
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 20 December 2022
Sec. Neonatology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1080212
 Zhe Yang1
Zhe Yang1 Fen Lin2
Fen Lin2 Jia-Xin Xu2
Jia-Xin Xu2 Hui Yang3
Hui Yang3 Yong-Hao Wu2
Yong-Hao Wu2 Zi-Kai Chen4
Zi-Kai Chen4 He Xie1
He Xie1 Bin Huang1
Bin Huang1 Wei-Hao Lin1
Wei-Hao Lin1 Jian-Peng Wu1
Jian-Peng Wu1 Yu-Bin Ma1
Yu-Bin Ma1 Jian-Dong Li1
Jian-Dong Li1 Li-Ye Yang5*
Li-Ye Yang5*
Background: This study aimed to investigate the influence of a variant of the UGT1A1 gene on the occurrence and severity of prolonged jaundice in Chinese infants at term.
Methods: 175 infants with prolonged jaundice and 149 controls were used in this retrospective case-control study. The infants with prolonged jaundice were subdivided into the mild-medium and severe jaundice groups (TSB ≥ 342 µmol/L). The frequency and genotype distribution of the UGT1A1 and G6PD genes, and clinical parameters including sex, birth weight, delivery mode, gestational age, and feeding mode, were analyzed, and the differences in the parameters between the two groups were compared.
Results: The allele frequency of UGT1A1*6 in the prolonged jaundice group was higher than that in the control group. Similarly, it was also higher in the severe jaundice group than in the mild-medium jaundice group. Homozygous and heterozygous UGT1A1*6 were also found more frequently in the prolonged jaundice group than in the control group. Exclusive breastfeeding, homozygous and heterozygous forms of UGT1A1*6 were significant risk indicators for prolonged jaundice. Moreover, UGT1A1*6 was the best predictor of prolonged severe jaundice.
Conclusion: UGT1A1*6 appears to be a risk factor for prolonged jaundice with hyperbilirubinemia in term infants of Chinese ancestry who are exclusively breastfed.
The UDP-glucuronosyltransferase 1A1 (UGT1A1) gene encodes UDP-glucuronosyltransferase (UDPGT), an enzyme that plays a crucial role in the metabolism of bilirubin. The UGT1A1 variants in hereditary unconjugated hyperbilirubinemia are distributed differently in different ethnicities and regions (1, 2). The homozygous mutation of the TATA box (A(TA)6TAA > A(TA)7TAA, UGT1A1*28, rs8175347) is identified as the primary genetic basis of Gilbert's syndrome in Caucasians and Africans (1). The incidence of c.211G > A (UGT1A1*6) in East Asia including China, Japan, and Korea, is high (16%–21%) (1, 2). The role of c.211G > A in prolonged jaundice was found in Japan and Iran (3, 4). Studies on the effects of c.211G > A on infants suffering from prolonged jaundice, especially severe prolonged jaundice, are limited in China.
Clinically, prolonged unconjugated jaundice was associated with glucose-6-phosphate dehydrogenase (G6PD) deficiency, breastfeeding, extravasated blood, sepsis, urinary tract infection, and hypothyroidism (5). The present study aimed to explore the contribution of the UGT1A1*6 variant to prolonged jaundice in term infants of Chinese ancestry. Meanwhile, we evaluated the potential confounding factors including sex, birth weight, delivery mode, gestational age, and the method of feeding. Our findings might provide information for assessing the risk of prolonged jaundice with hyperbilirubinemia, and aid genetic counseling in term infants of Chinese ancestry.
This retrospective case-control study was carried out at Chaozhou Central Hospital affiliated to Southern Medical University, China. In-hospital neonates from Department of Neonatology or General Pediatrics from February 2012 to November 2019 were selected; 175 cases (110 males and 65 females) were in the prolonged jaundice group, while 149 cases (78 males and 71 females) were set as the control group. All infants were full-term, had gestational age greater than 37 weeks, and weighed more than 2,500 g.
The diagnostic criteria for neonatal unconjugated hyperbilirubinemia were formulated based on previous studies (6, 7). Prolonged jaundice was defined as the presence of unconjugated hyperbilirubinemia beyond 14 days with total serum bilirubin (TSB) levels >150 µmol/L, or >100 µmol/L beyond 28 days of age (6). Severe hyperbilirubinemia was defined as prolonged jaundice newborns with TSB ≥ 342 µmol/L. The inclusion criteria for the control group were infants more than 14 days old without pathological jaundice, and hospitalized for mild pneumonia, umbilical infection, or other non-jaundice causes. The exclusion criteria were neonates with hemolysis due to incompatibility of the ABO or Rh blood group, sepsis, thalassemia, extravascular hemolysis, serious maternal diseases, organ dysfunction, and congenital malformation with clear etiology, or liver disease that might affect the total serum bilirubin level. These conditions were evaluated based on the medical history of the patients, clinical and laboratory tests such as whole blood cell count, liver function tests, reticulocyte count, blood groups and subgroups, and Coombs test.
All infants received standard clinical care. After conducting a routine clinical test, the remaining ethylenediaminetetraacetic acid (EDTA) anticoagulant blood was obtained. We stored the remaining blood from the neonates in the biobank of our hospital at −40°C for further analysis. Information on sex, age, birth weight, gestational age, delivery mode, feeding pattern, and bilirubin level was recorded at admission before phototherapy. The phototherapy protocol, reviewed from medical records, was based on the American Academy of Pediatrics guidelines (2004) (7).
The Medical Ethics Committee of Chaozhou Central Hospital approved this study (Approval No. 2011021 and No. 2015001). The samples of this retrospective case-control investigation were collected after standardized diagnosis and treatment; Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
The G6PD-deficient samples, determined by the clinically approved G6PD enzyme quantification assay (Guangzhou Micky Medical Instrument Co., Guangdong, China), were used for molecular analysis (8). Extraction of the genomic DNA and detection of the common G6PD mutation, including C.95A > G (Gaohe), C.871G > A (Viangchan), C.1004C > T (Foshan), C.1024C > T (Chinese-5), C.1376G > T (Canton), and C.1388G > A (Kaiping), were performed using a kit (Yaneng Biotechnology Limited Corp., Shenzhen, China) (8). For samples highly suspected of having G6PD gene mutations but not detected with this kit, 10 pairs of primers were designed for amplifying and sequencing exons 2–13, and the sequences were compared to those in NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (8).
Genomic DNA from peripheral blood samples was extracted using the blood genomic DNA extraction kits (Yaneng Biotechnology Limited Corp., Shenzhen, China). The primers were designed for exon 1 and the flanking sequence of the UGT1A1 gene; PCR amplification was performed using the TaKaRa Taq enzyme. The reaction conditions were 95°C for 5 min, followed by 94°C for 30 s, 60°C for 40 s, 72°C for 1 min, 35 cycles, and 72°C extension for 10 min. Finally, the sequences of the samples were analyzed using an ABI Genetic Analyzer (9).
Bilirubin levels at admission, gestational age, and birth weight were compared between two groups by conducting T-tests. To assess factors such as the ratio of male to female, the delivery method, the feeding methods, and the variation frequency of the G6PD and UGT1A1 genes, the χ2 test or Mann–Whitney U test was performed. The odds and odds ratios of prolonged jaundice and severe hyperbilirubinemia (TSB ≥ 342 µmol/L), associated with UGT1A1, G6PD, sex, weight at birth, delivery mode, gestational age, and feeding methods, were analyzed with multiple logistic regression performance. SPSS16.0 was used to conduct all statistical analyses, and all differences between groups were considered to be statistically significant at P < 0.05.
In total, data from 324 infants were included in the study, 175 cases (110 males and 65 females) were classified in the prolonged jaundice group and 149 cases (78 males and 71 females) were in the control group. The clinical characteristics of the infants are presented in Table 1. The sex ratio, gestational age, birth weight, and delivery mode ratio between the two groups were not significantly different (P > 0.05); however, feeding methods differed significantly (P < 0.001). In addition, the bilirubin levels of the infants in the jaundice group were higher than those of the infants in the control group (Table 1 and Figure 1).
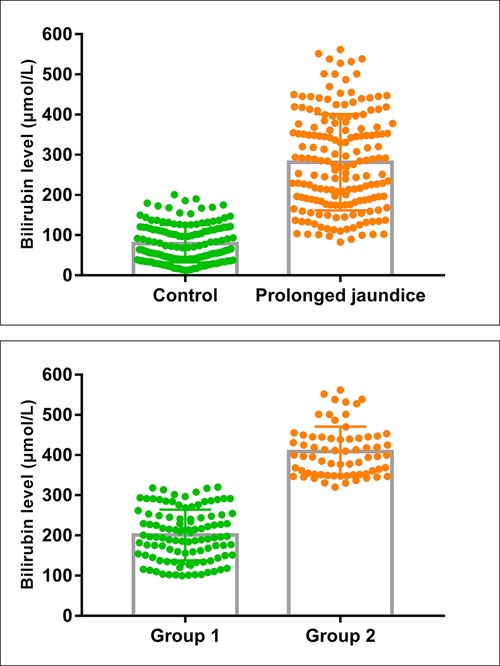
Figure 1. Bilirubin levels in the case and control groups. (Control, n = 145; Prolonged jaundice, n = 175; Group 1: mild-medium jaundice, n = 107; group 2: severe jaundice, n = 68).
The infants with prolonged jaundice (n = 175) were divided into mild-medium jaundice group 1 (TSB < 342 µmol/L) and severe jaundice group 2 (TSB ≥ 342 µmol/L) (Figure 1). The clinical characteristics, including sex, age, gestational age, birth weight, delivery mode, and feeding method, did not differ significantly between these two groups (P > 0.05) (Table 2). Some infants (52/107, 48.5%) in group 1 and all infants in group 2 (68/68, 100%) received phototherapy (χ2 = 50.974, P < 0.001). Three infants in group 2 developed bilirubin encephalopathy, with the highest total bilirubin levels of 469.9 µmol/L, 527.9 µmol/L, and 561.9 µmol/L, respectively.
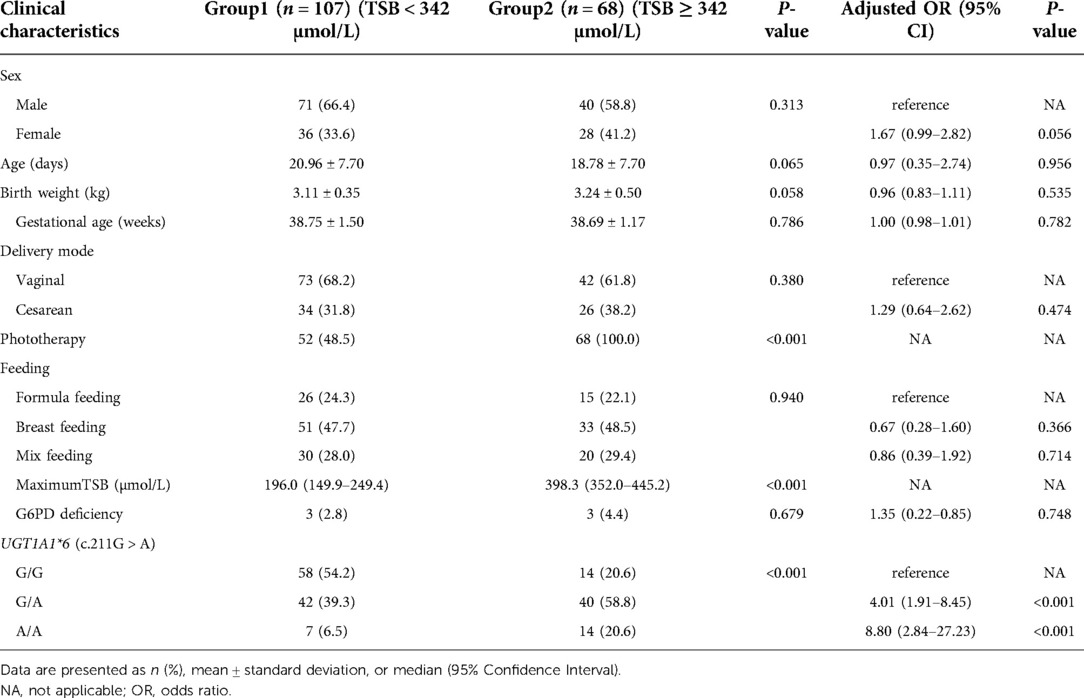
Table 2. Clinical characteristics and analysis of influencing factors for severe prolonged jaundice (TSB ≥ 342 µmol/L).
The genotype frequencies of homozygous and heterozygous c.211G > A variant of UGT1A1*6 were 12.0% (21/175) and 46.9% (82/175) in the case group, respectively, which were higher than those of the control group (Table 1). The allele frequency of the c.211G > A variant of UGT1A1*6 in the prolonged jaundice group was 58.9% while it was 21.5% in the control group (χ2 = 50.15, P < 0.001). Furthermore, the allele frequencies of c.211G > A in groups 1 and 2 were 46% and 79%, respectively (χ2 = 21.66, P < 0.001) (Table 2). The prevalence of homozygous and heterozygous UGT1A1*6 in group 1 was 6.5% (7/107) and 39.3% (42/107), respectively; and in group 2, their prevalence was 20.6% (14/68) and 58.8% (40/68), respectively (Table 2).
G6PD deficiency was identified in 6 cases of prolonged jaundice group (175 cases) by enzyme assay and no G6PD deficiency case was identified in the control group. The G6PD genotypes in the prolonged jaundice group were c.1388 G > A (2/6), c.1376 G > T (2/6), c.517 T > C (NanKang) (1/6), and c.95 A > G (1/6), respectively. The frequency of G6PD deficiency differed significantly between the prolonged jaundice group (3.4%, 6/175) and the control group (0%, 0/149) (χ2 = 5.21, P = 0.033) (Table 1).
Of the 68 infants with severe prolonged jaundice, three suffered from bilirubin encephalopathy, with peak bilirubin values of 469–561 µmol/L, two cases were homozygous 211G > A with G6PD mutation (c.1376 G > T and c.1388 G > A, respectively), and one was heterozygous 211G > A with G6PD deficiency (c.95 A > G).
Infants with prolonged jaundice, who were only breastfed, had a higher risk than formula-fed infants (OR = 2.27, 95% CI: 1.19–4.34, P = 0.012). Additionally, both homozygous (OR = 63.10, 95% CI: 5.30–751.63, P = 0.001) and heterozygous forms (OR = 4.43, 95% CI: 2.53–7.74, P = 0.000) of c.211G > A were significant risk assessment indicators for prolonged jaundice (Table 1). As summarized in Table 2, homozygous (OR = 8.80, 95% CI, 2.84–27.23, P < 0.001) and heterozygous (OR = 4.01.95% CI, 1.91–8.45, P < 0.001) UGT1A1*6 (c.211G > A) was the most significant predictor of severe prolonged jaundice (TSB ≥ 342 µmol/L).
Our results showed that the allele frequency of UGT1A1*6 was significantly different between the prolonged jaundice group (58.9%; 103/175) and the control group (21.5%; 32/149). Additionally, we found that heterozygous and homozygous forms of the UGT1A1*6 aggravated bilirubin levels in infants with prolonged jaundice. The variant UGT1A1*6 contributed to prolonged jaundice, consistent with the findings of previous studies (4, 6). A similar study in Taipei conducted by Weng et al. showed that UGT1A1*6 played a crucial role in prolonged jaundice (10). However, they only studied 30-day healthy infants, including preterm neonates, and severe prolonged jaundice was not included (10). Maruo et al. showed that UGT1A1*6, especially the homozygous variant, was associated with prolonged unconjugated hyperbilirubinemia, similar to our results (11). The variation 211 G > A leads to the transition of amino acid 71 from glycine to arginine, and the homozygous and heterozygous variants regulate the UDPGT activity by decreasing approximately 32.2%–60.2% of the enzyme activity, delaying bilirubin regression in infants with jaundice (6).
In this study, we also assessed multiple risk factors for prolonged jaundice in infants of Chinese descent, especially for severe jaundice, and we found that exclusive breastfeeding played a more significant role than other clinical parameters (sex, birth weight, gestational age, and delivery mode) for TSB levels. However, breastfeeding might not exacerbate the severity of prolonged jaundice as a risk factor. 5α-pregnane-3α and 20β-diol in breast milk could inhibit UGT1A1*6 bilirubin glucuronidation activity (11). Breast milk contains lipoprotein lipase, which produces nonesterified free fatty acids, thus decrease conjugation and excretion of bilirubin (12). Increased colostral interleukin-1β (IL-1β) concentrations in breast milk may repress genes involved in bilirubin metabolism (13). Thus, prolonged unconjugated hyperbilirubinemia may develop in infants with UGT1A1*6 who are fed with breast milk (10, 11, 14). While some substances in breast milk may be responsible for jaundice, on the other hand, there is an irrefutable evidence-based advantages conferred by continued breastfeeding, the breastfed infant benefits from fewer infections, increased brain growth and cognition, as well as the prospect of genetic modification of certain diseases (15). Hence, breastfeeding is encouraged even though breastfed infants experience more jaundice than those not breastfed (15).
Preterm, breastfeeding, and UGT1A1*6 are the primary risk factors for prolonged jaundice (10). For preterm neonates with prolonged jaundice, immature bilirubin metabolism has a stronger effect than genetic factors (4, 10). In this study, we investigated full-term infants with more mature bilirubin metabolism, and feeding patterns and genetic factors were significant predictors for prolonged jaundice. Some studies showed that UGT1A1 was more important in females than males for developing prolonged hyperbilirubinemia due to the higher expression of UGT1A1 in the female liver (6). In our study, however, sex was not a significant indicator of prolonged jaundice.
This was the first study to perform a detailed analysis of the influencing factors of severe prolonged jaundice (TSB ≥ 342 µmol/L) in mainland China. In severe prolonged jaundice, genetic regulation plays a key role in bilirubin metabolism, and homozygous and heterozygous UGT1A1*6 can aggravate the TSB levels of prolonged jaundice, the homozygous variant is more dominant than the heterozygous variant. UGT1A1*6 was also the most significant predictor of severe prolonged jaundice (TSB ≥ 342 µmol/L). Studies on UGT1A1*6 polymorphism and the severity of prolonged jaundice are limited. A study found that UGT1A1*6 homozygotes contributed to prolonged jaundice of breastfed East Asian infants, and serum bilirubin in homozygous neonates was considerably higher than that in heterozygous neonates (11). Our previous study on individuals from southern China found that the homozygous or heterozygous UGT1A1*6 was closely associated with the severity of bilirubin in the Chinese neonatal cohort (16). These studies suggest that UGT1A1*6 is a significant risk factor for severe prolonged jaundice in infants from southern China.
Most homozygous carriers of UGT1A1*6 have benign presentation without severe impairment of liver function, and have a good prognosis after follow-up (2). Therefore, we speculated that these cases with severe prolonged hyperbilirubinemia might have Gilbert syndrome in infancy. Further follow-up is required to determine whether recurrent jaundice occurs in adolescence (11).
This article emphasizes the importance of UGT1A1*6 in prolonged jaundice, especially for severe hyperbilirubinemia in Chinese term infants. We found that UGT1A1*6 was an important genetic modifier for prolonged jaundice in infants with severe hyperbilirubinemia (TSB ≥ 342 µmol/L) in mainland China. Our study improved the understanding of prolonged jaundice in the pediatric field, and provided valuable information for the etiological analysis and genetic counseling for prolonged hyperbilirubinemia.
The original datasheet used in the study are deposited and can be accessed publicly at https://GitHub.com, JingZhunLab/-neonatal-jaundice, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by The Medical Ethics Committee of Chaozhou Central Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Each author has met the Frontiers in Pediatrics authorship requirements. All authors made substantial contributions to the conception and design, data acquisition, and/or analysis and interpretation of data. LYY: designed the study. ZY: drafted the article. LYY, ZY, ZKC and FL: revised it critically for important intellectual content. FL and HY: performed the molecular test. ZY, HX, BH, WHL, JPW, YBM and JDL: performed the routine diagnosis and treatment for the infants of our study. HY, JXX, YHW, ZKC, HX, BH, WHL, JPW, YBM, JDL: all gave final approval of the version to be submitted for publication. All authors contributed to the article and approved the submitted version.
This study was supported by the Natural Science Foundation of Guangdong Province (grant no. 2016A030307035), Special Research Plan 2019 of Chaozhou (grant no. 2020xg01), High Level Development Plan of People’s Hospital of Yangjiang (grant no. G2020007), and Sailing Plan (Talent Culture) of Guangdong Province. The funder had no role in the study's design, data interpretation, and manuscript writing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Memon N, Weinberger BI, Hegyi T, Aleksunes LM. Inherited disorders of bilirubin clearance. Pediatr Res. (2016) 79:378–86. doi: 10.1038/pr.2015.247.
2. Mi XX, Yan J, Ma XJ, Zhu GL, Gao YD, Yang WJ, et al. Analysis of the UGT1A1 genotype in hyperbilirubinemia patients: differences in allele frequency and distribution. Biomed Res Int. (2019) 2019:6272174. doi: 10.1155/2019/6272174.
3. Alaee E, Bazrafshan B, Azaminejad AR, Fouladinejad M, Shahbazi M. The association between prolonged jaundice and UGT1A1 gene polymorphism (G71R) in Gilbert’s syndrome. J Clin Diagn Res. (2016) 10:GC05–GC08. doi: 10.7860/JCDR/2016/19004.8810
4. Yanagi T, Nakahara S, Maruo Y. Bilirubin uridine diphosphate-glucuronosyltransferase polymorphism as a risk factor for prolonged hyperbilirubinemia in Japanese preterm infants. J Pediatr. (2017) 190:159–162.e1. doi: 10.1016/j.jpeds.2017.07.014
5. Gundur NM, Kumar P, Sundaram V, Thapa BR, Narang A. Natural history and predictive risk factors of prolonged unconjugated jaundice in the newborn. Pediatr Int. (2010) 52:769–72. doi: 10.1111/j.1442-200X.2010.03170.x.
6. Chang PF, Lin YC, Liu K, Yeh SJ, Ni YH. Prolonged unconjugated hyperbiliriubinemia in breast-fed male infants with a mutation of uridine diphosphate-glucuronosyl transferase. J Pediatr. (2009) 155:860–3. doi: 10.1016/j.jpeds.2009.05.034.
7. Practice parameter: management of hyperbilirubinemia in the healthy term newborn. American Academy of Pediatrics. Provisional Committee for Quality Improvement and Subcommittee on Hyperbilirubinemia. Pediatrics. (1994) 94:558–65. doi: 10.1542/peds.94.4.558
8. Lin F, Lou ZY, Xing SY, Zhang L, Yang LY. The gene spectrum of glucose-6-phosphate dehydrogenase (G6PD) deficiency in Guangdong province, China. Gene. (2018) 678:312–7. doi: 10.1016/j.gene.2018.07.068.
9. Yang H, Wang Q, Zheng L, Lin M, Zheng XB, Lin F, et al. Multiple genetic modifiers of bilirubin metabolism involvement in significant neonatal hyperbilirubinemia in patients of Chinese descent. PLoS One. (2015) 10:e0132034. doi: 10.1371/journal.pone.0132034.
10. Weng YH, Cheng SW, Yang CY, Chiu YW. Risk assessment of prolonged jaundice in infants at one month of age: a prospective cohort study. Sci Rep. (2018) 8:14824. doi: 10.1038/s41598-018-33249-6.
11. Maruo Y, Morioka Y, Fujito H, Nakahara S, Yanagi T, Matsui K, et al. Bilirubin uridine diphosphate-glucuronosyltransferase variation is a genetic basis of breast milk jaundice. J Pediatr. (2014) 165:36–41.e1. doi: 10.1016/j.jpeds.2014.01.060
12. Ota Y, Maruo Y, Matsui K, Mimura Y, Sato H, Takeuchi Y. Inhibitory effect of 5β-pregnane-3α,20β-diol on transcriptional activity and enzyme activity of human bilirubin UDP-glucuronosyltransferase. Pediatr Res. (2011) 70:453–7. doi: 10.1203/PDR.0b013e31822f242e
13. Poland RL, Schultz GE, Garg G. High milk lipase activity associated with breast milk jaundice. Pediatr Res. (1980) 14:1328–31. doi: 10.1203/00006450-198012000-00011
14. Weng YH, Chiu YW, Cheng SW. Breast milk jaundice and maternal diet with Chinese herbal medicines. Evid Based Complement Alternat Med. (2012) 2012:150120. doi: 10.1155/2012/150120.
15. Prameela KK. Breastfeeding during breast milk jaundice - a pathophysiological perspective. Med J Malaysia. (2019) 74:527–33.31929480
Keywords: UGT1A1, prolonged jaundice, G6PD deficiency, breast feeding, neonatal hyperbilirubinemia
Citation: Yang Z, Lin F, Xu J, Yang H, Wu Y, Chen Z, Xie H, Huang B, Lin W, Wu J, Ma Y, Li J and Yang L (2022) UGT1A1*6 mutation associated with the occurrence and severity in infants with prolonged jaundice. Front. Pediatr. 10:1080212. doi: 10.3389/fped.2022.1080212
Received: 26 October 2022; Accepted: 28 November 2022;
Published: 20 December 2022.
Edited by:
Simone Pratesi, Careggi University Hospital, ItalyReviewed by:
Dazhi Fan, Foshan Women and Children Hospital, China© 2022 Yang, Lin, Xu, Yang, Wu, Chen, Xie, Huang, Lin, Wu, Ma, Li and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Ye Yang eWFuZ2xlZXllZUBzaW5hLmNvbQ==
Specialty Section: This article was submitted to Neonatology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.