- 1Chongqing Key Laboratory of Child Infection and Immunity, Children’s Hospital, Chongqing Medical University, Chongqing, China
- 2Neonatal Department, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 3National Clinical Research Center for Child Health and Disorders, Chongqing, China
- 4Ministry of Education Key Laboratory of Child Development and Disorders, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 5Neonatal Department, Chengdu Women’s and Children’s Central Hospital, Chongqing, China
- 6Ultrasonography Department, Chengdu Women’s and Children’s Central Hospital, Chongqing, China
Background: Necrotizing enterocolitis (NEC) is a devastating intestinal complication that occurs mainly in very-low-birth-weight infants (VLBWI). The study's aim was to investigate the possibility of early prediction of NEC on postnatal day 1 based on superior mesenteric artery (SMA) doppler ultrasonograpy.
Methods: A prospective, observational, nested case control study (ChiCTR1900026197) was conducted to enroll VLBWIs (birth weight <1,500 grams) between October 2019 and September 2021. Doppler ultrasound measurement was done during the first 12 h of life and before first feeding. Infants developing NEC (stage II or III) subsequently were included in NEC group and infants spare of NEC were included in control group.
Results: 370 VLBWIs were enrolled (30 NEC cases). Among the ultrasound parameters, S/D was significantly higher in the NEC group (OR: 2.081, 95% CI: 1.411–3.069, P = 0.000). The area under the receiver operating curve (AUROC) following the Logistic regression was 0.704 (95% CI: 0.566–0.842, P = 0.001). The sensitivity of S/D for predicting NEC was 52.2% and the specificity was 92.7%. The critical value of S/D was 6.944 and Youden index was 0.449. Preplanned subgroup analysis confirmed that NEC infants of different stages were characterized by different SMA bloodstream. Small for gestational age (SGA) might be a confounding factor affecting intestinal bloodflow. And infants with delayed initiation or slow advancement of feeding exhibited characteristic intestinal perfusion.
Conclusions: In VLBWI, early SMA ultrasound shows the potential to predict NEC. It is reasonable to speculate that SMA bloodstream is related to intestinal structural and functional integrity.
Introduction
Annually, about 15 million preterm infants (gestational age <37 weeks) are born globally (1), with an incidence of approximately 11%. Immaturity has been an important cause of neonatal mortality and even mortality of children under 5 years of age (2, 3). As an abrupt onset intestinal complication, neonatal necrotizing enterocolitis (NEC) is a multi-factor-related disease that mainly occurs in premature-birth infants. In very-low-birth-weight infants (VLBWI, birth weight <1,500 grams), the morbidity of NEC is 5.1%–9% (4–6), and surgical treatment can account for more than 50% of them (8,935/17,159) (7). The mortality rate of NEC is as high as 30%–50% (7–9), and complications such as intestinal stenosis and intestinal insufficiency may occur even if they survive. Moreover, the incidence of NEC-related long-term adverse neurological prognosis also reached 24%–59.3% (9–12). Generally, neonatology has been developing rapidly in recent decades, but it seems that efforts to control the onset of NEC have not been successful, let alone long-term outcomes.
Besides, there are also studies dedicated to predicting NEC and helping clinical work by identifying high-risk groups. The goal of these studies is to improve the prognosis of preterm infants, and ultrasound is one of the explorations. Superior mesenteric artery (SMA), a major branch of the descending aorta, is the main source of blood supply to the intestine (13). Our previous study tentatively confirmed that preterm infants with NEC have different SMA doppler ultrasound characteristics on first postnatal day, which indicates the potential of this technology to predict NEC (14). Next, we will focus on VLBWIs. This study intended to verify the predictive ability of subsequent NEC onset by SMA doppler ultrasound on the first day of life, and to evaluate whether the prediction model could be constructed through bloodstream indicators.
Materials and methods
Study design
This is a prospective, observational, nested case-control study (Chinese Clinical Trial Registry: ChiCTR1900026197). It was approved by hospital Ethics Committee [2019 (10)]. Enrollment took place between October 2019 and September 2021. Guardians, staffs of neonatal and radiological departments were blinded to the measurements of SMA doppler ultrasound.
Inclusion criteria: (1) These infants were born in our hospital, and admitted into neonatal department quickly after birth to receive complete support and treatment; (2) Birth weight should be less than 1,500 grams; (3) Written informed consents were obtained from guardians; (4) Doppler ultrasonography should be done within 12 h of birth, when the respiration and circulation were stable. In this study, cardiopulmonary stability refers to preductal SaO2 90%–95%, mean blood pressure ≥30 mmHg, arterial pH > 7.25, PaCO2 35–45 mmHg, PaO2 50–80 mmHg.
Exclusion criteria: (1) Refused to provide consent; (2) Participated in other studies; (3) Not admitted to neonatal department shortly after birth or just received limited supportive care at the family’s request; (4) Unable to achieve cardiopulmonary stability within 12 h; (5) Feeding couldn’t be initiated during hospitalization; (6) Major or lethal congenital anomalies.
Preterm infants management protocol of the neonatal intensive care unit (NICU) recommended that first feeding should be initiated no later than 12–24 h of life, and the ultrasound should be completed before it. Infants who developed NEC (stage II–III) during hospitalization were included in NEC group, and those without NEC were included in the control group.
Doppler ultrasound
SMA bloodstream doppler ultrasound were done by three experienced ultrasonologists, and interobserver agreement was tested prior to the study. Bloodstream were measured by CX50 ultrasound machine (Philips Healthcare, Bothell, WA) with L12–3 linear probe (3–12 MHz).
The probe was placed vertically and softly below the xiphoid process of the lying infant during the whole examination. The sample volume of doppler ultrasound was set near the origin of SMA with acceptable angle between soundwave and bloodstream (<30°). For measurement and calculation, continuous recording of at least 5 cardiac cycles was necessary. The average of three tests was the final result of the ultrasound examination. Direct indicators include: peak systolic velocity (PSV), end-diastolic velocity (EDV), time-averaged mean velocity (TAMV). Calculated parameters include: differential velocity (DV, DV = PSV–EDV), SD index (SD = PSV/EDV), pulsatility index [PI, PI = (PSV–EDV)/TAMV], and resistance index [RI, RI = (PSV–EDV)/PSV].
Clinical management
Breast milk is the default choice, and the formula is also an alternative when breast milk is unavailable.
According to modified Bell`s criteria (15, 16), NEC stage I (suspected) refers to nonspecific signs (heart rate <100 bpm, apnea, poor response, body temperature fluctuation) and intestinal abnormalities (feeding intolerance, bloody stool). The manifestations of stage II (definite) refers to abdominal muscular tension, disappearance of bowel sounds. Infants with x-ray confirmed pneumatosis intestinalis were diagnosed NEC IIA, and NEC IIB was featured by both x-ray confirmed portal venous gas and pneumatosis intestinalis. Infants progress to NEC IIIA could show obvious systemic complications (shock, sepsis, acidosis), severe abdominal conditions such as ascites. Infants with intestinal perforation are diagnosed NEC IIIB. To reduce risk of bias, the diagnosis of NEC should be made jointly by two neonatologists and one radiologist.
Study population
G*Power (Version 3.1.9.6) was used for sample size calculation. Historical data at our hospital showed an 8% incidence of NEC in VLBWIs. According to our previous study, it was assumed that the DV was 45 cm/s (standard deviation ±18 cm/s) in the NEC group and 34 cm/s (standard deviation ±13 cm/s) in the non-NEC group. With 5% significance, 90% power and 20% loss-to-follow, 328 VLBWIs (28 NEC infants and 300 Non-NEC infants) were required.
Data management
The endpoint of the study was a diagnosis of NEC (stage II or III) during hospitalization. Information of obstetric conditions, perinatal history, illnesses and treatments, ultrasound measurements, nutritions was collected. Given the excellent follow-up rate, the handling of missing data was done by removal of incomplete cases.
Continuous variables with non normal distribution were expressed as medians and quartiles, and compared by using the Mann–Whitney U test. Categorical variables (numbers and percentages) were tested by χ2 or Fisher's exact test as appropriate. Factors that had significant differences in the univariate test and passed the collinearity test were included in the logistic regression analysis. A receiver operating characteristic curve could be drawn based on the logistic regression analysis. All tests were two-sided and P < 0.05 were considered to be significantly different. All data analyses were performed using SPSS version 22.0 software (IBM Corporation, Armonk, NY).
Preplanned subgroup analyses include: (1) NEC stage II vs. stage III, to reveal if severity of NEC is related with intestinal bloodflow; (2) The control group was divided into two subgroups (1000–1,499 grams vs. <1,000 grams) to verify the relationship between birth weight and SMA bloodstream; (3) Control group was divided into ≥32 gestational weeks and <32 gestational weeks subgroups to test the relation between ultrasound and gestational age; (4) Infants diagnosed small for gestational age (SGA) (birth weight below the 10% of the same gestational week) were selected to evaluate the effect of fetal growth restriction on intestinal bloodflow.
As the major supply of blood perfusion in the gut, SMA is of great importance for the structural and functional integrity of the intestine. Thus we conducted further exploratory analyses to assess the association of intestinal perfusion stability with feeding of VLBWI.
Results
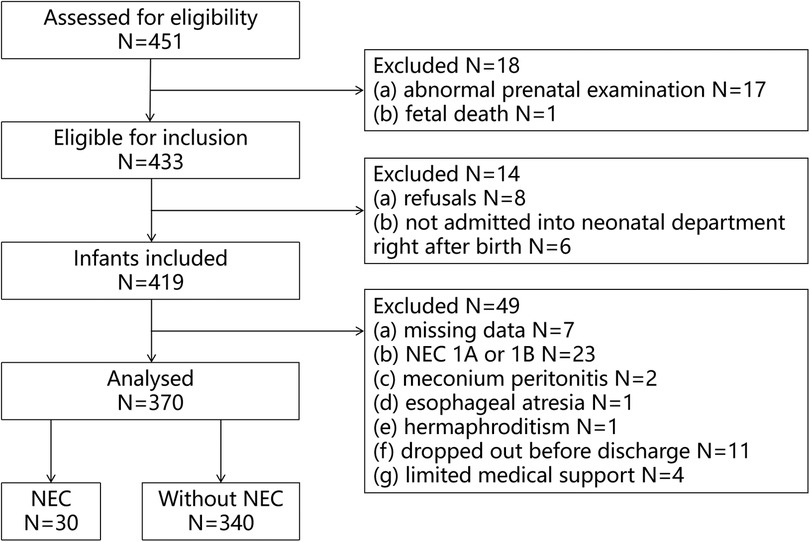
A total of 451 VLBWIs were screened for eligibility during the investigation, 433 infants met the inclusion criteria (17 abnormal pregnancy, 1 stillbirth). 419 VLBWIs were included (8 refused to participate, 6 not admitted quickly after birth). Finally there were 370 VLBWIs entered the data analysis (Figure 1).
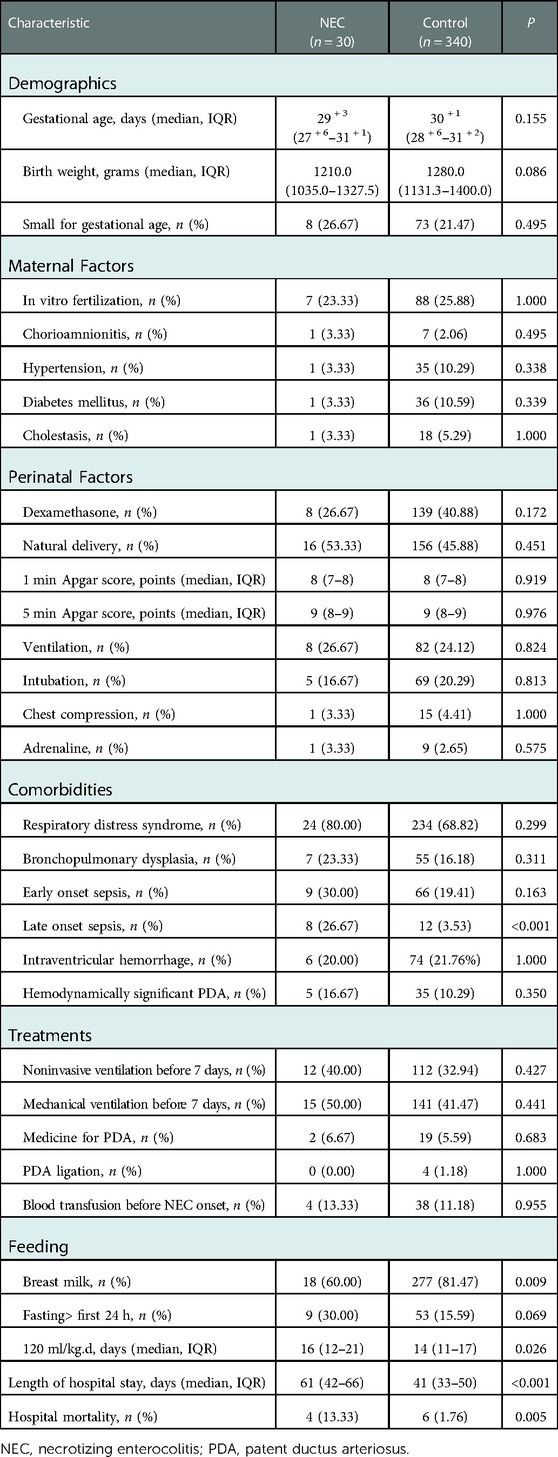
30 VLBWIs diagnosed with NEC stage II or III were included in the NEC group, while 340 VLBWIs without NEC served as control group. Details of NEC infants were listed in the Supplementary Material. Demographics, obstetric conditions, perinatal events, postnatal comorbidities and treatments were compared between two groups (Table 1). The breastfeeding rate in the NEC group was significantly lower than that in the control group [18 (60.00%) vs. 277 (81.47%), P = 0.009], and the NEC group infants needed longer days to reach 120 ml/kg.d of feeding (days) [16 (12, 21) vs. 14 (11, 17), P = 0.026]. According to other endpoints, the NEC group had significantly higher rates of late-onset sepsis [8 (26.67%) vs. 12 (3.53%), P = 0.000], longer hospital stays (days) [61 (42, 66) vs. 41 (33, 50), P = 0.000], and significantly higher in-hospital mortality than the control group [4 (13.33%) vs. 6 (1.76%), P = 0.005].
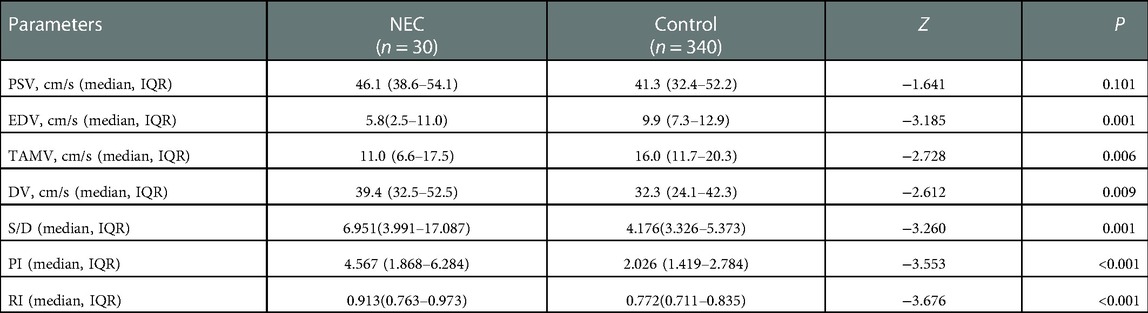
Univariate analysis of SMA doppler ultrasound between two groups detected significant differences in multiple parameters (Table 2). The risk of NEC was significantly higher as EDV and TAMV decreased. The DV, S/D, PI and RI of the NEC group were significantly higher than those of the control group. There was no significant difference in the proportion of absent SMA blood flow during diastole [4 (13.33%) vs. 23 (6.76%), χ2 = 0.921, P = 0.337], and there was also no significant difference in reverse flow during diastole [3 (10.00%) vs. 14 (4.12%), χ2 = 1.041, P = 0.308].
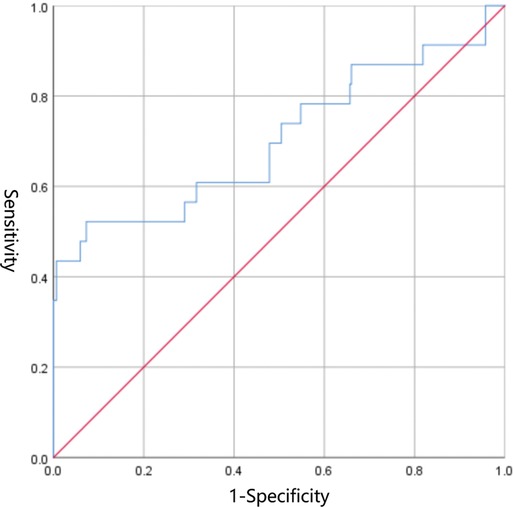
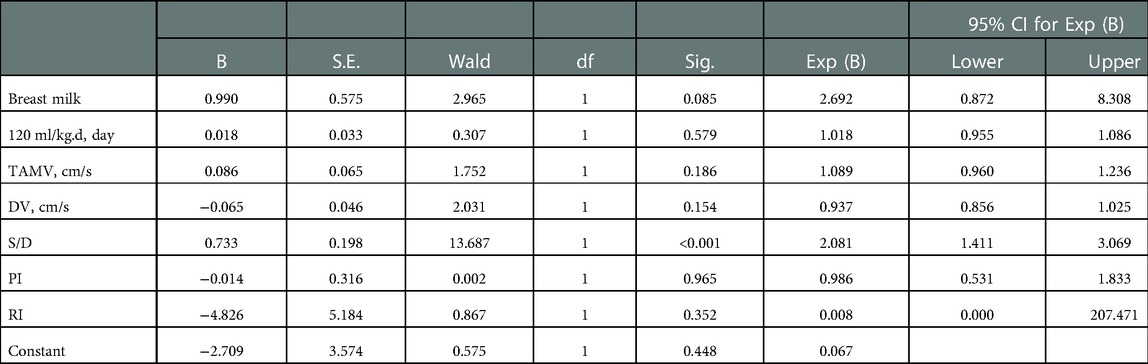
EDV was eliminated in the collinearity test of the regression analysis (see Supplementary Material). Subsequent Logistic regression analysis confirmed that S/D was significantly associated with NEC (OR: 2.081, 95% CI: 1.411–3.069, P = 0.000) (Table 3). The area under the receiver operating curve (AUROC) following the Logistic regression analysis was 0.704 (95% CI: 0.566–0.842, P = 0.001) (Figure 2). The sensitivity of S/D for predicting NEC was 52.2% and the specificity was 92.7%. The critical value of S/D was 6.944 and Youden index was 0.449.
Preplanned subgroup analyses were detailed in the Supplementary Material. (1) 30 VLBWIs were diagnosed NEC (stage II or III), the age of onset was 22 (17, 28) days, 23 (76.67%) patients presented with pneumatosis intestinalis on AXR, 17 (56.67%) presented with portal venous gas on AXR, 14 (46.67%) received surgical treatment. In the comparison of ultrasound parameters, NEC patients of stage II presented lower PSV compared with stage III (cm/s) [44.0 (36.2, 48.5) vs. 54.8 (41.2, 63.8) (cm/s), P = 0.039], and stage II patients also had lower DV (cm/s) [34.8 (32.5, 40.3) vs. 52.8 (33.2, 60.6), P = 0.039]. (2) Among the control group of 340 VLBWIs spared of NEC, there were 35 extremely low birth weight infants (ELBWI, birth weight <1,000 grams), other infants’s birth weight are larger than ELBWIs (1000–1,499 grams). Although significant differences naturally existed between the two subgroups in terms of demographics, diseases, treatments, and nutritions, but there was no significant difference in ultrasound parameters between them. (3) Among the control group of 340 VLBWIs spared of NEC, there were 57 infants with gestational age ≥32 weeks and 283 infants with gestational age <32 weeks. Although there were significant differences in multiple indicators of baseline characteristics between the two subgroups, ultrasound parameters did not show significant differences between them. (4) Among the control group of 340 VLBWIs spared of NEC, there were 73 SGA infants and 267 non-SGA infants, differences existed in a number of demographics between the two subgroups. Furthermore, obvious differences could be detected in ultrasound parameters.
Exploratory analyses were also detailed in the Supplementary Material. (1) Of the 370 VLBWIs, 308 infants started feeding within 24 h, and 62 infants’ feedings were introduced after 24 h. There were significant differences in multiple ultrasound parameters between the two groups. (2) The feeding volume of 86 infants reached 120 ml/kg.d within 10 days of age, while 284 infants did not reach it until 10 days or even later. Significant differences in multiple ultrasound parameters could be seen between the two groups.
During the ultrasound examination, no infant had adverse reactions.
Discussion
In this prospective, observational, nested case-control study of 370 VLBWIs, we found differences in multiple parameters of SMA ultrasound between 2 groups, and we finally confirmed the relation between S/D and incidence of NEC by Logistic regression analysis. The receiver operating characteristic curve also preliminarily verified the reasonable predictive power of this ultrasound parameter. Subgroup analysis unveiled that SMA bloodstream was related to the severity of NEC.
Murdoch et al. reported that EDV, TAMV, and PI of first day’s SMA ultrasound were related to NEC of preterm infants (17). 104 preterm infants were included in our previous study, DV was significantly associated with NEC (14). Both studies highlighted the importance of hemodynamic stability in terms of early stage SMA ultrasound parameters. This study further demonstrated that in VLBWI, the instability of gut perfusion in the early postnatal days was closely related to the incidence of NEC.
Since NEC seriously affects the prognosis of preterm infants, studies have been devoted to predicting this devastating disease. Alice et al. included 40 extremely preterm infants (gestational age <28 weeks), 11 developed NEC, 29 did not, none of 189 serum biomarkers within 2 days of age could effectively detect the risk (18). Although there have been many studies on biomarkers, none succeeded to exhibit good predictive power (19, 20). Ultrasound is another research direction. Prior studies have suggested the possible relationship between early postnatal ultrasound and the onset of NEC (14, 17). This is only a preliminary study, but the hypothesis has also been confirmed in VLBWIs. The result will undoubtedly encourage more similar studies to further explore the potential of early postnatal SMA ultrasound in predicting NEC.
In neonatal (21, 22) or animal (23, 24) studies, ultrasonography in the early stages of NEC suggests greater intestinal circulatory resistance and hemodynamic instability. Consistent with other studies, our finding extrapolates the relationship between high-resistance intestinal bloodstream and NEC to early postnatal stage. In addition to cardiac function and blood volume, local perfusion is also regulated by some factors (25). Miyake et al. found that mice knocked out of endothelin receptor B (to regulate vasoconstriction) exhibited less severe NEC, which underscores the important role of perfusion (26). It is worth noting the uncertainty of a direct connection between early intestinal perfusion and subsequent onset of NEC. Further exploration is required to reveal the pathophysiological basis for early prediction of NEC by ultrasound.
SMA provides major gut perfusion, and blood supply is crucial for structural and functional integrity, which is also confirmed by our exploratory analysis. Robel et al. found that early feeding volume was affected by intestinal hemodynamic stability after birth, but VLBWIs with poor feeding tolerance were mostly SGA infants in their analysis (27). Jain et al. included 63 VLBWIs, they could not confirm a clear correlation between SMA ultrasound and feeding intolerance, however, they also found significant differences in SMA bloodstream characteristics in SGA infants (28). In our study, multiple parameters of SMA ultrasound of SGA subgroup were very specific. In view of the heterogeneity of these studies in aspects such as subject inclusion, clinical management, ultrasonography, etc, we cannot yet say with certainty that SGA is a key variable affecting SMA blood flow, although SGA itself does represent some abnormalities in fetal growth and development.
VLBWIs are in need of sophisticated and hierarchical management, and SMA ultrasound in early postnatal period provides this possibility. Patel et al. (29) reported that severe anemia was associated with increased risk of NEC rather than red blood cell transfusion itself in a multicenter observational study of 598 VLBWIs. Currently, blood transfusion for VLBWIs was mainly based on age and respiratory support. This study requires us to evaluate the relationship between anemia and NEC especially in high-risk infants, and discuss the benefits and risks of blood transfusion with their families to protect VLBWIs as much as possible. Besides, if the infant's intestinal blood flow is unstable (such as high S/D), umbilical artery catheter (UAC) may need to be removed. Pranav et al. reported that UAC is one of the factors affecting intestinal blood perfusion (30). We can even imagine whether it is possible to adopt individualized feeding plan under ultrasound monitoring for NEC high-risk infants to avoid relative ischemia and reperfusion caused by feeding? (31).
Although our study confirms the relationship between SMA bloodstream and NEC in VLBWIs, obviously there are limitations: (1) NEC stage I (suspected) infants were not included, which helped to distinguish NEC group from control group, but in fact, the in-hospital treatment, nutrition of VLBWIs with suspected NEC are also affected, even not so severe as confirmed NEC. (2) Although no significant difference was observed in the proportion of SGA in 2 groups, subgroup analysis confirmed that SGA was more prone to high-resistance bloodstream, which is consistent with other studies (27, 28). SGA could be an critical factor affecting intestinal blood supply and may also be a confounding factor for intestinal complications, which should be considered in future studies. (3) S/D is of good specificity (92.7%) but suboptimal sensitivity (52.2%), which also results in a imperfect predictive power (AUROC 0.704). It allows us to effectively identify infants with low risk of developing NEC. However, the prediction inevitably has a relatively high false-positive rate. We expect this shortcoming could be overcome after newly developed study and model construction, or even through machine learning. (4) Due to the lack of adequate understanding of the intestinal blood flow in the early postnatal period of VLBWIs, we can only remove the missing data in ultrasonic measurement. (5) Intestinal blood flow changes rapidly in the early postnatal period, so ultrasound should completely reflect both the stability and adaptability. Feeding is one of the key factors affecting gut perfusion (32). Our study reflects the stability of intestinal blood flow before feeding, but it is obviously not an intact mirror, which does not reflect the full picture of intestinal blood flow. Subsequent studies should monitor blood flow before and after feeding.
In conclusion, there is now a potential for early prediction of NEC via SMA ultrasonography, which if timely managed, could reduce morbidity and mortality of VLBWIs. We hope that future well-designed studies will further confirm this and ultimately contribute to improving outcomes in preterm infants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Chengdu Women’s and Children’s Central Hospital, Chengdu, China. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
GY: is the first author. He was responsible for the initiation, trial registration, inclusion of infants, collection of data, he was also the main writer of the manuscript. JW: is the second author. He was in charge of statistical analysis, he was also responsible for the ethical review submission of the project. SY, YD and YW: are ultrasonologists, they completed ultrasound examination of all infants. WJ and HC: were responsible for screening and inclusion of infants, informed consent and clinical data collection. RJ: worked for study design, ethics review, and statistical analysis. YS: is the corresponding author. He was responsible for study design, ethical review, data analysis, manuscript review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1102238/full#supplementary-material.
References
1. Organization W H. Born too soon: the global action report on preterm birth. WHO. (2012) 380(9855):1713. doi: 10.1016/S0140-6736(12)61970-9
2. Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. (2013) 10(Suppl 1):S2.1-14. doi: 10.1186/1742-4755-10-S1-S2
3. Walani SR. Global burden of preterm birth. Int J Gynaecol Obstet. (2020) 150(1):31–3. doi: 10.1002/ijgo.13195
4. Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Canadian neonatal network. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. (2012) 129(2):e298–e304. doi: 10.1542/peds.2011-2022
5. Zozaya C, García González I, Avila-Alvarez A, Oikonomopoulou N, Sánchez Tamayo T, Salguero E, et al. Incidence, treatment, and outcome trends of necrotizing enterocolitis in preterm infants: a multicenter cohort study. Front Pediatr. (2020) 8:188. doi: 10.3389/fped.2020.00188
6. Zhu Z, Yuan L, Wang J, Li Q, Yang C, Gao X, et al. Mortality and morbidity of infants born extremely preterm at tertiary medical centers in China from 2010 to 2019. JAMA Netw Open. (2021) 4(5):e219382. doi: 10.1001/jamanetworkopen.2021.9382
7. Hull MA, Fisher JG, Gutierrez IM, Jones BA, Kang KH, Kenny M, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: a prospective cohort study. J Am Coll Surg. (2014) 218(6):1148–55. doi: 10.1016/j.jamcollsurg.2013.11.015
8. Henry MC, Moss RL. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. (2008) 17(2):98–109. doi: 10.1053/j.sempedsurg.2008.02.005
9. Jones IH, Hall NJ. Contemporary outcomes for infants with necrotizing enterocolitis-A systematic review. J Pediatr. (2020) 220:86–92.e3. doi: 10.1016/j.jpeds.2019.11.011
10. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. (2005) 115(3):696–703. doi: 10.1542/peds.2004-0569
11. Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. (2007) 92(3):F193–8. doi: 10.1136/adc.2006.099929
12. Fullerton BS, Hong CR, Velazco CS, Mercier CE, Morrow KA, Edwards EM, et al. Severe neurodevelopmental disability and healthcare needs among survivors of medical and surgical necrotizing enterocolitis: a prospective cohort study. J Pediatr Surg. (2017). S0022-3468(17)30651-6. doi: 10.1016/j.jpedsurg.2017.10.029.
13. D’Souza D, Bell D. Superior mesenteric artery. Reference article, Radiopaedia.org. doi: 10.53347/rID-5206 (Accessed Feb 14, 2022).
14. Guang Y, Ying D, Sheng Y, Yiyong F, Jun W, Shuqiang G, et al. Early doppler ultrasound in the superior mesenteric artery and the prediction of necrotizing enterocolitis in preterm neonates. J Ultrasound Med. (2019) 38(12):3283–9. doi: 10.1002/jum.15064
15. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. (1978) 187(1):1–7. doi: 10.1097/00000658-197801000-00001
16. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. (1987) 17(4):213–88. doi: 10.1016/0045-9380(87)90031-4
17. Murdoch EM, Sinha AK, Shanmugalingam ST, Smith GC, Kempley ST. Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. (2006) 118(5):1999–2003. doi: 10.1542/peds.2006-0272
18. Hoffsten A, Markasz L, Lilja HE, Olsson KW, Sindelar R. Early postnatal comprehensive biomarkers cannot identify extremely preterm infants at risk of developing necrotizing enterocolitis. Front Pediatr. (2021) 9:755437. doi: 10.3389/fped.2021.755437
19. Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: a review. JAMA Pediatr. (2017) 171(1):83–8. doi: 10.1001/jamapediatrics.2016.2708
20. D’Angelo G, Impellizzeri P, Marseglia L, Montalto AS, Russo T, Salamone I, et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital J Pediatr. (2018) 44(1):84. doi: 10.1186/s13052-018-0528-3
21. Urboniene A, Palepsaitis A, Uktveris R, Barauskas V. Doppler flowmetry of the superior mesenteric artery and portal vein: impact for the early prediction of necrotizing enterocolitis in neonates. Pediatr Surg Int. (2015) 31(11):1061–6. doi: 10.1007/s00383-015-3792-y
22. Hashem RH, Mansi YA, Almasah NS, Abdelghaffar S. Doppler ultrasound assessment of the splanchnic circulation in preterms with neonatal sepsis at risk for necrotizing enterocolitis. J Ultrasound. (2017) 20(1):59–67. doi: 10.1007/s40477-016-0228-z
23. Choi YH, Kim IO, Cheon JE, Kim JE, Kim EK, Kim WS, et al. Doppler sonographic findings in an experimental rabbit model of necrotizing enterocolitis. J Ultrasound Med. (2010) 29(3):379–86. doi: 10.7863/jum.2010.29.3.379
24. Ganji N, Koike Y, Li B, Zhu H, Lau E, Lok MJ, et al. Doppler ultrasound assessment of splanchnic perfusion and heart rate for the detection of necrotizing enterocolitis. Pediatr Surg Int. (2021) 37(3):347–52. doi: 10.1007/s00383-020-04819-5
25. Koike Y, Li B, Ganji N, Zhu H, Miyake H, Chen Y, et al. Remote ischemic conditioning counteracts the intestinal damage of necrotizing enterocolitis by improving intestinal microcirculation. Nat Commun. (2020) 11(1):4950. doi: 10.1038/s41467-020-18750-9
26. Miyake H, Seo S, Fujiwara N, Miyahara K, Lee C, Li B, et al. Endothelin receptor B affects the perfusion of newborn intestine: possible mechanism of necrotizing enterocolitis development. Pediatr Surg Int. (2019) 35(12):1339–43. doi: 10.1007/s00383-019-04559-1
27. Robel-Tillig E, Knüpfer M, Pulzer F, Vogtmann C. Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radiol. (2004) 34(12):958–62. doi: 10.1007/s00247-004-1285-6
28. Jain N, Ramji S, Jain A, Modi M, Sharma P. Early prediction of feed intolerance in very low birth weight preterm infants using superior mesenteric artery blood flow velocity. J Matern Fetal Neonatal Med. (2019):1–6. doi: 10.1080/14767058.2019.1591362. [Epub ahead of print]30888887
29. Patel RM, Knezevic A, Shenvi N, Hinkes M, Keene S, Roback JD, et al. Association of red blood cell transfusion, anemia, and necrotizing enterocolitis in very low-birth-weight infants. JAMA. (2016) 315(9):889–97. doi: 10.1001/jama.2016.1204
30. Jani P, Hinder M, Badawi N, Galea C, Goodwin A, Tracy M. Are there changes to regional tissue oxygenation and circulation following umbilical artery catheter placement? A prospective cohort study in newborn infants. J Paediatr Child Health. (2020) 56(4):550–6. doi: 10.1111/jpc.14679
Keywords: doppler ultrasound, necrotizing enterocolitis, very low birth weight infant, prediction, superior mesenteric artery
Citation: Yue G, Wang J, Yang S, Deng Y, Wen Y, Jia W, Cao H, Ju R and Shi Y (2023) Prediction of necrotizing enterocolitis in very low birth weight infants by superior mesenteric artery ultrasound of postnatal day 1: A nested prospective study. Front. Pediatr. 10:1102238. doi: 10.3389/fped.2022.1102238
Received: 18 November 2022; Accepted: 30 December 2022;
Published: 16 January 2023.
Edited by:
Jeroen J. van Vonderen, Leiden University Medical Center (LUMC), NetherlandsReviewed by:
Hidehiko Nakanishi, Kitasato University, JapanPradeep Suryawanshi, Bharati Vidyapeeth Deemed University, India
© 2023 Yue, Wang, Yang, Deng, Wen, Jia, Cao, Ju and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang Yue WUdtb29ubGlnaHRAMTM5LmNvbQ== Yuan Shi c2hpeXVhbkBob3NwaXRhbC5jcW11LmVkdS5jbg==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Guang Yue
Guang Yue Jun Wang5
Jun Wang5 Huiling Cao
Huiling Cao Rong Ju
Rong Ju Yuan Shi
Yuan Shi