- Department of Congenital Heart Surgery and Pediatric Cardiology Mediterranean Congenital Heart Center, “ Bambino Gesù”—San Vincenzo Hospital, Taormina, Italy
A limiting factor in using vascular conduits in the pediatric/newborn population is their inability to grow. Many complex congenital heart diseases require palliative surgery, but using rigid and nonexpandable conduits does not allow the structures to grow and anticipates the need for redo surgery. In newborns, a way to increase the palliation time according to the patient's growth is desirable. In recent years, expandable shunts (exGraft™ PECA) have been developed. According to recent material studies, a shunt could increase diameter after endovascular balloon dilatation. In this case report, we describe the first case of endovascular Blalock-Thomas-Taussig shunt (mBT) shunt expansion in a Tetralogy of Fallot / atrial-ventricular Septal Defect complete (TOFAVSDc) patient with trisomy 21 who went to palliative treatment for tracheomalacia (noncardiac lesion association), severe pulmonary arteries hypoplasia, and low weight. This case introduces the “Smart Palliation concept” in the clinical scenario of selected growing patients where the lifetime of the Blalock-Thomas-Taussig (BT) shunt, anatomic substrates, and complexity of clinical status may require an additional palliation time. The limitation of endovascular conduit expansion is the fragility of the anastomosis site. The anastomosis site is a lesser strength structure of the conduit, and dilatation could develop procedure complications. For this reason, in this paper, we introduced our project design: a new technique (Clepsydra Shape) that consists, before surgical implantation, of pre-expansion of the proximal and distal anastomotic parts of the shunt to obtain an increase of 30% in size of both anastomotic sides, preventing stress- and stretch-related lesion of future balloon dilatation.
Introduction
Using conduits is common in the treatment of congenital heart diseases. Recently, the PECA exGraft™ shunt landed in clinical practice offering a percutaneous expandable option, which in selected cases could be used as a customized palliation strategy. Qadir et al. (1), in a single-ventricle disease neonate patient, described using a 6-mm PECA exGraft on the right ventricle–pulmonary artery conduit (Sano shunt). Instead, we report the first human case of PECA exGraft balloon dilatation in a newborn receiving an Blalock-Thomas-Taussig shunt (mBT) shunt for complex congenital heart disease. A 2.5-kg girl (trisomy 21) was referred to Mediterranean Congenital Heart Center with a diagnosis of Tetralogy of Fallot / atrial-ventricular Septal Defect complete (TOFAVSDc), severe pulmonary artery hypoplasia (Z score: 4.24), and subglottic stenosis in the tracheomalacia context. After 6 days of life, she underwent a 3.0-mm mBT shunt implantation without cardiopulmonary bypass; the postoperative period was free of complications. At 6 months of age, due to the saturation drop value of up to 75%, the patient was admitted to our center. Echocardiographic examination showed good biventricular function, patent but restrictive BT shunt flow pattern, and evidence of small pulmonary arteries. Cardiac catheterization was subsequently planned (Figure 1). Vascular access was taken on the right femoral artery: due to restriction of the PECA exGraft BT shunt (Figure 2), multiple dilatations were performed by mini-Tyshak (NuMED For Children, Orlando, FL, United States), raising from 3 mm to up to 4 mm × 20 mm balloon. The control shunt size was 4 mm in diameter, with a 33% increase in size (Figure 3). No signs of dissection or proximal and distal anastomosis distortion were detected. In our experience, “step-by-step dilatation” was preferred to reduce complications (especially at the anastomotic level) without increasing eccentric intensity in a short time, reducing the microstructural tension of the shunt. Our experience confirms the mechanical and structural parameters reported by Loneker et al. analyses (2). The patient was successfully extubated during the first postoperative day.
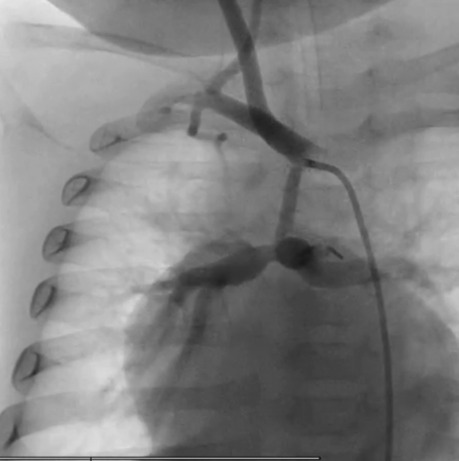
Figure 1. Angiography evidence of the restrictive flow pattern and the reduction size of the MRBT shunt (PECA exGraft).
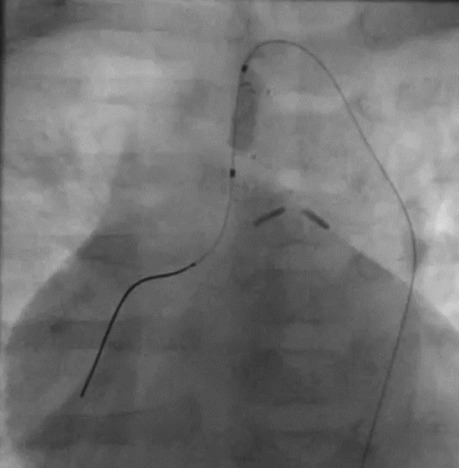
Figure 2. Intraprocedural frame of the mini-Tyshak balloon (NuMED For Children, Orlando, FL, United States) and MRBT shunt radiopaque reference alignment.
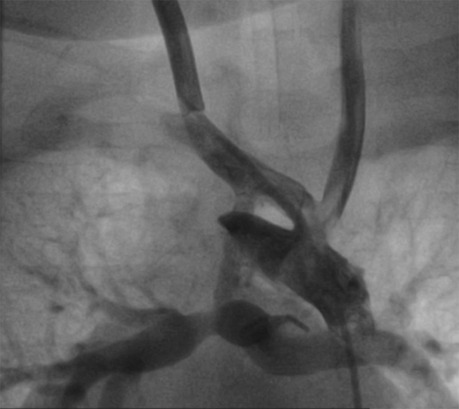
Figure 3. Hypoplastic diffusion BT shunt (2 mm) without significant stenosis. After guide placement, multiple dilations with 3/3.5/4 mm balloons are performed. The control BT shunt size was 4 mm.
Comment
A combination of tetralogy of Fallot and cAVSD is a well-recognized CHD that occurs in 5%–10% of patients with AVSD and 1.7% of patients with TOF (3). Controversies still exist about the timing of complete repair and the different surgical strategies (4). A palliative treatment is a well-accepted strategy in cyanotic patients or additional noncardiac lesions,. The choice of palliative treatment is influenced by various factors including age, low weight, clinical presentation severity, size of pulmonary arteries, tracheomalacia, and center preference. Due to the lack of clinical experience with PECA exGraft, we decide to start a clinical evaluation focusing on short and midterm results in the neonatal population. Since March 2020, 21 patients (mean shunt size: 3.59 mm, mean weight: 3.89 kg, and mean age: 28.63 days) received PECA exGraft shunts as palliation for complex CHD. A prospective analysis confirmed the efficacy and safety of the PECA exGraft shunt without complication or mortality rate. During our experience, due to the mechanical properties of the PECA exGraft™ shunt (1, 2, 5) and patient adaptation to the dilatation strategy, we were able to delay definitive correction 6 months after the balloon procedure without interstage-related complications and maintain hemodynamic stability. We suppose that type of syndrome, low birth weight, hypoplasia of the pulmonary arteries, and respiratory comorbidity were additional risk factors for one stage of complete surgical repair. This case introduces the Smart Palliation concept in the clinical scenario of selected growing patients where the lifetime of the BT shunt, anatomic substrates, and complexity of clinical status may require an additional palliation time. This approach may also be helpful in the adaptation of continuous pathophysiology changes directly related to congenital cardiac disease. This can reduce, delay, and personalize cardiac surgery procedures associated with high morbidity and mortality. Regarding the dilation procedure, several critical points have been advanced. It is well known that the distal and proximal anastomotic sites are the “Achilles heels” of percutaneous ballooning. Anastomotic shunt fragility and complication have already been reported in the literature (6). For this reason, the authors of this article are planning a method that could reduce this anastomosis tension and improve safety in endovascular dilation treatment.
We are drawing and designing a new technique that consists, before surgical implantation, in pre-expansion (Advance Low-Profile PTA Balloon Catheter—Cook Medical Europe Ltd.) of the proximal and distal anastomotic parts of the shunt to obtain an increase of 30% in size of both anastomotic sides (Figure 4). Preflaring—called by us “Clepsydra Shape”—will respect the necessary pulmonary flow, preventing balloon stress- and stretch-related lesions of the suture line on the proximal and distal anastomosis, fixing the final conduit diameter. Shunt dilatation with a “step-by-step” balloon system was performed for the best dynamic control, increasing the proximal and distal diameters and dosing the concentric distribution forces. The Clepsydra Shape project is in the design phase, but the preclinical results are very encouraging and will be proposed for publication when available. With PECA BT shunt endovascular dilatation, we are able to decide the right dilation or complete surgical correction performing timing. This methodology makes it possible to rethink indications for palliative surgery. The anastomotic implantation technique is no different from that used with other shunt devices. Our center does not use a different anticoagulation protocol, but we are analyzing new clinical and histological dates that, in the future, could give new indications and directions. In our center, the PECA exGraft™ shunt has become the first choice in neonatal patients, where a small diameter increase allows a longer palliative window time. Extending palliation time is an “arrow in our quiver” that can be used according to the different clinical cases. This will allow us to perform the correction surgery with an adequate weight and pulmonary artery Z score increase. Our research group is working to develop a specific registry that could help understand the clinical impact of the Smart Palliation approach.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors contributed to the clinical case management and drafting of the paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garven E, Rodell CB, Shema K, Govender K, Forbess JM, Nugent A. Use of dilatable exGraft conduit in single-ventricle pallation. Ann Thorac Surg. (2020) 110:e131–3. doi: 10.1016/j.athoracsur.2019.12.024
2. Loneker AE, Luketich SK, Bernstein D, Kalra A, Nugent AW, D'Amore A, et al. Mechanical and microstructural analysis of a radially expandable vascular conduit for neonatal and pediatric cardiovascular surgery. J Biomed Mater Res B Appl Biomater. (2018) 106(2):659–71. doi: 10.1002/jbm.b.33874
3. Najm HK, Van Arsdell GS, Watzka S, Hornberger L, Coles JG, Williams WG. Primary repair is superior to initial palliation in children with atrioventricular septal defect and tetralogy of fallot. J Thorac Cardiovasc Surg. (1998) 116:905–13. doi: 10.1016/S0022-5223(98)70040-6
4. Prifti E, Crucean A, Bonacchi M, Bernabei M, Luisi VL, Murzi B, et al. Total correction of complete atrioventricular septal defect with tetralogy of fallot. J Heart Valve Dis. (2003) 12:640–8. doi: 10.21037/tp.2017.01.01
5. Feins EN, Emani SM. Expandable valves, annuloplasty rings, shunts, and bands for growing children. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. (2020) 23:17–23. doi: 10.1053/j.pcsu.2020.02.002
Keywords: pediatric cardiosurgery, pediatric cardiologist, shunt, palliation surgery, innovation cardiologist
Citation: Bellanti E, Calaciura RE, Andriani I, Saitta M and Agati S (2023) Case Report: “Smart Palliation” and “Clepsydra Shape”: A new approach in complex congenital heart disease. Front. Pediatr. 10:1073412. doi: 10.3389/fped.2022.1073412
Received: 18 October 2022; Accepted: 7 December 2022;
Published: 6 January 2023.
Edited by:
Antonio Francesco Corno, Children’s Memorial Hermann Hospital, United StatesReviewed by:
Bahaaldin Alsoufi, University of Louisville, United StatesKenji Baba, Okayama University, Japan
© 2023 Bellanti, Calaciura, Andriani, Saitta and Agati. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ermanno Bellanti ZXJtYW5ub2JlbGxhbnRpQGljbG91ZC5jb20=
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Ermanno Bellanti
Ermanno Bellanti Rita E. Calaciura
Rita E. Calaciura Salvatore Agati
Salvatore Agati