- 1Department of Otorhinolaryngology, Head and Neck Surgery, Shenzhen University General Hospital, Shenzhen, China
- 2Department of Otorhinolaryngology, Head and Neck Surgery, Huizhou Third People's Hospital, Huizhou, China
- 3Shenzhen University, Shenzhen, China
Allergic rhinitis (AR) is one of the popular childhood diseases, bringing physical and metal burdens to the children and their families. The study was performed to detect common allergens eliciting AR in children, to investigate the prevalence of allergens in different age and gender cohorts, and to provide a reliable basis for clinical prevention and treatment of AR during childhood. We measured serum-specific IgE and performed inhalant and ingestion allergen examinations in 2,316 children with AR, in collaboration with BioSciTec GmbH. The prevalence of different allergens was determined according to gender, age, severity, and season. Among the 2,316 AR cases, the top five inhalant allergens were Dermatophagoides pteronyssinus (1,674 cases, 72.3%), Dermatophagoides farinae (1,520 cases, 65.6%), Blomia tropicalis (1,477 cases, 63.8%), Cockroach (602 cases, 26.0%), and Dog hair (602 cases, 26.0%). The top five ingestive allergens were Milk (1,111 cases, 48.0%), Egg white (543 cases, 23.4%), Shrimp/Crab (425 cases, 18.4%), Beef/Mutton (422 cases, 18.2%), and Egg yold (329 cases, 14.2%). AR severity analyses showed that 50.9% (1,180 cases) of D. pteronyssinus allergies were above level three, 47.9% (1,109 cases) of D. farinae allergies were above level three, only 23.3% (539 cases) of B. tropicalis allergies were level three, and B. tropicalis allergies were mainly of level 2. Other AR-inducing allergens mainly produced level one or two reactions. Regarding ingestion allergens, 7.9% (183 cases) of milk allergies and 4.7% (108 cases) of Shrimp/Crab allergies were above level three, and other allergens induced AR mainly of level one or two. The study investigated the major allergens eliciting AR in children from Guangdong, China, assessed the prevalence and severity among cohorts regarding age, gender, and season, and produced essential information on childhood AR, laying important references for AR prevention and treatment in the future.
Introduction
Allergic diseases constitute a series of reactions of the immune system to allergens. Such reactions greatly reduce the life quality of patients and sometimes are life-threatening (1). Allergic rhinitis (AR) is a common form of allergy, which is a non-infectious chronic inflammatory disease of the nasal mucosa mediated by immunoglobulin E (IgE) due to exposure to allergens (2).
AR is an allergic disease with a globally increasing trend, and it is estimated that there are more than 500 million AR patients worldwide (3), thus constituting a substantial social and economic burden. Additionally, AR is the most common allergic disease in children, with a prevalence of approximately 3%–38%, respectively (4, 5). Hence, identifying allergens in children with AR plays is key for effective treatment and prevention schemes (6). Inhalation allergens are the main cause of AR, with typical clinical symptoms including sneezing attacks, runny nose, nasal itching, and nasal congestion. Ingestion allergens are a further main cause of AR in children, and recent studies have shown that ingestion allergens in childhood are associated with AR during adulthood (7). Serum-specific IgE determination is a basic method for detecting allergens in AR patients, which provides valuable evidence for AR diagnosis and individual-specific immunotherapy. Identifying the factors that contribute to allergic reactions is essential to improve public health, and determining the distribution of allergens is key to understanding such afflictions (8, 9). However, allergens vary widely, depending on location, climate, and lifestyle, with variability occurring even between different regions of the same country (10–15). A previous study suggested that the prevalence of AR has increased in adults and children in China in the past 20 years, which may be attributed to “Western” lifestyle, industrialization, and air pollution (16). China has a large population and covers a vast territory. Differences in climate, environment, dietary habits, living conditions, and economic development between regions may affect the effects of potential allergens, in addition to differences between genders and age classes. However, the main allergen sources, respective AR severities, and seasonal effects in children from southern China remain to be revealed.
In this study, we retrospectively analyzed 2,316 children with AR who resided in Shenzhen, Guangdong, China, between April 2019 and March 2022, and we explored sensitization differences between genders, age classes, and seasons. The results of the present study provide a clinical basis for scientific research and clinical prevention and treatment of AR in children.
Materials and methods
Ethics
This study was reviewed and approved by the institutional research ethics committee of Shenzhen University (NO:M202200341). Written informed consent was waived by the Institutional Review Board. All analyses adhered to the institutional guidelines and requirements of the Ethics Committee.
Ar participant enrollment
A total of 2,316 patients showing AR clinical symptoms of AR were examined in Shenzhen University General Hospital from April 2019 to March 2022, including 1,480 male and 836 female children aged 1–14 years (mean age 7.61 ± 3.03 years). I)All patients should satisfy at least two of the following clinical symptoms: paroxysmal sneezing, runny nose, nasal itching, nasal congestion, and a history of sudden and recurrent episodes with a daily duration of more than 1 h; Physical signs: pale nasal mucosa, edema, abundant watery secretion, turbinate enlargement, allergy-associated black eye circles, and allergic folds in severe AR children; and at least one allergen was positive in a serum-specific IgE test. All patients had lived in Shenzhen for at least one year and had no history of long-term foreign travel.
Clinical examination and data collection
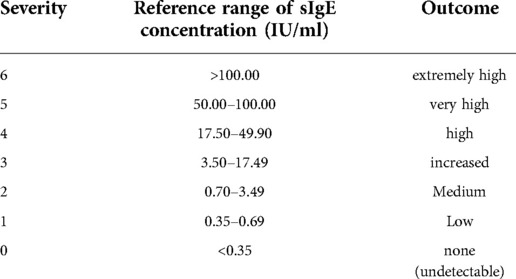
Serum specific IgE (sIgE) concentrations of inhaled and ingested allergens were measured using a serum specific IgE assay (AllergyScreen, Mediwiss-Analytic GmbH, Moers, Germany). Serum specific IgE values in response to 18 inhalable allergens (D.pteronyssinus, D.farinae, B. tropicalis, cat hair, etc.) and ingested allergens (Egg white, Milk, Beef, Mango, Cashew nut, Shrimp, Crab, etc.) were detected. Responses to allergen concentrations >0.3 5IU/ml were considered positive. Moreover, severities were divided into six levels according to the concentration of allergens (Table 1).
Statistical analyses
SPSS Statistics 26.0 (IBM, Armonk, NY, USA) was used to analyze the data. Count data were presented as an example (%), and comparison between groups was performed using a χ2 test. P < 0.05 was considered statistically significant.
Results
Distribution of allergens
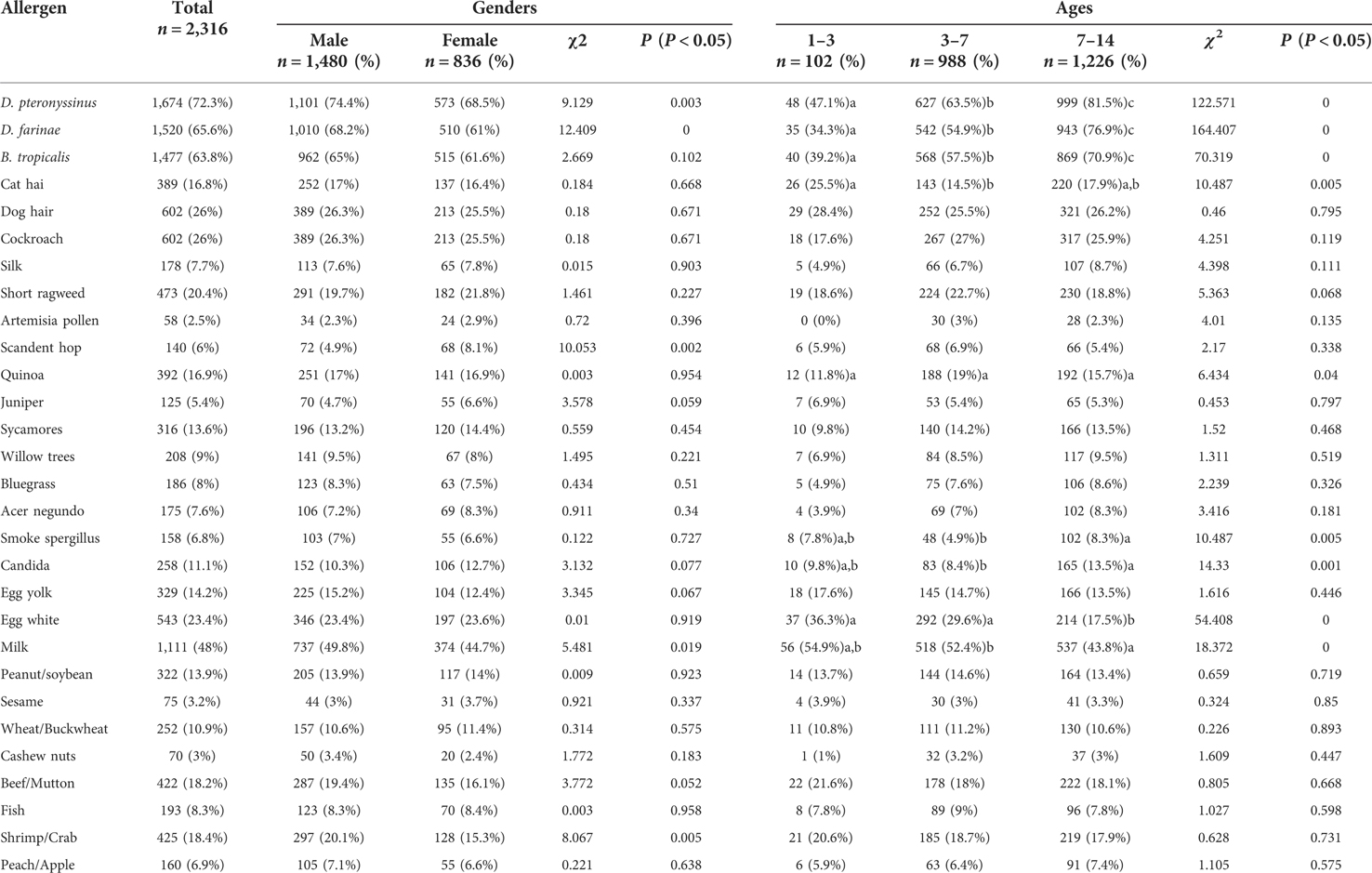
All 2,316 children with AR, aged 1–14 years and living in Shenzhen, were positive for at least one allergen. Among them, 1,337 were positive for more than five allergens (inhalation and food), 336 were positive for four allergens, 337 were positive for three allergens, 222 were positive for two allergens, and 84 were positive for one allergen. The distribution of various inhalation allergens is shown in Figure 1, among which the top five most common types of inhalation allergens were D. pteronyssinus (1,674 cases, 72.3%), D. farinae (1,520 cases, 65.6%), B. tropicalis (1,477 cases, 63.8%), cockroach (602 cases, 26.0%), and dog hair (602 cases, 26.0%). The distribution of various ingestion allergens is shown in Figure 2. The top five most common ingestion allergens were milk (1,111 cases, 48.0%), egg white (543 cases, 23.4%), shrimp/crab (425 cases, 18.4%), beef/lamb (422 cases, 18.2%), and Egg yolk (329 cases, 14.2%) (Figures 1, 2).
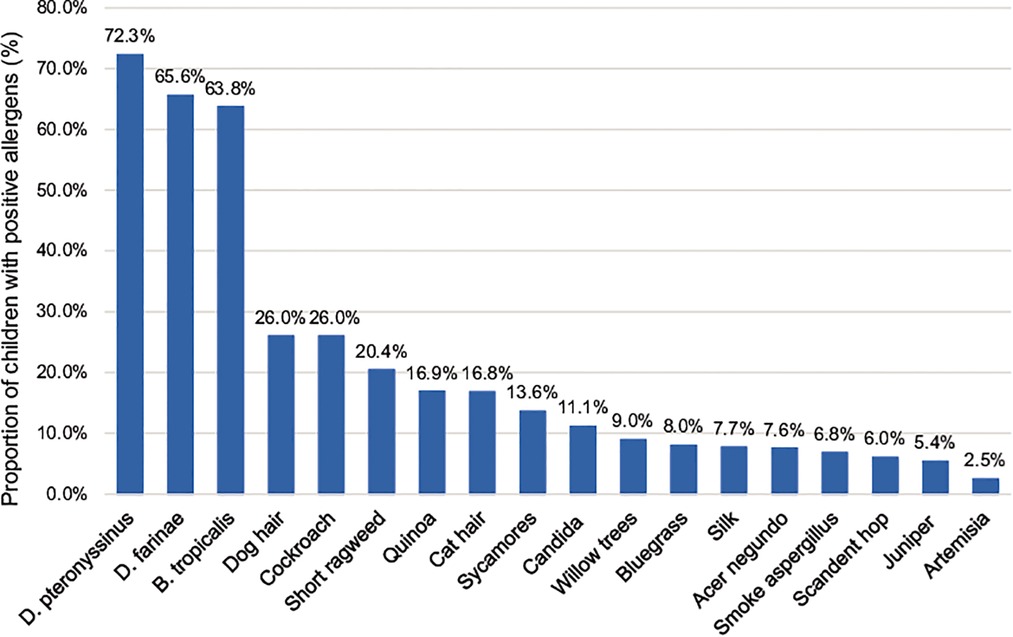
Figure 1. Distribution of inhaled allergens in 2,316 children with AR. The abscissa represents different inhaled allergens, and the ordinate exhibits their distributions in the AR children.

Figure 2. Distribution of ingestive allergens in 2,316 children with AR. The abscissa represents different ingestive allergens, and the ordinate exhibits their distributions in the AR children.
Differences between male and female participants
Among 2,316 children with AR and aged 1–14 years, the top five common inhalation allergens in 1,480 male AR children were D. pteronyssinus, D. farinae, B. tropicalis, cockroach, and dog hair. The top five common ingestion allergens were milk, egg white, shrimp/crab, beef/mutton, and egg yolk. The top five common inhalation allergens in 836 female AR children were D. pteronyssinus, B. tropicalis, D. farinae, cockroach and dog hair. The top five common ingestion allergens were milk, egg white, beef/lamb, shrimp/crab, and egg yolk. There were significant differences between genders in the positive rates regarding D. pteronyssinus (P = 0.003), D. farinae (P = 0.000), B. tropicalis (P = 0.002), milk (P = 0.019), and shrimp/crab (P = 0.005) among different genders, whereas no significant differences in the positive rates of inhaled allergens and ingested allergens were observed between other cohorts (Table 2).
Distribution of allergens in different age groups
The distribution of positive inhalation allergens and ingestion allergens in children of different ages is shown in Table 2. The children were divided into three age groups: ≤3 years old (infant group; 102 cases), 3–7 years old (preschool group; 988 cases), and 7–14 years old (school-age group; 1,226 cases). There were 102 cases in the group, among which the top five most common inhalant allergens were D. pteronyssinus, B. tropicalis, D. farinae, dog hair, and cockroach. The top five most common ingestion allergens were milk, egg white, beef/lamb, shrimp/crab, and egg yolk. Among the 988 preschool group cases, 627 cases were attributed to D. pteronyssinus, 568 cases to B. tropicalis, 542 cases to D. farinae, 267 cases to cockroach, and 252 cases to dog hair. The top five most common ingestion allergens were milk, egg white, shrimp/crab, beef/lamb, and egg yolk. There were 1,226 cases of school-age group, with 999 cases attributed to D. pteronyssinus, 869 cases to B. tropicalis, 943 cases to D. farinae, 321 cases to dog hair, and 317 cases to cockroach; The top five most common ingestion allergens were milk, beef/lamb, shrimp/crab, egg white, and egg yolk. There were significant differences in allergens among age classes, including D. pteronyssinus, D. farinae, B. tropicalis, cat hair, Quinoa, smoke aspergillus, and Candida egg white, and milk, whereas no significant differences were observed in the other allergens (Table 2).
Distribution of positive degree of allergens in children with AR
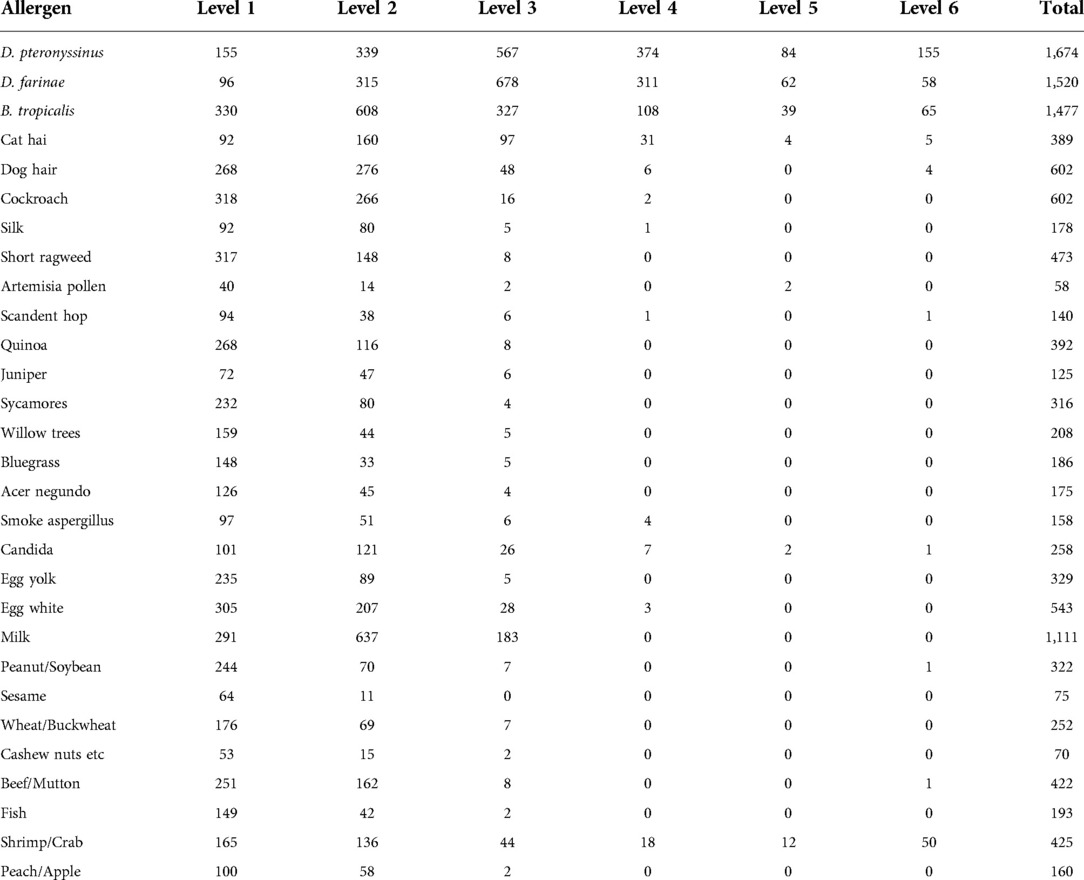
Among the inhaled allergens of children with AR aged 1–14 years, the top three D. pteronyssinus and D. farinaes were mainly of level 3 or above, 50.9% (1,180 cases) of D. pteronyssinus were ≥3, 47.9% (1,109 cases) of D. farinaes ≥3, 23.3% (539 cases) of B. tropicalis ≥3. The main allergens were level 2, and the other allergens were level 1 and 2. Among the allergens of children with AR aged 1–14 years, 7.9% (183 cases) had milk level ≥3, 4.7% (108 cases) had shrimp/crab level ≥3, and other allergens were mainly level 1 and 2, as shown in Table 3.
Seasonal distribution of positive allergens in children with AR
As shown in Figures 3, 4, the peak period of AR children's visits in Shenzhen was concentrated in July and August, with the largest number of visits in August, and relatively few visits in March and November. Most children with AR visited hospitals in summer. In addition, the allergens D. pteronyssinus, B. tropicalis, D. farinae, and milk in children with AR were generally increasing (Figures 3, 4).

Figure 3. Distribution of cases of children with AR in different months. The abscissa represents different months, and the ordinate represents the number of children with AR.
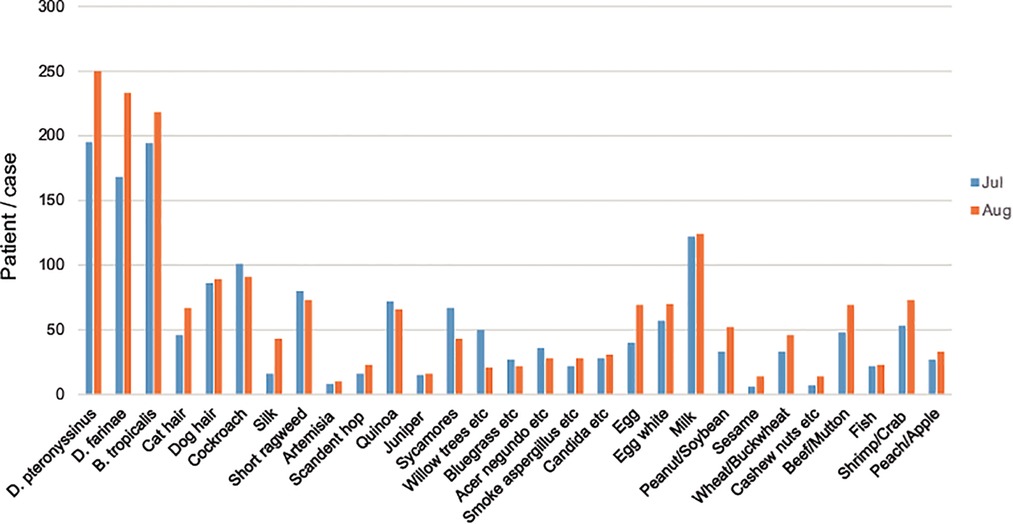
Figure 4. Distribution of allergens in children with AR in July and August. The abscissa represents different allergens, and the ordinate represents the number of children with AR. The blue color stands for July, and the orange color stands for August.
Discussion
Distribution of allergens
Our study showed that the most common allergens were D. pteronyssinus, D. farinae, and B. tropicalis. Milk, egg white, and shrimp/crab were the most common ingestion allergens. A previous study found that atopy and allergic sensitization to D. pteronyssinus and D. farinae were more common in Guangzhou than in Beijing (17), which is in line with the results of the present study. In the past, ingestion allergens in children with AR were frequently ignored; recently, however, it was found that the symptoms of AR may be aggravated by ingestion allergens (7). Inhaled allergens were mainly D. pteronyssinus and D. farinae; the reason may be that Shenzhen area experiences a subtropical monsoon climate, which is warm and humid, with long summers, short winters, and long rainy seasons, which is conducive to the propagation of mites and cockroaches. In particular, the B. tropicalis is an endemic allergen in southern China, which is in line with the geographical characteristics of Shenzhen. Ingestion allergens are mainly milk, egg white, and shrimp/crab. Shenzhen has a high average living standard, and its residents prefer nutritious foods such as milk and egg white, whereas the Pearl River Delta region is rich in aquatic resources, thus seafood allergens such as crab and shrimp are important parts of the local diet and thus act as allergens more frequently. This suggests that the sensitization of ingested allergens is closely related to local economic level and dietary habits. At the same time, the results showed that 1,337 cases of children with allergies (aspiration and ingestion) were positive for five or more allergens: 336 patients were positive for four allergens, 337 patients were positive for three allergens, 222 patients were positive for two allergens, and 84 patients were positive for one single allergen. Since the number of AR children positive for multiple allergens were more than those positive for single allergen, we deduced that allergies may be a kind of constitution. Few AR patients are allergic to one single allergen, and most patients are allergic to multiple allergens. Patients with allergies to mites, in particular, are generally allergic to one mite and show positive reactions to other mite allergens, but the severity of the reactions is different because the main determinants of mite allergens differ between species (18).
Difference of distribution of allergens between male and female participants
The results of this study showed that the common inhalation allergens of 2,316 children with AR aged 1–14 years were D. pteronyssinus, D. farinae, and B. tropicalis. The most common ingestion allergens of male children with AR were milk, egg white, and shrimp/crab. The most common inhalation allergens in 836 female children with AR were D. pteronyssinus, B. tropicalis, and D. farinae. The most common ingestion allergens were milk, egg white, and beef/mutton. We found that the positive rate of serum sIgE in children with AR was higher in boys than in girls, and it has been reported that the gender difference in the positive rate of allergen sIgE may be related to different inflammatory pathways in different sexes (19). Studies from other countries reported that the prevalence of AR was higher in males than in females, and nasal congestion was the main symptom in males and runny nose and nasal itching in females (20). The positive rate of serum sIgE in male children with AR is higher than that in female children, indicating that boys are more likely to suffer from allergic diseases, which may be related to the regulation of estrogen on the immune system; however, the underlying mechanisms remain to be elucidated (21). The study showed that the main different allergens between different gender of children in Shenzhen region were D. farinaes, and boys with AR exhibited significantly higher inhaled allergen positive rates than girls with AR, probably due to different preferences of toys. There is a dose-dependent relationship between the exposure concentration of D. farinaes in the environment and the symptoms of AR. Reducing the concentration of D. farinaes in the environment can help reduce the incidence of AR in children (22). Therefore, it is necessary to pay more attention to the sensitization of male patients to inhalation allergens in clinical practice. Among the ingestion allergens, only milk and shrimp/crab showed statistical differences between genders, which was firstly related to similar dietary habits of children, and secondly was related to intestinal immune functions of children.
Distribution of allergens in different age–groups
Different age groups exhibited different allergens. The prevalence of AR increased with age, and the number of children with AR in the school-age group was the highest. Previous studies have shown that AR increases from preschool children and peaks at age 17–18 years (22). Some studies have reported that age is a protective factor for allergic diseases, and the positive rate of allergens gradually decreases with age (23–25). In the three age groups of the present study, the top three inhaled allergens were D. pteronyssinus, D. farinae, and B. tropicalis, while the top three ingestion allergens were milk, egg white, beef/mutton, and shrimp/crab, respectively. The main allergen thus differed between age groups. We speculated that the inhaled allergens of mites are more important for the Shenzhen region with regard to climate and environmental characteristics favoring mite propagation, and mites are typically most abundant in indoor areas, with textiles and objects such as quilts, bed sheets, pillows, cloth sofas, carpets, and stuffed toys being preferred mite hiding places, which are typically present in the main activity areas of children. With increasing age, the range and areas of children's' activities change, and the overlap of allergens caused by seasonal changes may be the reason for the largest number of AR patients in the school group. The order of the ingestion allergen spectrum differed between age groups, which may be due to changes in dietary habits and dietary structure. Overall, avoiding D. farinae and improving the family environment is particularly important. With regard to ingestion allergens, parents should be familiar with the most common ingestion allergens in children and should prevent ingestion of allergens by the children, i.e., the children's diet should be monitored and adjusted, and alternatives to the allergenic food sources should be provided.
Distribution of positive degree of allergies in AR patients
Children with AR showed different severities of responses to inhaled and ingestive allergens. The three common inhaled allergens were D. pteronyssinus, D. farinaes, and B. tropicalis; the frequency of allergies to D. pteronyssinus and D. farinaes was obviously higher than those to B. tropicalis. In addition, the severity of AR children positive for B. tropicaliss was mainly on levels 1, 2, 3, or lower. First, this may be affected by the climate in Shenzhen. Exposure to mites at high abundances is typically long-lasting, especially during the rainy season. Previous studies have shown that the time of exposure to allergens is the key factor of AR (26, 27). Second, the prevalence of AR in children may be related to increased exposure to indoor allergens, especially D. farinae, due to indoor temperatures, humidity, and living environments (28).
Seasonal distribution of allergens in children with AR
Season is one of the main factors affecting the occurrence of AR. The results showed that the peak visits by children with AR children were in July and August, and the number of visits was relatively small in March and November. Shenzhen is a coastal city in southern China, adjacent to Hong Kong. It has a subtropical monsoon climate with long summers and short winters, which is generally mild with abundant sunshine and rainfall. According to the Shenzhen Meteorological Bureau report released in 2021, the four seasons of Shenzhen in recent years are roughly divided into spring (early February to mid-April), summer (late April to early November), autumn (early November to mid-January of the following year), and winter (mid-January to early February). The present results showed that AR was most common in July and August, which in Shenzhen corresponds to warm moist air flows of the summer, with high temperatures and high humidity. These conditions are conducive to the propagation of mites and cockroaches, thus markedly increasing the abundances of mites. In addition to the increase of mite exposure, this season also coincides with the pollen peak. For AR patients in the summer, short ragweed、quinoa, Amaranthus retroflexus are important allergens, in addition to mites; short ragweed flowers from July to September, quinoa flowers for 5–10 months, and Amaranthus retroflexus flowers from July to August. In line with our results and additive effects of allergens, this may explain the observed high prevalence of AR in children during summer. The number of hospitalized AR children is relatively small in March and November. Considering the climate change, dropping temperature, and humidity at these times, we hypothesized that the exposure chance and concentration of mites have decreased, explaining the relieving symptoms in the AR children.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by This study was reviewed and approved by the institutional research ethics committee of Shenzhen University (NO:M202200341). Written informed consent was waived by the Institutional Review Board. All analyses adhered to the institutional guidelines and requirements of the Ethics Committee.. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Conceptualization: NNZ and QFZ. Compilation of literature: NNZ, YWW, JS and JEL. Article writing and editing: NNZ, YWW and JEL. Figure organization: NNZ, YWW, and ZQW. Specimen collection: RC, HLH and SYOY. Supervision: NNZ and QFZ. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Sanming Project of Medicine in Shenzhen Municipality (Project No.: SZSM202003005).
Acknowledgments
We thank all recruited children and their guardians for their participation. We are also grateful to the nurses at the Shenzhen University General Hospital who provided helps in clinical examinations and sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. The following formatting styles are meant as a guide, as long as the full citation is complete and clear, Frontiers referencing style will be applied during typesetting,. Yao Y, Chen CL, Yu D, Liu Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy. (2021) 76:456–70. doi: 10.1111/all.14639
2. Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Chinese Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association,. Chinese Guidelines for the Diagnosis and Treatment of allergic rhinitis (revised edition, 2022). Chin J Otorhinolaryngol Head Neck Surg. (2022) 57(2):106–29.
3. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. (2008) 63(suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
4. Brozek JL, Bousquet J, Agache I, Agarwal A, Bachert C, Bosnic-Anticevich S, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. (2017) 140:950–8. doi: 10.1016/j.jaci.2017.03.050
5. Aaron SD, Boulet LP, Reddel HK, Gershon AS. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med. (2018) 198:1012–20. doi: 10.1164/rccm.201804-0682
6. Alvaro-Lozano M, Akdis CA, Akdis M, Alviani C, Angier E, Arasi S, et al. EAACI Allergen immunotherapy user’s guide. Pediatr Allergy Immunol. (2020) 31(25):1–101. doi: 10.1111/pai.13189
7. Fong WCG, Chan A, Zhang H, Holloway JW, Roberts G, Kurukulaaratchy R, et al. Childhood food allergy and food allergen sensitisation are associated with adult airways disease: a birth cohort study. Pediatr Allergy Immunol. (2021) 32(8):1764–72. doi: 10.1111/pai.13592
8. Park HJ, Lim HS, Park KH, Lee JH, Park JW, Hong CS. Changes in allergen sensitization over the last 30 years in Korea respira-tory allergic patients: a single-center. Allergy Asthma Immunol Res. (2014) 6(5):434–43. doi: 10.4168/aair.2014.6.5.434
9. Farrokhi S, Gheybi MK, Movahed A, Tahmasebi R, Iranpour D, Fatemi A, et al. Common aeroallergens in patients with asthma and allergic rhinitis living in southwestern part of Iran: based on skin prick test reactivity. Iran J Allergy Asthma Immunol. (2015) 14(2):133–8.25780879
10. Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. (2019) 11(2):156–69. doi: 10.4168/aair.2019.11.2.156
11. Bousquet PJ, Burbach G, Heinzerling LM, Edenharter G, Bindslev-Jensen C, Bousquet-Rouanet L, et al. GA2 LN skin test study III: minimum battery of test inhalent allergens needed in epidemiological studies in patients. Allergy. (2009) 64(11):1656–62. doi: 10.1111/j.1398-9995.2009.02169.x
12. Boulet L-P, Turcotte H, Laprise C, Lavertu P-M, Bedard A, Lavoie J, et al. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. (1997) 27(1):52–9. doi: 10.1111/j.1365-2222.1997.tb00672.x
13. Lumia M, Luukkainen P, Kaila M, Tapanainen H, Takkinen H-M, Prasad M, et al. Maternal dietary fat and fatty acid intake during lactation and the risk of asthma in the offspring. Acta Paediatr. (2012) 101(8):e337–43. doi: 10.1111/j.1651-2227.2012.02718.x
14. Black PN, Sharpe S. Dietary fat and asthma: is there a connection? Eur Respir J. (1997) 10(1):6–12. doi: 10.1183/09031936.97.10010006
15. D’Amato G, Liccardi G, D’Amato M. Environmental risk factors (outdoor air pollution and climatic changes) and increased trend of respiratory allergy. J Investig Allergol Clin Immunol. (2000) 10(3):123–8.
16. Zhang Y, Zhang L. Prevalence of allergic rhinitis in China. Allergy Asthma Lmmunol Res. (2014) 6(2):105–13. doi: 10.4168/aair.2014.6.2.105
17. Wong GW, Hui DS, Chan HH, Fok TF, Leung R, Zhong NS, et al. Prevalence of respiratory and atopic disorders in Chinese schoolchildren. Clin Exp Allergy. (2001) 3(8):1225–31. doi: 10.1046/j.1365-2222.2001.01140.x
18. Hong H, Qianhui Q, Shaohua C. Comparative analysis of the results of aspiration allergen skin test for allergic rhinitis. Chin J Integr Tradit Chin West Med Otolaryngol. (2006) 14(5):283–5.
19. Barrenas F, Andersson B, Cardell LO, Langston M, Mobini R, Perkins A, et al. Gender differences in inflammatory proteins and pathways in seasonal allergic rhinitis. Cytokine. (2008) 42(3):325–9. doi: 10.1016/j.cyto.2008.03.004
20. Hong SN, Won JY, Nam EC, Kim TS, Ryu YJ, Kwon JW, et al. Clinical manifestations of allergic rhinitis by age and gender: a 12-year single-center study. Ann Otol Rhinol Laryngol. (2020) 129(9):910–7. doi: 10.1177/0003489420921197
21. Wang C, Xu ZB, Peng YQ, Zhang HY, Yu QN, Guo YB, et al. Sex differences in group 2 innate lymphoid cell-dominant allergic airway inflammation. Mol Immunol. (2020) 128:89–97. doi: 10.1016/j.molimm.2020.09.019
22. Wang Y, Xiong L, Yin X, Wang J, Yu Z, Gong G, et al. Dermatophagoides pteronyssinus allergen levels in households and correlation with allergic rhinitis symptoms. Am J Rhinol Allergy. (2014) 28(5):193–6. doi: 10.2500/ajra.2014.28.4095
23. Sakurai Y, Nakamura K, Teruya K, Shimada N, Umeda T, Tanaka H, et al. Prevalence and risk factors of allergic rhinitis and cedar pollinosis among Japanese men. Prev Med. (1998) 27(4):617–22. doi: 10.1006/pmed.1998.0336
24. Wuthrich B. Epidemiology of the allergic diseases: are they truly on the increase? Int Arch Allergy Appl Immunol. (1989) 90(Suppl. 1):3–10. doi: 10.1159/000235067
25. Dawei Z, Xiaoshan Q, Jianrong H. Clinical analysis of inhaled allergens in 579 pediatric allergic rhinitis patients in the Guangzhou region. J Mol Diagn Ther. (2015) 7(3):171–5.
26. Song Hongmao H. Allergspectrum analysis of 452 allergic rhinitis patients in huai’an, Jiangsu province. Chin J Integr Tradit Chin West Med Otolaryngol. (2020) 28(2):116–23.
27. Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. (2014) 134(3):593–601. doi: 10.1016/j.jaci.2014.04.018
Keywords: allergenic rhinitis, children, allergens, specific IgE, epidemiology
Citation: Zhang N, Wu Y, Wei Z, Li J, Shi J, Cai R, Huang H, Ouyang S and Zhang Q (2022) Investigation of the allergens in 2,316 children with allergic rhinitis from Guangdong, China. Front. Pediatr. 10:1051993. doi: 10.3389/fped.2022.1051993
Received: 23 September 2022; Accepted: 7 November 2022;
Published: 24 November 2022.
Edited by:
Dongfang Li, BGI-Shenzhen, ChinaReviewed by:
Kaihu Yao, Capital Medical University, ChinaYinhu LI, City University of Hong Kong, Hong Kong SAR, China
© 2022 Zhang, Wu, Wei, Li, Shi, Cai, Huang, Ouyang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingfeng Zhang enh5eWViaEAxNjMuY29t
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Nannan Zhang
Nannan Zhang Yunwen Wu1,†
Yunwen Wu1,† Qingfeng Zhang
Qingfeng Zhang