta Cir Bras. (2017) 32:270–9. doi: 10.1590/s0102-865020170040000002
21. Zhai X, Lin H, Chen Y, Chen X, Shi J, Chen O, et al. Hyperbaric oxygen preconditioning ameliorates hypoxia-ischemia brain damage by activating Nrf2 expression in vivo and in vitro. Free Radic Res. (2016) 50:454–66. doi: 10.3109/10715762.2015.1136411
22. Bennett MH, Weibel S, Wasiak J, Schnabel A, French C, Kranke P. Hyperbaric oxygen therapy for acute ischaemic stroke. Cochrane Database Syst Rev. (2014) Cd004954. doi: 10.1002/14651858.CD004954.pub3
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
24. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. (2015) 8:2–10. doi: 10.1111/jebm.12141
25. Han X, Sun D. Ganglioside combined with hyperbaric oxygen in the treatment of moderate to severe neonatal hypoxic-ischemic encephalopathy. Chin J Pract Nerv Dis. (2015) 22:97–8. doi: 10.3969/j.issn.1673-5110.2015.20.068
28. Gapal A, Dorikun Z. Clinical treatment and prognostic analysis of hyperbaric oxygen therapy for neonatal hypoxic-ischemic encephalopathy. World Latest Med Inf. (2020) 20:72–4. doi: 10.19613/j.cnki.1671-3141.2020.2.042
29. Chen Y. Efficacy of ganglioside sodium combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. Cap Food Med. (2018) 25:29. doi: 10.15887/j.cnki.13-1389/r.2018.35.097
30. Dai Y. Clinical study of hyperbaric oxygen combined with ganglioside in the treatment of neonatal hypoxic-ischemic encephalopathy. In: Proceedings of the 26th National Hyperbaric Oxygen Medicine Academic Paper Collection of Chinese Medical Association. Tianjing: Chinese Medical Association (2017). p. 94–6.
31. Du Y, Gao S, Li B, Yang X. Effects of hyperbaric oxygen combined with GM-1therapy on serum Bcl-2,NSE and NF-κBof neonates with hypoxic-ischemic encephalopathy. Med Pharm J Chin PLA. (2017) 29:61–3. doi: 10.3969/j.issn.2095-140X.2017.12.017
32. Guo J. Evaluation of the effect of ganglioside sodium combined with hyperbaric oxygen in the treatment of neonatal hypoxic-ischemic encephalopathy and its influence on nervous system function. J Med Aesthet Cosmetol. (2020) 29:1672–9463.
33. Han X. Clinical value of hyperbaric oxygen therapy for hypoxic ischemic brain injury in neonates and its influence on prognosis. Contemp Med. (2016) 22:40–1. doi: 10.3969/j.issn.1009-4393.2016.12.026
34. He R. Analysis of the clinical efficacy of hyperbaric oxygen combined with ganglioside in the treatment of neonatal hypoxic-ischemic encephalopathy. Med Health. (2015) 23:13–4.
35. Jiang L, Li Y, Yu W, Zeng G, Yang X. Therapeutic effect of hyperbaric oxygen on 120 neonates with hypoxic ischemic encephalopathy. Intern Med China. (2015) 10:482–3. doi: 10.16121/j.cnki.cn45-1347/r.2015.04.17
36. Jin L. Clinical observation of hyperbaric oxygen in the treatment of neonatal ischemic hypoxic encephalopathy. Modern Health Clin Exp. (2015) 8:97.
37. Li M. Clinical efficacy of acupoint injection combined with hyperbaric oxygen in the treatment of moderate and severe neonatal hypoxic-ischemic encephalopathy. Med Innov China. (2019) 16:14–8. doi: 10.3969/j.issn.1674-4985.2019.29.004
38. Liu Z, He Z. The efficacy and safety hyperbaric oxygen treatment of neonatal ischemic hypoxic encephalopathy. In: Proceedings of the 2015 Clinical Emergency and Critical Care Experience Exchange Summit. Beijing: Chinese Medical Association (2015). p. 645.
39. Wang Y, Guo Y, Yuan X. Curative effect of ganglioside combined with hyperbaric oxygen in treatment of neo- natal hypoxic-ischemic encephalopathy. Matern Child Health Care China. (2017) 32:1490–2. doi: 10.7620/zgfybj.j.issn.1001-4411.2017.07.47
40. Zhang G. Effect observation of ganglioside combined with hyperbaric oxygen on neonatal hypoxic and ischemic encephalopathy. J Med Forum. (2019) 40:1672–3422.
41. Cai Z. Efficacy of ganglioside sodium combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. Mod Diagn Treat. (2017) 28:249–50. doi: 10.3969/j.issn.1001-8174.2017.02.033
42. Cao L. Efficacy of ganglioside combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. China Reflexolocy. (2017) 26:115–7. doi: 10.19589/j.cnki.issn1004-6569.2017.16
43. Chao H. Observation of the clinical effect of ganglioside sodium combined with hyperbaric oxygen therapy on neonatal hypoxic-ischemic encephalopathy. Psychologist. (2018) 24:118.
44. Deng Z, Liu Y. Clinical effect of hyperbaric oxygen therapy on neonatal hypoxic ischemic encephalopathy. Med Inf. (2015) 28:93. doi: 10.3969/j.issn.1006-1959.2015.31.111
45. Du L. Clinical observation of gangliosides and hyperbaric oxygen therapy in neonates with hypoxic ischemic encephalopathy. China For Med Treat. (2015) 5:134–5. doi: 10.3969/j.issn.1674-0742.2015.20.067
46. Fan X. The effect of hyperbaric oxygen therapy on neonatal hypoxic-ischemic encephalopathy. Contemp Med Forum. (2015) 16:290–1.
47. Gao H. Efficacy of ganglioside combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. Chin J Pract Nerv Dis. (2015) 18:63–4. doi: 10.3969/j.issn.1673-5110.2015.15.038
48. Ji R. Observation of the therapeutic effect of gangliosides combined with hyperbaric oxygen chamber on neonatal hypoxic-ischemic encephalopathy. In: Proceedings of the 2nd Summit Forum for the Exchange of Clinical Acute and Severe Experience in 2015. Beijing: Chinese Medical Association (2015). p. 1.
49. Jia B, Guo G. The clinical observation of effects of hyperbaric oxygen on hemorheology in neonates with hypoxic ischemic encephalopathy. Chin J Mod Drug Appl. (2015) 9:59–60. doi: 10.14164/j.cnki.cn11-5581/r.2015.06.045
50. Jiang X. Effects of hyperbaric oxygen on hemorheology in neonates with hypoxic ischemic encephalopathy. Chin J Pract Nerv Dis. (2017) 20:105–7. doi: 10.3969/j.issn.1673-5110.2017.11.042
51. Li J, Zhang Y, Su Y, Liu J. Effect analysis of ganglioside combined with hyperbaric oxygen in the treatment of neonatal ischemic hypoxic encephalopathy. Contemp Med. (2015) 21:27–8. doi: 10.3969/j.issn.1009-4393.2015.7.018
52. Li N, Lv Y, Meng X, Guo D, Pan S. The clinical study of hyperbaric oxygen in the treatment of neonatal hypoxic ischemic encephalopathy. Med J Chin PAP. (2018) 29:282–6. doi: 10.3969/j.issn.1004-3594.2018.03.019
53. Liu J, Tang C, Xu C. Clinical observation of hyperbaric oxygen in the treatment of neonatal hypoxic-ischemic encephalopathy in Hexi area. China Health Care Nutr. (2018) 28:45. doi: 10.3969/j.issn.1004-7484.2018.30.030
54. Liu J. The clinical value of ganglioside sodium combined with hyperbaric oxygen in the treatment of neonatal hypoxic-ischemic encephalopathy. World Clin Med. (2017) 11:127.
55. Meng W. Clinical observation of monosialic ganglioside combined with hyperbaric oxygen in the treatment of neonatal hypoxic-ischemic encephalopathy. China Prac Med. (2015) 10:149–50. doi: 10.14163/j.cnki.11-5547/r.2015.24.108
56. Ni Y. Curative effect of cerebroside carnosine combined with hyperbaric oxygen on neonatal hypoxic ischemic encephalopathy. Pract Clin Med. (2018) 19:60–2. doi: 10.13764/j.cnki.lcsy.2018.03.022
57. Shao M. Curative effect of ganglioside combined with hyperbaric oxygenin treatment of neonatal hypoxic ischemic encephalopathy and its in fluence on serum NSE. Chin J Prim Med Pharm. (2015) 22:1980–2. doi: 10.3760/cma.j.issn.1008-6706.2015.13.021
58. Shu L. The value of applying hyperbaric oxygen chamber in the treatment of moderate neonatal hypoxic-ischemic encephalopathy. Matern Child World. (2018) 2:69–70. doi: 10.3969/j.issn.1671-2242.2018.15.066
59. Tian Y, Long C, Cui S, Xu N. Effects of hyperbaric oxygen on serum sICAM-1 and S100B protieins in neonatal hypoxic ischemic encephalopathy. Chin J Naut Med Hyperbar Med. (2019) 26:396–9. doi: 10.3760/cma.j.issn.1009-6906.2019.05.003
60. Wang H, Fan X. Clinical effect of hyperbaric oxygen therapy on neonatal hypoxic ischemic encephalopathy in 74 cases. Case Rep. (2017) 4:60–1.
61. Wei G, Guo J. Clinical observation on treatment of neonatal HIE with hyperbaric oxygen combined with cerebroside carnosine. Med J Natl Defend Forces Southwest China. (2016) 26:35–8. doi: 10.3969/j.issn.1004-0188.2016.01.013
62. Weng H. Analysis on the clinical effect of hyperbaric oxygen assisted therapy on neonatal hypoxic encephalopathy. Med Eng Technol. (2018) 33:23–4.
63. Yan T, Zhang J, Lin H, Lu S, Que G. The curative effect and prognosis of hyperbaric oxygen on neonatal hypoxic ischemic encephalopathy. Med Innov China. (2015) 12:24–6. doi: 10.3969/j.issn.1674-4985.2015.29.008
64. Yang Q. Effect of comprehensive rehabilitation course of treament interventions for neonatal hypoxic ischemic encephalopathy. J North Sichuan Med Coll. (2016) 31:373–6. doi: 10.3969/j.issn.1005-3697.2016.03.024
65. Yang R. The therapeutic effect of ganglioside and hyperbaric oxygen on neonatal hypoxic-ischemic encephalopathy. Matern Child World. (2017) 11:95. doi: 10.3969/j.issn.1671-2242.2017.14.089
66. Yang Z, Long Z. Analysis of the clinical effect of hyperbaric oxygen on neonatal hypoxic-ischemic encephalopathy. Health Matern Child Health. (2016) 10:200–1. doi: 10.3969/j.issn.1009-6019.2016.11.267
67. Chen Y. Clinical observation of hyperbaric oxygen in the treatment of neonatal ischemic hypoxic encephalopathy. Chin J Clin Ration Drug Use. (2018) 25:29. doi: 10.3969/j.issn.1005-8257.2018.22.025
68. Zhou Y. Effects of hyperbaric oxygenation therapy on hemorheology hypoxic ischemic encephalopathy of neonates. World Clin Med. (2016) 10:140–3.
69. Zhang G. Curative effect of ganglioside combined with hyperbaric oxygen in treatment of neo- natal hypoxic ischemic encephalopathy. J Baotou Med Coll. (2016) 32:94–5.
70. Zhang N. Efficacy of ganglioside combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. Clin Res Pract. (2016) 1:111–5.
71. Zhang X, Liao ZJ. Effect of hyperbaric oxygen combined with GM-1 on neonatal hypoxic ischemic encephalopathy and influence on serum NSE, Bcl-2 and NF- kappa B in children. Xinjiang Med J. (2019) 49:46–8.
72. Zhang Z. Efficacy of ganglioside combined hyperbaric oxygen in treatment of neonatal hypoxic ischemic encephalopathy. J Clin Med. (2016) 3:6346–7.
73. Zhang ZM, Liu ZQ, Wu XQ, Zhang AH, Mo J, Li XH, et al. Hyperbaric oxygen therapy on serum hypoxic-ischemic encephalopathy of Bcl-2 after the neonatal period. Shanxi Med J. (2016) 45:379–82. doi: 10.3969/j.issn.0253-9926.2016.04.003
74. Xiong T, Li H, Zhao J, Qu Y, Mu D, Dong W, et al. Hyperbaric oxygen for term newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. (2019) 2019, CD009248.
75. Davidson JO, Dean JM, Fraser M, Wassink G, Andelius TC, Dhillon SK, et al. Perinatal brain injury: mechanisms and therapeutic approaches. Front Biosci (Landmark Ed). (2018) 23:2204–26. doi: 10.2741/4700
76. Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. Neurorehabilitation. (2010) 26:5–13. doi: 10.3233/nre-2010-0531
77. Zhang H. The imaging diagnostic value of MRI inneonata1 hypoxic-ischemic encephalopathy. Chin J Comput Tomogr MRI. (2018) 16:40–2; 57. doi: 10.3969/j.issn.1672-5131.2018.04.013
78. Huang L, Zhang L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog Neurobiol. (2019) 173:1–17. doi: 10.1016/j.pneurobio.2018.05.004
79. Chen S, Xiao N, Zhang X. Effect of combined therapy with ephedrine and hyperbaric oxygen on neonatal hypoxic-ischemic brain injury. Neurosci Lett. (2009) 465:171–6. doi: 10.1016/j.neulet.2009.09.011
80. Zhang QC, Lv XP, Fu B. Nursing care of patients with tracheal intubation after hyperbaric oxygen therapy. Heilongjiang Med Pharm. (2011) 34:106. doi: 10.3969/j.issn.1008-0104.2011.01.074
81. Benedetti S, Lamorgese A, Piersantelli M, Pagliarani S, Benvenuti F, Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin Biochem. (2004) 37:312–7. doi: 10.1016/j.clinbiochem.2003.12.001
82. Feng Z, Liu J, Ju R. Hyperbaric oxygen treatment promotes neural stem cell proliferation in the subventricular zone of neonatal rats with hypoxic-ischemic brain damage. Neural Regen Res. (2013) 8:1220–7. doi: 10.3969/j.issn.1673-5374.2013.13.007
83. Liu Z, Xiong T, Meads C. Clinical effectiveness of treatment with hyperbaric oxygen for neonatal hypoxic-ischaemic encephalopathy: systematic review of Chinese literature. BMJ. (2006) 333:374. doi: 10.1136/bmj.38776.731655.2F
84. Zhang G, Luo Y, Yu H, Min L. Meta-analysis of the effect of hyperbaric oxygen on hypoxic ischemia encephalopathy. Chin Gen Pract. (2013) 16:1641–5.
85. Xu Y, Ji R, Wei R, Yin B, He F, Luo B. The efficacy of hyperbaric oxygen therapy on middle cerebral artery occlusion in animal studies: a meta-analysis. PLoS One. (2016) 11:e0148324. doi: 10.1371/journal.pone.0148324
86. Wang F, Wang Y, Sun T, Yu HL. Hyperbaric oxygen therapy for the treatment of traumatic brain injury: a meta-analysis. Neurol Sci. (2016) 37:693–701. doi: 10.1007/s10072-015-2460-2
87. Song Z, Ding B, Shen Q, Jing WG. Prevention and treatment of adverse reactions and complications of hyperbaric oxygen therapy. Clin Misdiagn Misther. (2012) 25:106–8. doi: 10.3969/j.issn.1002-3429.2012.05.055
88. Shankaran S. Therapeutic hypothermia for neonatal encephalopathy. Curr Treat Opt Neurol. (2012) 14:608–19. doi: 10.1007/s11940-012-0200-y
89. Guo SC, Zhao SG. Research progress in the treatment of hypoxic ischemic encephalopathy. Inner Mongol Med J. (2015) 47:813–5. doi: 10.16096/J.cnki.nmgyxzz.2015.47.07.017
Keywords: neonate, hypoxic-ischemic encephalopathy, hyperbaric oxygen therapy, randomized controlled trials, meta-analysis
Citation: Gong X-B, Feng R-H, Dong H-M, Liu W-H, Gu Y-N, Jiang X-Y, Lou Y-H, Xu J and Dou Q-L (2022) Efficacy and Prognosis of Hyperbaric Oxygen as Adjuvant Therapy for Neonatal Hypoxic-Ischemic Encephalopathy: A Meta-Analysis Study. Front. Pediatr. 10:707136. doi: 10.3389/fped.2022.707136
Received: 09 May 2021; Accepted: 09 March 2022;
Published: 21 April 2022.
Edited by:
Letizia Capasso, Federico II University Hospital, ItalyReviewed by:
Dick Tibboel, Erasmus Medical Center, NetherlandsCopyright © 2022 Gong, Feng, Dong, Liu, Gu, Jiang, Lou, Xu and Dou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Xu, eHVqdW5mcmVlQDEyNi5jb20=; Qing-Li Dou, ZG91cWluZ2xpQDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.