- 1Department of Nursing, College of Medicine and Health Sciences, Dilla University, Dilla, Ethiopia
- 2School of Nursing, College of Health Sciences, Mekelle University, Mekelle, Ethiopia
Background: In the year 2015, more than one-third of neonatal deaths caused by prematurity was recorded worldwide. Despite different kinds of efforts taken at the global and local levels to reduce neonatal mortality, it remains high with low reduction rates, especially in low- and middle-income countries like sub-Saharan Africa and South Asia. Therefore, this study aims to assess the survival status and predictors of mortality among preterm neonates.
Methods: A retrospective follow-up study was conducted on randomly selected 561 preterm neonates. Data were extracted from patient records using a pretested checklist. Data entry and analysis were done using Epi-Data Version 4.4.2.1 and Stata version 14, respectively. The Cox proportional hazard regression model was fitted to identify the predictors of mortality. A hazard ratio with a 95% confidence interval (CI) was estimated and p-values < 0.05 were considered statistically significant.
Result: The proportion of preterm neonatal deaths was 32.1% (180) with an incidence of 36.6 (95% CI: 31.6–42.4) per 1,000 person days. The mean survival time was 18.7 (95% CI: 17.7–19.9) days. Significant predictors for time to death of preterm neonates were respiratory distress syndrome [adjusted hazard ratio (AHR): 2.04; 95% CI: 1.48–2.82], perinatal asphyxia (AHR: 2.13; 95% CI: 1.32–3.47), kangaroo mother care (AHR: 0.14; 95% CI: 0.08–0.24), and gestational age (AHR: 0.85; 95% CI: 0.80–0.90).
Conclusion: Preterm neonatal death is still a major public health concern. Respiratory distress syndrome, perinatal asphyxia, kangaroo mother care, and gestational age were independent significant predictors for time to death, as found in this study. Hence, priority must be given to neonates with the above illnesses and strengthen the management and care of preterm neonates.
1. Introduction
Neonatal mortality is a global burden that affects both developing and developed countries. Globally, approximately 2.7 million neonatal deaths were reported in the year 2015, accounting for 45·1% of under-five mortality. Prematurity was responsible for 1.05 million deaths that occurred in under-five children worldwide in 2015, of which 0.356 million deaths occurred in sub-Saharan Africa (1). In 2010, the leading cause of under-five mortality was infectious disease (64%), and prematurity-related deaths accounted for only 14% (2). Then, after 5 years, prematurity became the second leading cause of under-five mortality (3), and from 2015 until now, prematurity has been the foremost cause of under-five and neonatal mortality (1).
Despite such mortality affecting all countries of the world, there is a difference in terms of survival between developed and developing countries; neonates born in Africa have 12 times high risk of mortality compared with those born in Europe (4). Sub-Saharan Africa has a high neonatal mortality rate (27%) compared with European countries (5%) but has a low annual neonatal mortality reduction rate (2.4%) compared with the global reduction rate (3%) (5).
Preterm neonates who are born before 37 completed weeks of gestation (6) have a high risk of mortality (7, 8) and adverse health outcomes (9). Preterm birth is a major contributor to the loss of human potential (10) and to hospital admission (11). Moreover, preterm neonates who survive after the neonatal period have a high risk of neurodevelopmental and learning impairment, visual disorders, long-term cardiovascular disease, and other non-communicable diseases (12, 13). In 2010, 3.1% of disability-adjusted life years occurred because of prematurity (14).
According to the 2017 United Nations International Children's Emergency Fund (5) report, the neonatal mortality rate in Ethiopia was 27% (5). Furthermore, the 2013 UNICEF report clustered Ethiopia among the top 25 countries with high under-five mortality (3); prematurity was one of the foremost causes (15). In addition, the Ethiopian Demographic Health survey (EDHS) report indicated the trends of neonatal mortality—29% in 2016 (16) and 30% in 2019 (17). Moreover, previous studies conducted in Ethiopia showed a high neonatal mortality rate (18–21) and a low reduction rate (20).
Previous studies identified respiratory distress syndrome (RDS) (19, 22), asphyxia (18, 22, 23), sex (24–27), maternal residency (19, 28), gestational age (25, 29, 30), birth weight (18, 27, 31), neonatal sepsis (19, 23, 26), jaundice (18, 23), hyaline membrane disease (18, 23), hypothermia at admission (23), hypoglycemia (18), maternal chronic disease (19, 22), and parity (22) as a predictor of mortality for preterm neonates.
Although the above predictors have been identified, the mortality of preterm neonates continues to be high and is on an increasing trend. Furthermore, if Sustainable Development Goal 3 is to be achieved by the year 2030, conducting relevant studies on crucial topics such as neonatal mortality is essential. Such studies will also support the realization of the goal of the National Newborn and Child Survival Strategy. Therefore, this study aims to assess the survival status and predictors of mortality among preterm neonates in Northern Ethiopia.
The results of this study will help program planners, decision makers, and implementers to know the gaps in current practices of preterm neonate management, focus on the identified gaps, and take action.
2. Methods and materials
2.1. Study area and design
An institution-based retrospective follow-up study was conducted in comprehensive specialized hospitals in the Tigray region. The Tigray region is one of the nine federal administrative regions in Ethiopia. It covers an estimated area of 41,409.95 km2 with the capital city of Mekelle, which is approximately 781 km away from Addis Ababa, the capital city of Ethiopia. The region has an estimated total population of 5,377,144, with 2,651,167 (49.3%) males and 2,725,977 (50.7%) females. Among the total population, 159,164 are under -1-year infants. There are 2 comprehensive specialized hospitals, 15 general hospitals, 23 primary hospitals, 245 health centers, and 750 health posts in the Tigray region (32). This study was conducted in these comprehensive specialized hospitals.
The Ayder comprehensive specialized hospital (ACSH) has 45 neonatal beds in the neonatal intensive care unit (NICU) and more than 170,000 patient flows per year. The Aksum comprehensive specialized hospital provides its service to a population of over 3.6 million from the central, northwest, and western zones of the Tigray regional state. It has a total capacity of 173 beds, including 13 neonatal beds. In both hospitals, intubation, vasopressors, IV antibiotics, and gavage feeding are offered.
2.2. Population
In this study, the target population was all preterm neonates who were treated in the two comprehensive specialized hospitals of the Tigray region. Preterm neonates who were treated in the ACSH and Aksum comprehensive specialized hospital between February 1, 2017, and January 30, 2019, constituted the study population.
2.3. Eligibility criteria
Preterm neonates (born before 37 completed weeks of gestation) who were admitted to the ACSH and Aksum NICUs between February 1, 2017, and January 30, 2019, were included in this study. Preterm neonates who had incomplete medical records relating to the mainly required variables (date of birth, date of admission, outcome status, and date at which outcome was determined) were excluded.
2.4. Sample size determination and sampling technique
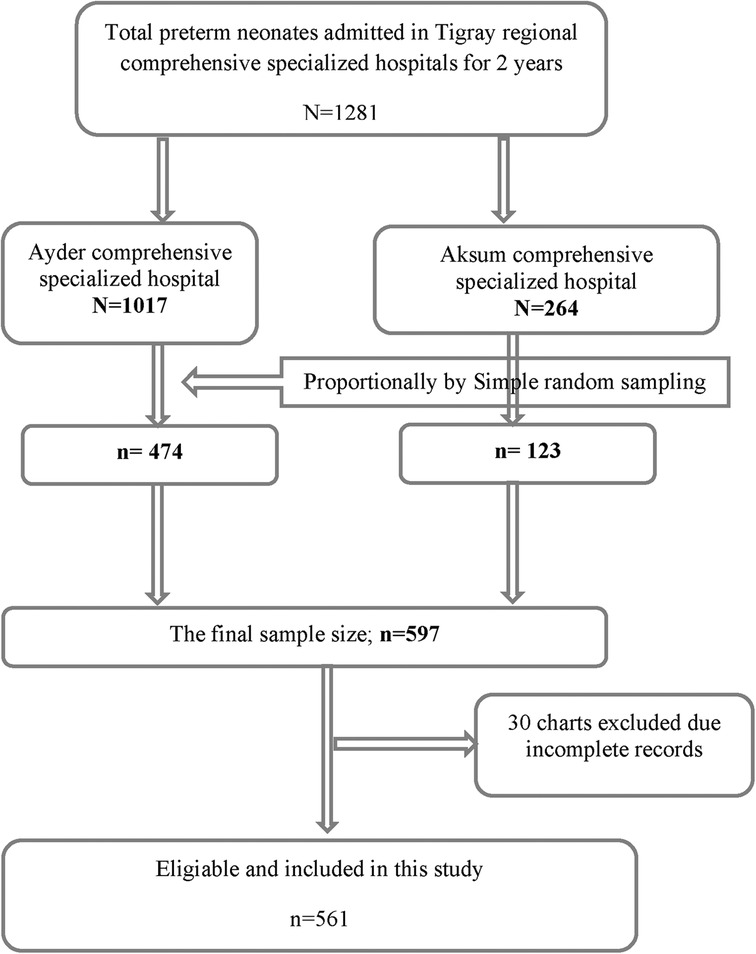
The required sample size for this study was determined by using the Stata statistical package (Cox model), version 14, and the following assumptions: a hazard ratio (HR) of 1.55 for selected covariate of interest (perinatal asphyxia, PNA) from a study done in Gondar, Ethiopia (18), a variability (SD) of 0.5, a probability of failure (death) of 0.288 (18), a margin of error of 5%, and a confidence interval (CI) of 95% to achieve 80% power. After the addition of a 5% non-response rate, the final sample size for this study was 597. A simple random sampling technique was employed. The required number of subjects were proportionally allocated for both hospitals based on their population size (Figure 1).
2.5. Operational definitions and measurements
The date of admission was the initial follow-up time and the follow-up was done on 28 days of life.
2.5.1. Survival time
The time from admission to the event (death).
2.5.2. Event
Death of preterm neonates after admission to the NICU.
2.5.3. Time scale
The survival time was measured in days.
2.5.4. Censored
Preterm neonates who were discharged after recovery, lost to follow-up, subjected to parental refusal to medical advice, transferred to other health institutions, and remained in the NICU after 28 days were considered censored.
2.5.5. Congenital anomalies
In this study, congenital anomalies represent neural tube defects, encephalocele, anencephaly, duodenal and choanal atresia, cleft lip and palate, and gastroschisis (33).
2.5.6. Respiratory distress syndrome
This condition is characterized by grunting while breathing, rapid or shallow breathing, and flaring of the nostrils (34).
2.5.7. Apnea of prematurity
Respiratory pauses >20 s or pauses <20 s that are related to bradycardia (<80 beats/minute), central cyanosis, and/or oxygen saturation <85% in neonates born at <37 gestation weeks and without causal disorders that induce apnea (34).
Perinatal asphyxia: an Apgar score that remained at less than 7 (at 5 min after birth) and evidence of acute hypoxic compromise with acidemia (35).
2.5.8. Neonatal jaundice
This is defined as elevated total serum bilirubin (TSB) and clinically manifests as a yellowish discoloration of the skin, sclera, and mucous membrane.
2.6. Data collection tool and procedures
The data were extracted from the medical records of preterm neonates who were admitted to the NICUs. A pretested checklist adapted from a study done in Addis Ababa, Ethiopia (36), was used for data collection. After permission was obtained from both hospitals, the list was prepared by investigators based on the information obtained from the NICU health management system registration book. Then, the data were scouted by some select people in this study. Finally, to fill the checklist, all obtained medical records were assessed by data collectors with supervision.
2.7. Data quality control
A pretest was conducted on 5% of the sample size in Mekelle general hospital. Data were collected by two trained and experienced diploma nurses and four BSc nurses, and two master's students acted as supervisors. During the data collection period, a close follow-up, monitoring, and guidance were carried out. The completeness and consistency of the data were checked daily. Before the entry of data, the checklist was patterned for tracking errors and a checklist that contained errors was excluded from the analysis.
2.8. Data processing, analysis, and presentation
The data were coded and entered by using Epi-Data manager version 4.4.2.1 and transferred to Stata statistical software version 14 for clearance and analysis. The Cox proportional hazard regression model was used. After Cox proportional hazard regression was done for each variable, a variable with a p-value <0.25 was entered for multivariate analysis. Multicollinearity was checked by using the variance inflation factor. The Cox proportional hazard regression assumption was tested by using the Schoenfeld residual test (global test). The overall goodness of model fitness was checked graphically by using the Cox Snell residual graph (Supplementary Annex S1). The overall model adequacy was checked through Harrell's C test. Lastly, after multivariate analysis, an association was expressed through the hazard ratio and statistically significant predictors were identified using 95% CI and p-value. The results of this study were presented by way of tables, texts, and graphs.
2.9. Ethics approval and consent to participate
The study protocol was evaluated and approved by the Institution of Review Board (IRB) of the College of Health Sciences, Mekelle University, and ethical clearance was obtained. A letter of cooperation was written to the chief executive managers of the ASCH and Aksum comprehensive specialized hospital. The hospital managers provided the right to access the chart of neonates. Finally, confidentiality and anonymity of the data were secured.
3. Result
3.1. Sociodemographic predictors
In this study, out of 597 preterm neonate medical charts, only 561 were complete and were included, with a 94% response rate. Out of the 561 preterm neonates, 444 (79.1%) were admitted to the ACSH, and of these, 140 (31.5%) died. Approximately 310 (55.3%) preterm neonates were males; among these, 98 (31.6%) died. Only 26 (4.6%) preterm neonates were born at home, and 12 (46.1%) of these died. A total of 406 (72.4%) moderate-to-late preterm neonates were born; among these, 95 (23.4%) died. The median gestational age at birth was 33 weeks and 6 days (IQR: 31 weeks and 3 days, 35 weeks). The median maternal age was 26 with an IQ of (22, 30) years (Table 1).
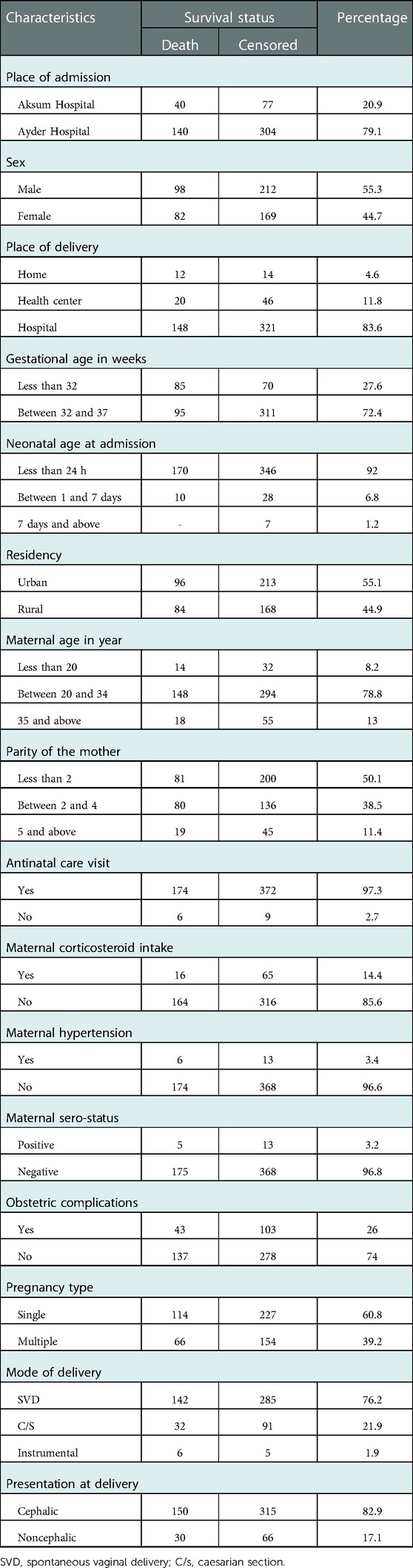
Table 1. Distribution of sociodemographic and obstetric-related characteristics in Northern Ethiopia, 2022 (n = 561).
3.2. Neonatal-related predictors
In this study, 344 (61.3%) preterm neonates had a birth weight between 1,500 and 2,500 g; 72 (20.9%) died. A total of 191 (34%) preterm neonates did not receive any feeding after delivery; 127 (66.5%) of these died. Moreover, 375 (66.8%) babies did not receive kangaroo mother care (KMC). Of these, 166 (42.3%) died. The Apgar score of 386 (68.8%) babies was known at birth. Among these, the median Apgar score at the 1st and 5th minute were 7 (IQR: 2) and 8 (IQR: 2), respectively (Table 2).
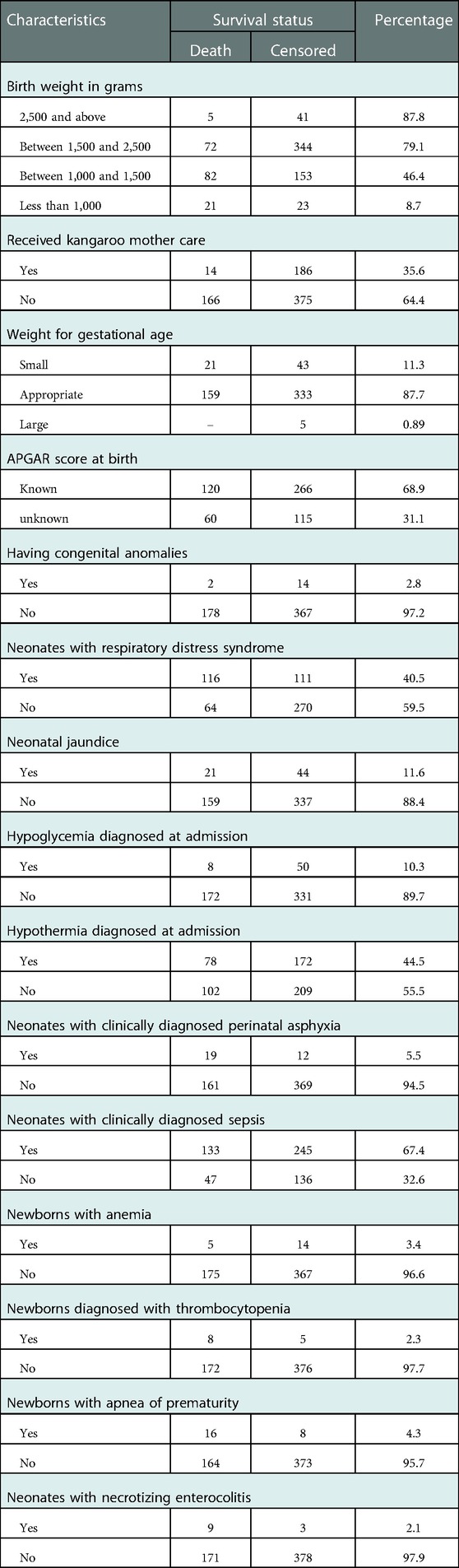
Table 2. Distribution of neonatal-related characteristics for preterm neonates in Northern Ethiopia, 2022 (n = 561).
3.3. Preterm birth–related predictors
Only 41 (7%) preterm neonates did not have medical and surgical complications. Among those who had such complications, 227 (40.5%), 378 (67.4%), 13 (2.3%), and 12 (2.1%) of them had RDS, sepsis, thrombocytopenia, and necrotizing enterocolitis (NEC), respectively (Table 3).
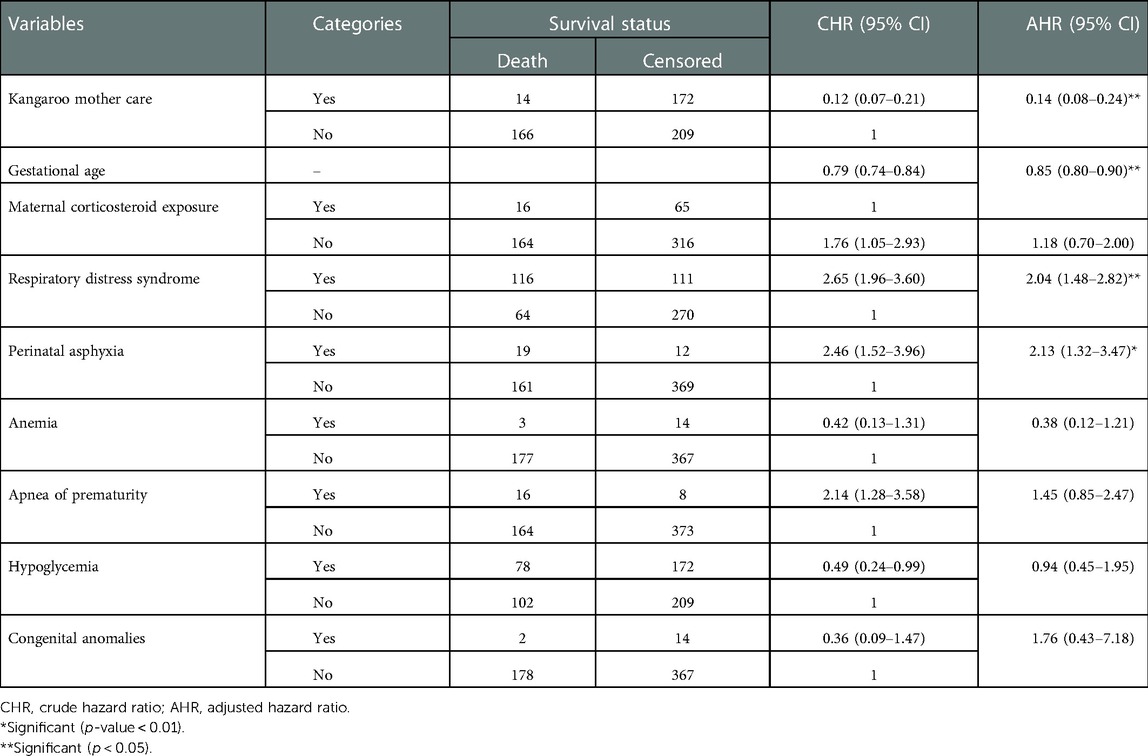
Table 3. Bivariable and multivariate Cox proportional hazard regression output for predictors to the time to death of preterm neonates in Northern Ethiopia, between February 1, 2017, and January 30, 2022 (n = 561).
3.4. Maternal- and obstetric-related predictors
Out of 561 preterm neonates, 50% were born to mothers who had a parity of less than 2, and 546 (97.3%) were born to mothers who had antenatal care (ANC) follow-up. A total of 460 (86.2%) babies were born to mothers who had three and more ANC visits. A majority (85.6%) of preterm neonates were born to mothers who had not taken corticosteroids before delivery; of these, 164 (34.2%) died. A total of 146babies (26%) were born to mothers who had obstetric complications; among these, 40 (27.4%) were born to those who suffered antepartum hemorrhage (Table 1).
3.5. Overall survival function
According to Kaplan–Meier survival estimates, 160 (88.9%) deaths occurred in the first week of admission, of which 53 (33.1%) died within the first 24 h of admission. In this study, the survival function vs. survival time was a decreasing step function (Figure 2). The survival probabilities for preterm neonates at the end of the first day, 7th day of admission, 14th day of admission, 21 days, and at the end of 28 days of admission were 90.5%, 67.7%, 62.8%, 60.4%, and 56.4%, respectively. Kaplan–Meier survival function estimation graphs were prepared for categorical covariates to observe survival differences (Figure 3).
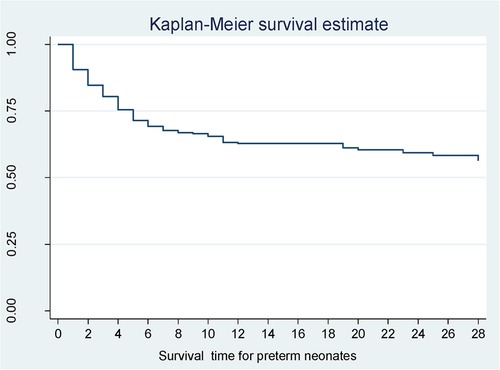
Figure 2. Overall survival function for preterm neonates in Northern Ethiopia, between February 1, 2017, and January 30, 2022 (n = 561).
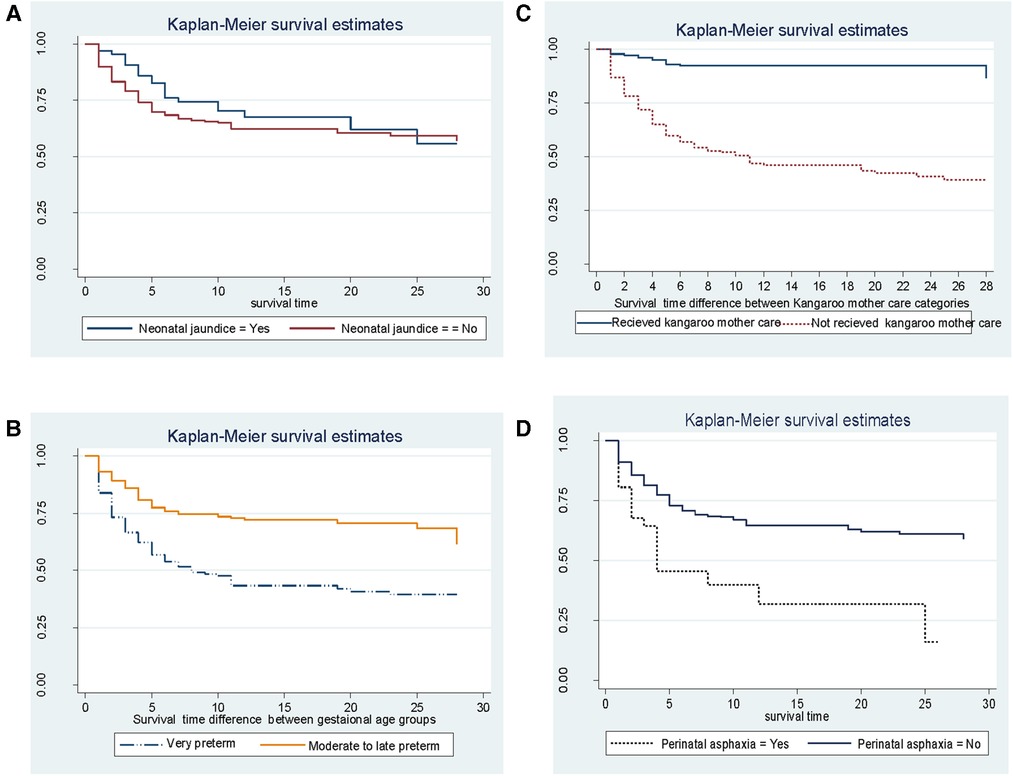
Figure 3. Kaplan–Meier estimated survival difference for selected covariates (A–D) of preterm neonates in Northern Ethiopia, between February 1, 2017, and January 30, 2019, and 2022 (n = 561).
3.6. Survival status of preterm neonates
In this study, 180 (32.1%) preterm neonates died during the follow-up period (Figure 4). The median length of follow-up was 6 days. The total person-day-observations were 4,917 days. The overall incidence of mortality for preterm neonates was 36.6 (95% CI: 31.6, 42.4) per 1,000 person days. Furthermore, the overall mean survival time of preterm neonates was 18.8 (95% CI: 17.7–19.9) days.
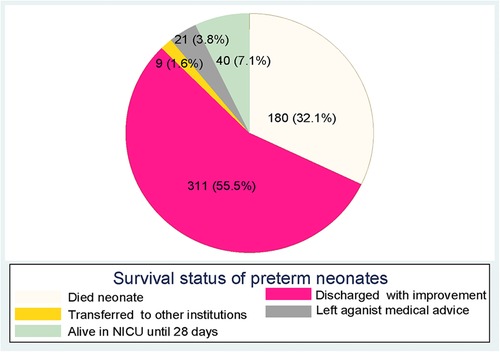
Figure 4. Survival status of preterm neonates at the end of follow-up in Northern Ethiopia, between February 1, 2017, and January 30, 2019, and 2022 (n = 561).
3.7. Predictors for time to death of preterm neonates
Bivariable and multivariate Cox proportional hazard regression were fitted to identify predictors for the time to death of preterm neonates. Variables that had a p-value <0.25 in the bivariable analysis were entered into the multivariate analysis. Findings from the bivariable analysis showed that respiratory distress syndrome, birth weight, apnea of prematurity, anemia, perinatal asphyxia, kangaroo mother care, congenital anomalies, gestational age, maternal corticosteroid intake, and hypoglycemia at admission were significantly associated with the time to death of preterm neonates. Before multivariate analysis, the global test was checked and it did not violate the assumption; the overall global test = 0.53. The model adequacy test (Harrell's C test) was 0.8 (80%). Multicollinearity was checked by VIF and it was 1.1. In multivariate analysis, four variables, respiratory distress syndrome, perinatal asphyxia, gestational age, and kangaroo mother care, were identified as independent predictors for the time to death of preterm neonates.
Preterm neonates having respiratory distress syndrome were twice [adjusted hazard ratio (AHR): 2.04; 95% CI: 1.48–2.82] more likely to die compared with their counterparts. Neonates having perinatal asphyxia were twice (AHR: 2.13; 95% CI: 1.32–3.47) more likely to die compared with their counterparts in the comparison group. As the gestational age increased in 1 week, the death rate decreased by 15% (AHR = 0.85; 95% CI: 0.80–0.90). Preterm neonates who received kangaroo mother care were 86% (AHR: 0.14; 95% CI: 0.08–0.24) less likely to die compared with their counterparts (Table 3).
4. Discussion
This study aimed to assess the survival status and predictors of mortality among preterm neonates admitted in the comprehensive specialized hospitals located in Northern Ethiopia, Tigray region. The incidence of mortality and the mean survival time for preterm neonates were 36.6 (95% CI: 31.6–42.4) per 1,000 person-day-observations and 18.8 days, respectively. RDS, PNA, KMC, and gestational age were identified as independent significant predictors for the time to death of preterm neonates.
The mean survival time of preterm neonates was lower than that in a study done in the University of Gondar (UOG) comprehensive specialized hospital, Ethiopia, which was 20.4 (22). This difference might be due to a high mortality rate in this study. Furthermore, the mean survival time of preterm neonates in this study was also lower than that in a study conducted in Iran, which was 43.0 (29). This variation might be due to differences in the length of follow-up. In a study conducted in Iran, preterm neonates were followed up until discharge with a maximum hospital stay of 105 days. However, in this study, neonates were followed up until 28 days, which made the survival time lower than that in the above study finding.
Preterm neonates who had RDS during hospital stay had increased hazards or risks of death compared with their counterparts. This finding was in line with a study conducted at Jimma University specialized hospital in Ethiopia (23). Another study that was conducted in Ethiopia also supported this finding (22). These study agreements might be attributed to the fact that the main cause of respiratory distress syndrome in preterm neonates is hyaline membrane deficiency, and preterm neonates who have RDS mostly develop acute complications such as pulmonary hemorrhage, apnea of prematurity, and intraventricular hemorrhage (IVH), which increase the risk of mortality (37).
Neonates who had perinatal asphyxia were more likely to die compared with their counterparts. This finding was consistent with that of studies done at the UOG comprehensive specialized hospital (18, 22) and Jimma University specialized hospital (23). This similarity might be attributed to the same levels of quality care provision for asphyxiated preterm neonates because all hospitals were comprehensive specialized hospitals. Also, perinatal asphyxia is one of the leading causes of neonatal mortality in Ethiopia (16), and perinatal asphyxia has the potential to cause damage to all organs of the body, including the brain and kidneys, and to impair gas exchange, in addition to causing organ immaturity in preterm neonates that facilitates neonatal mortality. Moreover, this similarity might be attributed to perinatal asphyxia causing severe complications such as hypoxic ischemic encephalopathy (HIE) and IVH, which have a high level of incidence in preterm neonates (15).
Preterm neonates who received KMC had less risks of mortality, and this result was similar to that of the study conducted in the UOG comprehensive specialized hospital (18). This similarity might be due to the majority of preterm neonates not receiving kangaroo mother care, as found in both studies, and KMC also protects neonates from infection, effectively treat hypothermia, improve gastrointestinal function and cardiorespiratory stability, and initiate/encourage breastfeeding. One systematic review and meta-analysis also supported this finding (38). In view of the important benefits of KMC, the World Health Organization (WHO) has strongly recommended its use as a package to treat preterm neonates (39).
In this study, as noted previously, it was found that when the gestational age increased by 1 week, the risk of mortality decreased by 15%. This finding was in line with that of a study conducted at the University of Gondar, Ethiopia (18). Also, this finding was supported by studies conducted in Ethiopia (23), Iran (29), and East Africa (30). Clinical evidence also supports this finding. The link between gestational age and mortality could be traced to the fact that organ immaturity leads to higher mortality; the more preterm, the more organ immaturity.
Because of the retrospective nature of the study design, an incomplete recording of data was a major limitation of this study.
5. Conclusion
The incidence of preterm neonatal mortality was high in this study. The most critical period for preterm neonatal mortality was the first week of admission, especially the first 24 h. The mean survival time of preterm neonates in this study was found to be lower than that in previous studies. Respiratory distress syndrome, perinatal asphyxia, gestational age, and kangaroo mother care were identified as predictors for the time to death of preterm neonates. To decrease the risk of mortality, health professionals should strengthen their follow-up regimens for very preterm neonates and neonates who have PNA and RDS. Moreover, health professionals should make the implementation of kangaroo mother care more stringent, since our study was retrospective study institutional related factors were not assessed. Therefore, we recommend prospective studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institution of Review Board (IRB) of the College of Health Sciences, Mekelle University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
BG conceptualized the study, wrote the proposal, and performed data analysis and manuscript preparation. HB, FM, and JN supervised subsequent drafts of the paper. All authors contributed to the article and approved the submitted version.
Funding
The authors acknowledge the financial support provided by the College of Health Sciences, Mekelle University.
Acknowledgments
The authors would like to thank the supervisors and data collectors who participated in this study. The authors also thank the neonatal intensive care unit and record room staff as well as the administrators of the Ayder and Aksum comprehensive specialized hospitals for their cooperation during the process of data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1083749/full#supplementary-material.
Abbreviations
ACSH, Ayder comprehensive specialized hospital; AHR, adjusted hazard ratio; ANC, antenatal care; C/s, caesarian section; CHR, crude hazard ratio; CI, confidence interval; CSH, Comprehensive specialized hospital; EDHS, Ethiopian Demographic Health Survey; HIE, hypoxic ischemic encephalopathy; IVH, intraventricular hemorrhage; KMC, kangaroo mother care; NEC, necrotizing enterocolitis; PNA, perinatal asphyxia; RDS, respiratory distress syndrome; SVD, spontaneous vaginal delivery; UNICEF, United Nations International Children's Emergency Fund; UOG, University of Gondar; WHO, World Health Organization.
References
1. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet. (2016) 388(10063):3027–35. doi: 10.1016/S0140-6736(16)31593-8
2. Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. (2012) 379(9832):2151–61. doi: 10.1016/S0140-6736(12)60560-1
3. Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. (2015) 385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6
4. Ashish K, Wrammert J, Nelin V, Ewald U, Clark R, Målqvist M. Level of mortality risk for babies born preterm or with a small weight for gestation in a tertiary hospital of Nepal. BMC Public Health. (2015) 15(1):877. doi: 10.1186/s12889-015-2232-1
5. UNICEF, WHO, World Bank Organization, UN. Levels & Trends in estimates developed by the UN inter-agency group for child mortality estimation child mortality 2018 report. Geneva, Switzerland (2018).
6. WHO. ICD-10: International statistical classification of diseases and related health problems: Tenth revision. 2nd ed. Geneva: Coherent Digital (2010).
7. Mengesha HG, Lerebo WT, Kidanemariam A, Gebrezgiabher G, Berhane Y. Pre-term and post-term births: predictors and implications on neonatal mortality in Northern Ethiopia. BMC Nurs. (2016) 15(1):48. doi: 10.1186/s12912-016-0170-6
8. FMOH. Neonatal intensive care unit (NICU) training participants manual: Ethiopia. Addis Ababa (2014). p. 46–7.
9. Stephens AS, Lain SJ, Roberts CL, Bowen JR, Nassar N. Survival, hospitalization, and acute-care costs of very and moderate preterm infants in the first 6 years of life: a population-based study. J Pediatr. (2016) 169:61–8.e3. doi: 10.1016/j.jpeds.2015.10.028
10. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. (2008) 111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73
11. Kuppusamy N, Balasubramanian M, Krithiga M. Magnitude of preterm admissions in neonatal intensive care unit of rural medical college hospital. Int J Sci Study. (2016) 4(1):284–7. doi: 10.17354/ijss/2016/629
12. Butler AS, Behrman RE. Preterm birth: causes, consequences, and prevention. Washington, DC: National Academies Press (2007).
13. WHO. March of dimes, PMNCH, save the children. In: Howson CP, Kinney MV, Lawn JE, editors. Born too soon: the global action report on preterm birth. Geneva: WHO Library (2012). p. 1–10.
14. Blencowe H, Lee AC, Cousens S, Bahalim A, Narwal R, Zhong N, et al. Preterm birth associated neurodevelopmental impairment estimates at regional and global level for 2010. Pediatr Res. (2013) 74(1):17–34. doi: 10.1038/pr.2013.204
15. FMOH. Neonatal intensive care unit (NICU) training participants manual: Ethiopia. Addis Ababa (2014). p. 46–7.
16. FMOH. Ethiopia demographic and health survey 2016: key indicators report. The DHS Program. Addis Ababa: ICF (2016). p. 364.
17. Institute EPH, ICF. Ethiopia Mini demographic and health survey 2019: key indicators. Addis Ababa: MD: EPHI and ICF Rockville (2019).
18. Yismaw AE, Gelagay AA, Sisay MM. Survival and predictors among preterm neonates admitted at University of Gondar comprehensive specialized hospital neonatal intensive care unit, Northwest Ethiopia. Ital J Pediatr. (2019) 45(1):4. doi: 10.1186/s13052-018-0597-3
19. Asmare Y. Survival status and predictor of mortality among premature neonate that was admitted to neonatal intensive care unit from 2013 to 2017 at Tikur Anbessa Hospital, Addis Ababa Ethiopia: conference presentation. Annual child and family healthcare nursing conference; August 13–14, 2018; Bali, Indonesia. Prim Health Care (2018). Open Access.
20. Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC Public Health. (2013) 13(1):483. doi: 10.1186/1471-2458-13-483
21. Mengesha HG, Sahle BW. Cause of neonatal deaths in Northern Ethiopia: a prospective cohort study. BMC Public Health. (2017) 17(1):62. doi: 10.1186/s12889-016-3979-8
22. Yehuala S, Teka Z. Survival analysis of premature infants admitted to neonatal intensive care unit (NICU) in northwest Ethiopia using semi-parametric frailty model. J Biom Biostat. (2015) 6(1):1. doi: 10.472/2155-6180.1000223
23. Wesenu M, Kulkarni S, Tilahun T. Modeling determinants of time-to-death in premature infants admitted to neonatal intensive care unit in Jimma University Specialized Hospital. Ann Data Sci. (2017) 4(3):361–81. doi: 10.1007/s40745-017-0107-2
24. Gargari SS, Kashanian M, Zendedel H, Nayeri F, Shariat M, Haghollahi F. Survival and risk factors of extremely preterm babies (<28 weeks) in the three Iranian hospitals. Acta Med Iran. (2018) 56(3):181–8.
25. de Castro ECM, Leite ÁJM, de Almeida MFB, Guinsburg R. Perinatal factors associated with early neonatal deaths in very low birth weight preterm infants in Northeast Brazil. BMC Pediatr. (2014) 14(1):312. doi: 10.1186/s12887-014-0312-5
26. Schindler T, Koller-Smith L, Lui K, Bajuk B, Bolisetty S. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC Pediatr. (2017) 17(1):59. doi: 10.1186/s12887-017-0810-3
27. Basiri B, Ashari FE, Shokouhi M, Sabzehei MK. Neonatal mortality and its main determinants in premature infants hospitalized in neonatal intensive care unit in Fatemieh Hospital, Hamadan, Iran. J Compr Pediatr. (2015) 6(3):1–6. doi: 10.17795/ijcp-26965
28. Abdel-Latif ME, Bajuk B, Oei J, Vincent T, Sutton L, Lui K. Does rural or urban residence make a difference to neonatal outcome in premature birth? A regional study in Australia. Arch Dis Child Fetal Neonatal Ed. (2006) 91(4):F251–6. doi: 10.1136/adc.2005.090670
29. Haghighi L, Nojomi M, Mohabbatian B, Najmi Z. Survival predictors of preterm neonates: hospital based study in Iran (2010–2011). Iran J Reprod Med. (2013) 11(12):957. PMID: 24639721; PMCID: PMC3941403.24639721
30. Marchant T, Willey B, Katz J, Clarke S, Kariuki S, Ter Kuile F, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. (2012) 9(8):e1001292. doi: 10.1371/journal.pmed.1001292.22904691
31. Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 and 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. (2016) 16(1):174. doi: 10.1186/s12887-016-0716-5
32. Tigray Regional Health Bureau. Demographic data of Tigray region and public health facilities. Mekelle, Ethiopia (2019).
33. Robert M, Mark J, Richard E, Bonita F, Joseph W, Nina F, et al. Nelson text book of pediatrics. 20th ed. Elsevier (2016).
34. McManus BM, Chambliss JH, Rapport MJ. Application of the NICU practice guidelines to treat an infant in a level III NICU. Pediatr Phys Ther. (2013) 25(2):204–13. doi: 10.1097/PEP.0b013e31828a4870
35. Carter BS, Haverkamp AD, Merenstein GB. The definition of acute perinatal asphyxia. Clin Perinatol. (1993) 20(2):287–304. doi: 10.1016/S0095-5108(18)30394-4
36. Asmare Y. Survival status and predictor of mortality among premature neonate admitted to neonatal intensive care unit from 2013 to 2017 in Tikur Anbesa specialized hospital, Addis Ababa, Ethiopia, 2018. Addis Ababa: Addis Ababa University (2018).
37. Kliegman MBR, Jenson H, Santon B. Nelson textbook of pediatrics. 18th ed. Philadelphia, PA: Saunders Elsevier (2007).
Keywords: survival, predictor, mortality, preterm neonate, north, Ethiopia
Citation: Girma B, Berhe H, Mekonnen F and Nigussie J (2023) Survival and predictors of mortality among preterm neonates in Northern Ethiopia: A retrospective follow-up study. Front. Pediatr. 10:1083749. doi: 10.3389/fped.2022.1083749
Received: 29 October 2022; Accepted: 19 December 2022;
Published: 13 January 2023.
Edited by:
Ranjan Pejaver, People Tree at Meenakshi Hospitals, IndiaReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, ChinaWubet Bayih, Debre Tabor University, Ethiopia
© 2023 Girma, Berhe, Mekonnen and Nigussie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bekahegn Girma YmVrYWhlZ25naUBnbWFpbC5jb20=
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
 Bekahegn Girma
Bekahegn Girma Hailemariam Berhe2
Hailemariam Berhe2