- 1Pediatric Department, King Hussein Cancer Center (KHCC), Amman, Jordan
- 2Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan
- 3Department of Pathology and Laboratory Medicine, King Hussein Cancer Center (KHCC), Amman, Jordan
According to the latest WHO classification of hematopoietic malignancies, myeloid and lymphoid neoplasms with eosinophilia and gene rearrangements include three specific rare diseases and one provisional entity. Myeloid/lymphoid neoplasms with platelet-derived growth factor receptor alpha (PDGFRA) rearrangements are the most frequent of these disorders and are usually present in adult males with a median age of the late 40s. Patients usually have chronic eosinophilic leukemia but can occasionally manifest as acute myeloid leukemia or extramedullary T- or B-lineage lymphoblastic lymphoma. We report a case of a previously healthy 2-year-old girl who presented with a right supraorbital swelling with no associated lymphadenopathy. Peripheral blood smear evaluation at initial presentation revealed microcytic hypochromic red blood cells and leukocytosis with marked eosinophilia, occasional myelocytes, and occasional blasts. Whole-body CT scans and PET scans revealed hypermetabolic potentially lymphomatous mass in the superior medial aspect of the right orbit in addition to splenomegaly but no evidence of hypermetabolic mediastinal, hilar, abdominal, or pelvic lymph nodes. Bone marrow aspirate and biopsy revealed hypercellular bone marrow with quantitatively decreased erythroid precursors and increased granulocytic precursors with 60% of the cells being eosinophilic cells in different stages of maturation. The diagnosis of myeloid neoplasm with eosinophilia and rearrangement of PDGFRA was made following confirmation by fluorescence in situ hybridization (FISH) test for FIP1L1-PDGFRA gene fusion. An incisional biopsy of the supraorbital mass revealed B-cell lymphoblastic lymphoma (B-LBL). FISH test for FIP1L1-PDGFRA gene fusion was positive in 70% of the cells studied. Thus, the final diagnosis was B-cell lymphoblastic lymphoma arising in the setting of myeloid/lymphoid neoplasm with eosinophilia and PDGFRA rearrangement. The patient was started on imatinib with concomitant therapy for B-LBL per the Children Oncology Group (COG) standard therapy for localized B-LBL and demonstrated a favorable outcome in the 2.5-year follow-up period. To our knowledge, this is the first pediatric case of myeloid/lymphoid neoplasm with PDGFRA rearrangement presenting with synchronous myeloproliferative disease and B-LBL. We present our diagnostic and management approach of this patient and review prior relevant pediatric cases of myeloid/lymphoid neoplasms with PDGFRA rearrangement.
Introduction
Myeloid/lymphoid neoplasms with eosinophilia (M/LN-Eo) and rearrangements of PDGFRA, PDGFRB, or FGFR1, or with PCM1-JAK2 genetic variants constitute a rare but well-defined category of hematologic malignancies recognized by the WHO revised classification (1). Despite having myeloproliferative neoplasm (MPN) and eosinophilia as common manifestations in most reported cases, individual patients may display remarkable heterogeneity at initial presentation and clinical course (2). Myeloid/lymphoid neoplasms with PDGFRA rearrangements are the most frequently encountered of these disorders and are usually present in adult males with a median age of the late 40s (1–3). Patients usually have chronic eosinophilic leukemia (CEL) but can also manifest as acute myeloid leukemia (AML), systemic mastocytosis (SM) with hypereosinophilia, or extramedullary T- or B-lineage lymphoblastic lymphoma (T-LBL/B-LBL) (2). Myeloid/lymphoid neoplasms with eosinophilia and gene rearrangements are extremely rare in the pediatric population with only few cases reported in the literature.
Given its exceptional therapeutic benefit demonstrated in previous reports, the tyrosine kinase inhibitor imatinib is currently considered first-line therapy for patients with M/LN-Eo harboring rearrangements of PDGFRA (2). However, due to the rarity of these diseases in the pediatric population, and the lack of reports describing imatinib monotherapy in cases presenting with T-LBL/B-LBL, treating physicians are often left with challenging decisions when formulating their management plans. In this report, we present the first pediatric case of myeloid/lymphoid neoplasm with PDGFRA rearrangement presenting with synchronous myeloproliferative disease and B-cell lymphoblastic lymphoma (B-LBL). We present our diagnostic and management approach of this patient and review prior relevant pediatric cases of myeloid/lymphoid neoplasms with PDGFRA rearrangements.
Case report
A previously healthy 2-year-old girl presented with right supraorbital swelling that developed over 1.5 months with no other symptoms at the time of initial presentation. Notable on examination was a right firm supraorbital swelling causing ptosis, with no overlying skin changes (Figure 1A). Examination was also notable for hepatosplenomegaly but not lymphadenopathy. Initial laboratory data were significant for anemia (Hb 8.2 g/dl) and leukocytosis (WBC 19.5 × 109/L) with eosinophilia of 32% and an absolute eosinophilic count of 6.3 × 109/L. Blood smear showed microcytic, hypochromic red blood cells (RBCs) in addition to leukocytosis with marked eosinophilia (32%), occasional myelocytes (2%), and occasional myeloblasts (less than 1%). Some of the eosinophils had three to four nuclear segments and polarized distribution of the cytoplasmic granules.
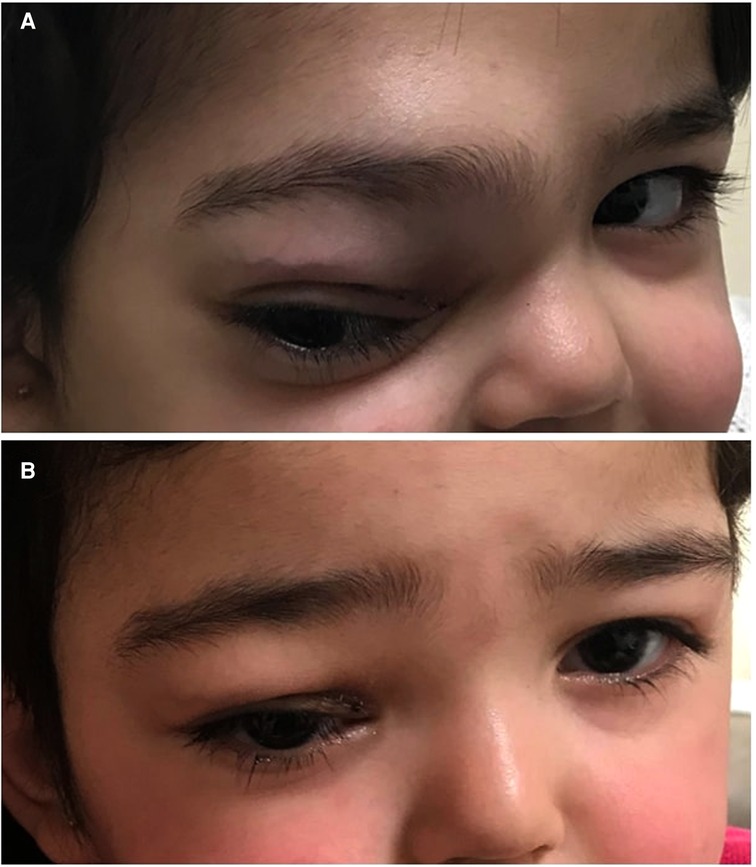
Figure 1. (A) Right supraorbital swelling at initial presentation. (B) Resolved right supraorbital swelling following 5 months of combined chemotherapy with imatinib.
Whole-body PET/CT scan revealed hypermetabolic potentially lymphomatous mass in the superior medial aspect of the right orbit in addition to splenomegaly, but no evidence of hypermetabolic mediastinal, hilar, abdominal or pelvic lymph nodes. MRI of the orbits revealed a lobulated well-defined nonhomogenous enhancing mass in the superior medial aspect of the right orbit measuring 2.2 cm × 0.6 cm × 2.8 cm. The lesion was mostly involving the anterior aspect of the medial rectus muscle, causing destruction of the medial aspect of the right orbital roof with mild extra-axial intracranial extension but no compression of the brain parenchyma. There was a mild extension of the subcutaneous tissue in the supraorbital space, with compression of the right eye globe but no evidence of invasion of the sclera or retro-ocular extension (Figure 2A).
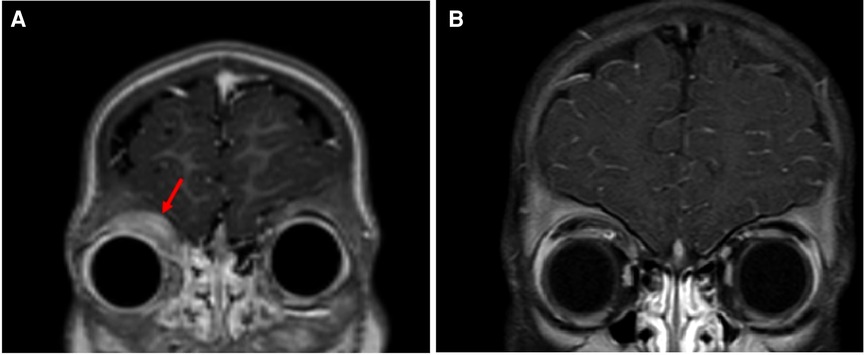
Figure 2. (A) Orbital MRI (coronal T1 fat sat postcontrast section) at initial presentation showing mass in the superior medial aspect of the right orbit (arrow). (B) Orbital MRI (Coronal T1 fat sat postcontrast section) following 5 months of combined chemotherapy with imatinib demonstrates marked improvement of tumor size.
Bone marrow (BM) aspirate and biopsy revealed hypercellular BM with quantitatively decreased erythroid precursors and quantitatively increased granulocytic precursors with 60% of the cells being eosinophilic cells in different stages of maturation. Megakaryocytes were quantitatively normal (Figure 3A). Flow cytometry showed less than 1% myeloblasts. No B-cell lymphoblasts were detected. The diagnosis of myeloid neoplasm with eosinophilia and rearrangement of PDGFRA was made following confirmation by fluorescence in situ hybridization (FISH) test for FIP1L1-PDGFRA gene fusion. Cerebrospinal fluid (CSF) examination showed an acellular specimen with no blast or atypical cells. An incisional biopsy of the supraorbital mass revealed B-cell lymphoblastic lymphoma (B-LBL) (Figure 3B–D). FISH test for FIP1L1-PDGFRA gene fusion was positive in 70% of the cells studied (Figure 3E). Thus, the final diagnosis was B-cell lymphoblastic lymphoma arising in the setting of myeloid/lymphoid neoplasm with eosinophilia and PDGFRA rearrangement.
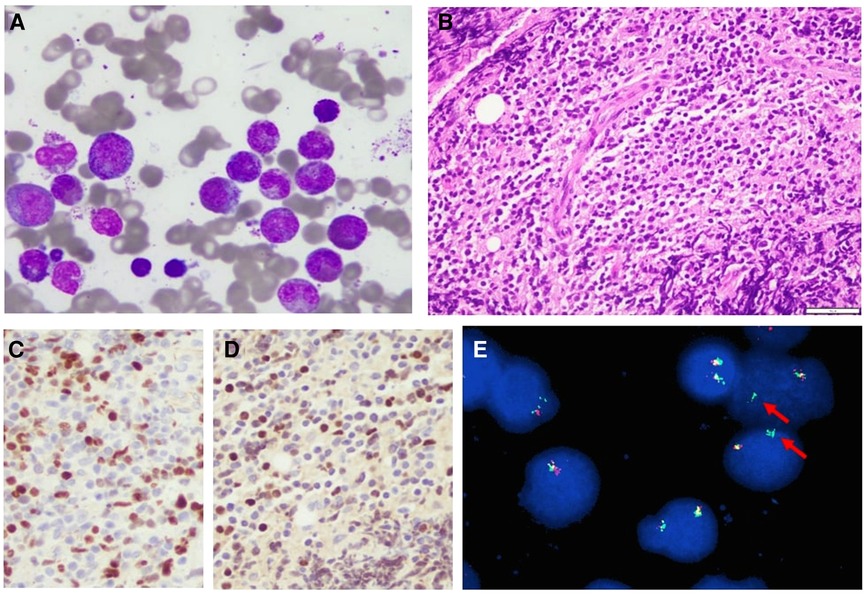
Figure 3. (A) Bone marrow aspirate showing mostly eosinophils, Wright–Giemsa stain, 40×. (B) Biopsy from the orbital mass demonstrating heavy lymphoid infiltrate composed of small- to medium-sized lymphoblasts, hematoxylin and eosin stain, 40×. (C,D) PAX5 and TdT immunostains showing positive brown nuclear stain in the tumor cells, respectively, indicating that these cells are B-cell lymphoblasts 40×. (E) Rearranged PDGFRA gene (arrows). Rearrangement results from fusion of FIPL1 and PDGFRA after CHIC2 deletion.
Cardiac evaluation was normal. Gastrointestinal evaluation that included upper endoscopy and colonoscopy showed chronic gastric, duodenal, and rectal inflammation but no evidence of eosinophilic infiltration or B-cell lymphoma.
The patient was started on imatinib 340 mg/m2/day with concomitant therapy for B-LBL per the Children Oncology Group (COG) standard therapy for localized B-LBL. WBC count and absolute eosinophilic count normalized after 4 days of imatinib. One month after the initiation of therapy, which corresponded to the end of chemotherapy induction phase, the supraorbital swelling decreased grossly to 50% of its original size. The BM evaluation was morphologically normal and the FIP1L1-PDGFRA gene fusion by FISH analysis was decreased to 2.5%. Five months following treatment, the supraorbital swelling had completely resolved (Figure 1B). Whole-body PET CT showed almost complete metabolic response, and the brain and orbital MRI showed almost complete resolution of the right orbital mass (Figure 2B). The BM evaluation was also in morphologic remission with negative FISH for PDFGRA rearrangement. After 2.5 years of treatment, the multiagent chemotherapy for B-LBL was completed and the patient is now on imatinib monotherapy with close follow-up.
Discussion
Myeloid/lymphoid neoplasms with eosinophilia and gene rearrangements are extremely rare in the pediatric population with only few cases reported in the literature. To the best of our knowledge, only a few pediatric cases were reported describing myeloid/lymphoid neoplasms harboring PDGFRA rearrangements (4–14) (Table 1).
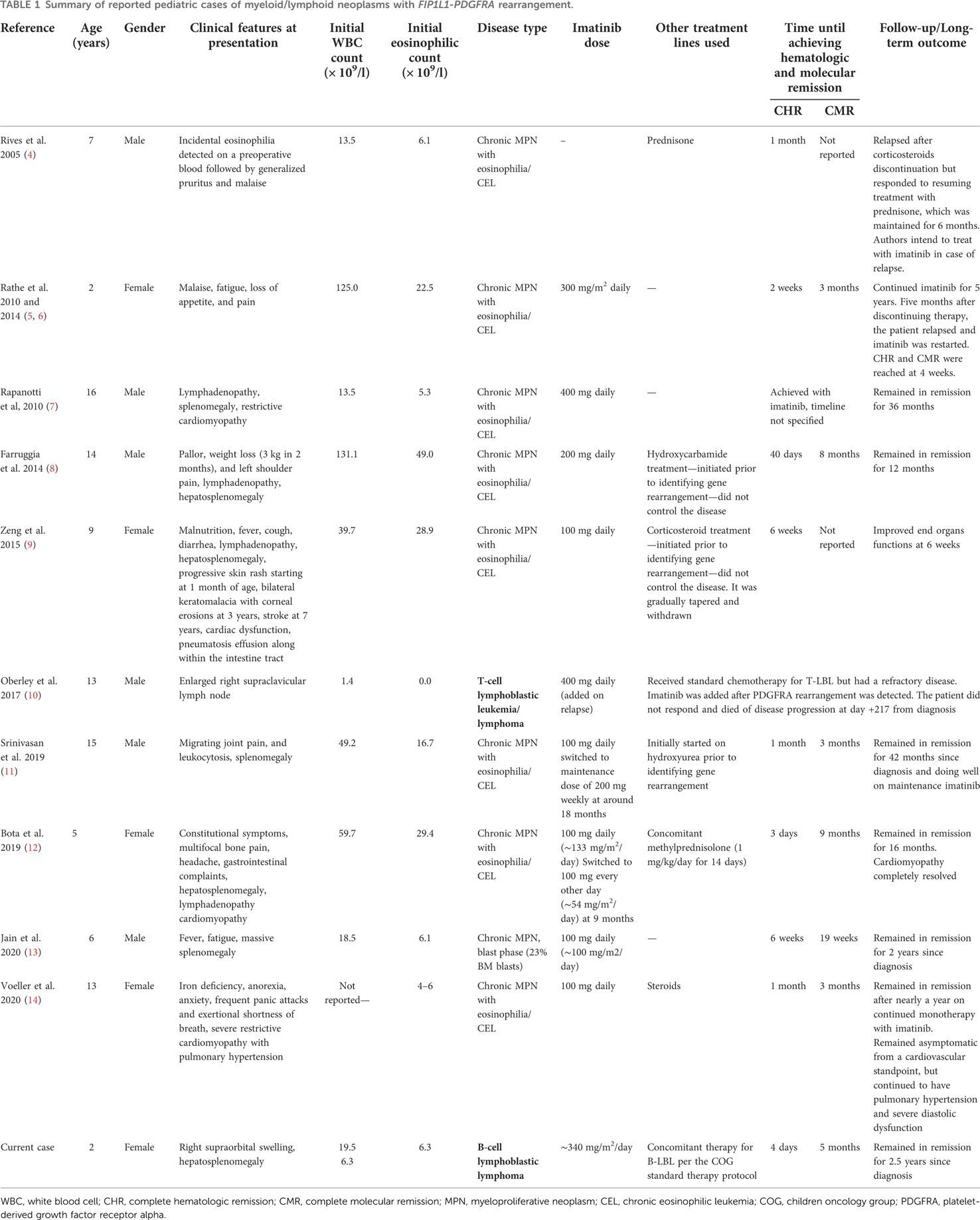
Table 1. Summary of reported pediatric cases of myeloid/lymphoid neoplasms with FIP1L1-PDGFRA rearrangement.
Despite the well-known and very strong male predominance in adult cases of hypereosinophilic syndromes (HES), in general, and myeloid/lymphoid neoplasms with PDGFRA rearrangements, in particular, pediatric cases demonstrate a less pronounced male predominance (13, 15). In adults, patients with M/LN-Eo with PDGFRA rearrangements usually present as CEL, but can rarely manifest as AML, SM with hypereosinophilia, or extramedullary T-LBL/B-LBL (2). All previously reported pediatric cases manifested as chronic MPN with eosinophilia/CEL with only one case presenting as T-lymphoblastic lymphoma (T-LBL) without eosinophilia (10). To the best of our knowledge, we report the first pediatric case of myeloid/lymphoid neoplasm with PDGFRA rearrangement presenting with synchronous myeloproliferative disease and B-lymphoblastic lymphoma (B-LBL).
The product of the FIP1L1-PDGFRA fusion gene is a constitutively activated tyrosine kinase, thus, making the tyrosine kinase inhibitor imatinib a well-known agent for targeting conditions with this specific genetic abnormality (16). Imatinib is currently considered the first-line therapy for patients with M/LN-Eo harboring rearrangements of PDGFRA (2). In 2005, Rives et al. reported the first child with HES/CEL harboring FIP1L1-PDGFRA rearrangement. However, this genetic abnormality was not identified at initial presentation. The patient had received corticosteroids for the diagnosis of primary hypereosinophilic syndrome and achieved complete hematologic response in 1 month. The patient relapsed after discontinuation of the corticosteroids but responded to resuming same treatment. After the FIP1L1-PDGFRA fusion gene was identified, the diagnosis was modified to FIP1L1-PDGFRA-positive CEL and the authors declared the intent of initiation of imatinib if their patient exhibits a second relapse (4). All subsequent reported pediatric cases included imatinib in their treatment plan (5, 7–14). Favorable outcomes were reported in all cases except the case presenting as T-LBL that was reported by Oberley et al. in 2017 (10). Their 13-year-old male patient had chemotherapy-resistant T-LBL, and once the FIP1L1-PDGFRA rearrangement was detected, imatinib was added. However, the patient did not respond and eventually died of his disease (10). Our patient was treated with both imatinib and standard chemotherapy for the B-LBL due to the lack of reports describing imatinib monotherapy in cases presenting with T-LBL/B-LBL in all age groups, in addition to the serious initial presentation of B-LBL that posed an imminent threat to the patient's vision and central nervous system as evident by the aforementioned imaging findings. Five months following treatment, our patient achieved complete clinical, hematologic, and molecular remission. After 2.5 years of treatment, multiagent chemotherapy for B-LBL was completed and the patient is now on imatinib monotherapy and close follow-up.
Discontinuation of imatinib has been associated with relapse in FIP1L1/PDGFRA-positive chronic eosinophilic leukemia in both adult and pediatric cases (6, 17, 18). Although few authors reported maintained remission after decreasing imatinib maintenance dose, there is a lack of consensus regarding the optimal maintenance duration (11, 12). It is advised that patients remain on regular follow-up by molecular monitoring to dictate optimal duration of continuation therapy. Moreover, it is generally presumed that patients may require lifelong therapy (6, 13).
Conclusion
Myeloid/lymphoid neoplasms with eosinophilia and gene rearrangements are extremely rare in the pediatric population with only few cases reported in the literature. In this report, we presented the first pediatric case of myeloid/lymphoid neoplasm with PDGFRA rearrangement presenting with synchronous myeloproliferative disease and B-LBL. Our patient demonstrated a favorable outcome after initiating combined chemotherapy with imatinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. editors. WHO classification of tumours of haematopoietic and lymphoid tissues (revised 4th edition). Lyon: IARC (2017).
2. Pozdnyakova O, Orazi A, Kelemen K, King R, Reichard KK, Craig FE, et al. Myeloid/lymphoid neoplasms associated with eosinophilia and rearrangements of PDGFRA, PDGFRB, or FGFR1 or with PCM1-JAK2. Am J Clin Pathol. (2021) 155(2):160–78. doi: 10.1093/ajcp/aqaa208
3. Vega F, Medeiros LJ, Bueso-Ramos CE, Arboleda P, Miranda RN. Hematolymphoid neoplasms associated with rearrangements of PDGFRA, PDGFRB, and FGFR1. Am J Clin Pathol. (2015) 144(3):377–92. doi: 10.1309/AJCPMORR5Z2IKCEM
4. Rives S, Alcorta I, Toll T, Tuset E, Estella J, Cross NC. Idiopathic hypereosinophilic syndrome in children: report of a 7-year-old boy with FIP1L1-PDGFRA rearrangement. J Pediatr Hematol Oncol. (2005) 27(12):663–5. doi: 10.1097/01.mph.0000193467.06938.77
5. Rathe M, Kristensen TK, Moller MB, Carlsen NL. Myeloid neoplasm with prominent eosinophilia and PDGFRA rearrangement treated with imatinib mesylate. Pediatr Blood Cancer. (2010) 55(4):730–2. doi: 10.1002/pbc.22655
6. Rathe M, Schomerus E, Wehner PS, Kristensen TK, Moller MB. Relapse of myeloid neoplasm with eosinophilia and PDGFRA rearrangement after imatinib discontinuation in a pediatric patient. Pediatr Blood Cancer. (2014) 61(12):2328. doi: 10.1002/pbc.25199
7. Rapanotti MC, Caruso R, Ammatuna E, Zaza S, Trotta L, Divona M, et al. Molecular characterization of paediatric idiopathic hypereosinophilia. Br J Haematol. (2010) 151(5):440–6. doi: 10.1111/j.1365-2141.2010.08394.x
8. Farruggia P, Giugliano E, Russo D, Trizzino A, Lorenzatti R, Santoro A, et al. FIP1L1-PDGFRalpha-positive Hypereosinophilic syndrome in childhood: a case report and review of literature. J Pediatr Hematol Oncol. (2014) 36(1):e28–30. doi: 10.1097/MPH.0b013e31827e6386
9. Zeng K, Li L, Huang L, Liang YH. Newly identified phenotypes in a FIP1L1/PDGFRA-associated paediatric HES patient: thrombocytosis, mHPA, young stroke and blindness. J Eur Acad Dermatol Venereol. (2015) 29(3):614–6. doi: 10.1111/jdv.12416
10. Oberley MJ, Denton C, Ji J, Hiemenz M, Bhojwani D, Ostrow D, et al. A neoplasm with FIP1L1-PDGFRA fusion presenting as pediatric T-cell lymphoblastic leukemia/lymphoma without eosinophilia. Cancer Genet. (2017) 216–217:91–9. doi: 10.1016/j.cancergen.2017.07.007
11. Srinivasan A, Scordino T, Baker A. Myeloid neoplasm with eosinophilia and FIP1L1-PDGFRA rearrangement treated with imatinib mesylate: a pediatric case with long-term follow-up. J Pediatr Hematol Oncol. (2019) 41(4):334–5. doi: 10.1097/MPH.0000000000001446
12. Bota S, Alves P, Constantino C, Maia R. Hypereosinophilia and severe bone disease in an African child: an unexpected diagnosis. BMJ Case Rep. (2019) 12(4):e227653. doi: 10.1136/bcr-2018-227653
13. Jain J, Weinzierl EP, Saxe D, Bergsagel J, Gotlib J, Reiter A, et al. Sustained complete molecular remission with imatinib monotherapy in a child presenting with blast phase FIP1L1-PDGFRA-associated myeloid neoplasm with eosinophilia. Hemasphere. (2020) 4(6):e486. doi: 10.1097/HS9.0000000000000486
14. Voeller J, DeNapoli T, Griffin TC. Two pediatric oncologic cases of hypereosinophilic syndrome and review of the literature. Cancer Rep. (2022) 5:e1710. doi: 10.1002/cnr2.1710
15. Katz HT, Haque SJ, Hsieh FH. Pediatric hypereosinophilic syndrome (HES) differs from adult HES. J Pediatr. (2005) 146(1):134–6. doi: 10.1016/j.jpeds.2004.09.014
16. Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. (2003) 348(13):1201–14. doi: 10.1056/NEJMoa025217
17. Breccia M, Cilloni D, Cannella L, Stefanizzi C, Tafuri A, Fama A, et al. Isolated molecular relapse in FIP1L1-PDGFRalpha hypereosinophilic syndrome after discontinuation and single weekly dose of imatinib: need of quantitative molecular procedures to modulate imatinib dose. Cancer Chemother Pharmacol. (2009) 63(6):1161–3. doi: 10.1007/s00280-008-0858-8
Keywords: myeloid/lymphoid neoplasms, eosinophilia, PDGFRA, B-cell lymphoblastic lymphoma (B-LBL), imatinib
Citation: Akiely R, Almasri F, Almasri N and Abu-Ghosh A (2022) Case Report: Pediatric myeloid/lymphoid neoplasm with eosinophilia and PDGFRA rearrangement: The first case presenting as B-lymphoblastic lymphoma. Front. Pediatr. 10:1059527. doi: 10.3389/fped.2022.1059527
Received: 1 October 2022; Accepted: 22 November 2022;
Published: 14 December 2022.
Edited by:
Daniele Zama, Sant'Orsola-Malpighi Polyclinic, ItalyReviewed by:
Barbara Buldini, Università degli Studi di Padova, ItalyYana Pikman, Dana-Farber Cancer Institute, United States
© 2022 Akiely, Almasri, Almasri and Abu-Ghosh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amal Abu-Ghosh bW91c2ExOTkxQG1zbi5jb20=
†This author shares first authorship
Specialty Section: This article was submitted to Pediatric Hematology and Hematological Malignancies, a section of the journal Frontiers in Pediatrics
 Reem Akiely
Reem Akiely Farah Almasri2
Farah Almasri2 Nidal Almasri
Nidal Almasri Amal Abu-Ghosh
Amal Abu-Ghosh