- 1Department of Health Sciences, University of Florence, Florence, Italy
- 2Allergy Unit, Department of Pediatrics, Meyer Children's Hospital, Florence, Italy
- 3Pediatric Allergy Group, Department of Women and Children's Health, School of Life Course Sciences, King's College London, London, United Kingdom
- 4Section of Pediatrics, Department of Medicine and Surgery, University of Perugia, Perugia, Italy
- 5Pediatric Allergy Group, Peter Gorer Department of Immunobiology, School of Immunology & Microbial Sciences, King's College London, London, United Kingdom
- 6Children's Allergy Service, Evelina London Children's Hospital, Guy's and St Thomas’ NHS Foundation Trust, London, United Kingdom
- 7Unit for Pediatric & Population-Based Dermatology Research, St John's Institute of Dermatology, Guy's and St Thomas’ NHS Foundation Trust and King's College London, London, United Kingdom
Introduction
Atopic dermatitis (AD), or atopic eczema, is an inflammatory, pruritic, chronic or chronically relapsing skin disease, often occurring in families with atopic diseases.
AD is a common non-communicable skin disease, affecting up to 20% of children (1) and 8% of adults (2). It often begins in early childhood, but about one-third of cases may develop in adulthood. The disease is mild in most affected children and may resolve before the age of 2 years old in about 40% of the cases (3). Other cases may persist into adulthood (4), e.g., more severe cases, which account for about 7% of pediatric cases (5), those with early onset or concomitant allergic sensitization (6). AD may be the first step which leads to the development of other atopic diseases, such as food allergies, allergic rhinoconjunctivitis and asthma (7). Some authors also consider eosinophilic esophagitis as a possible late-onset manifestation of this “allergic march” (8).
The core clinical manifestations of AD are skin dryness and pruritic, poorly defined eczematous lesions, which vary from acute, red and exudative papules and vesicles to subacute-chronic and often lichenified erythematous lesions. The distribution and morphology of the lesions is highly variable across patients and across different age groups (9).
Quality of life of the patients is influenced by the psychological and physical burden intrinsic to AD lesions, which can lead to sleep deprivation due to the itch and social embarrassment due to lesion visibility. These factors further promote a higher prevalence of depression and anxiety in AD patients (10). There is also a potential association with mental disorders, which is not fully understood (10–12).
Diagnosis of AD is primarily clinical (13–16). There are a number of physician-assessed severity scales, commonly used both during a first evaluation and in the follow-up of AD patients, such as the Eczema Area Severity Index (EASI) and the SCORing Atopic Dermatitis (SCORAD) Index, which are the recommended systems to measure clinical manifestations (17–19). In clinical practice, patient-assessed scores are more commonly used (e.g., the Patient-Orientated Eczema Measure – POEM) (17).
As regards pathophysiology, AD is a multifactorial disorder characterized by an impaired skin barrier and immune dysregulation, both contributing to skin inflammation (20, 21), which can be further perpetuated by superinfection with bacteria such as Staphylococcus aureus (22). Like many other immune-mediated disorders, AD is not a single disease entity but has several endotypes characterized by discrete immunological and molecular mechanisms (23–25). This concept is becoming relevant for disease treatment, potentially leading in the future to a more personalized approach in AD management, also regarding novel therapies.
Standard of treatment for AD consists of the regular use of emollients to preserve skin barrier function, and topical corticosteroids or calcineurin inhibitors to treat acute flares, but often also as maintenance (e.g., 2–3 times weekly) therapy. Severe or refractory cases can benefit from systemic immunomodulatory therapy, including immuno-suppressive drugs, biologics and small molecules, such as Janus kinase (JAK) inhibitors (26). A role for allergen immunotherapy (AIT) in AD management has been suggested, but clear indications for its application are still lacking (27, 28).
AIT is an allergen-based, tolerance-inducing treatment for allergic diseases, nowadays used in the management of allergic rhinitis, as well as, e.g., food and venom allergies. It consists in the repeated administration of definite quantities of allergen extracts, mainly via the subcutaneous (SCIT), sublingual (SLIT) (29) or oral route (OIT). AIT modifies the immune regulation of allergic responses (30), by inducing regulatory T (Treg) cells to produce, e.g., interleukin (IL)-10, transforming growth factor (TGF)-beta and IL-35 and to express surface molecules as cytotoxic T-lymphocyte-associated protein-4 (CTLA4) and programmed cell death protein-1 (PD1), all of which contribute to suppression of Th2 cells and cytokines, basophils, and eosinophils. Treg cells also induce allergen-specific regulatory B (Breg) cells (31). The suppressive milieu limits the production of IgE and induces production of IgG4 from B cells, which can act as a decoy for allergen binding (32). Breg cells, regulatory natural killer (NKreg) cells, and regulatory innate lymphoid cells (ILCregs) all contribute to the induction and maintenance of allergen-specific tolerance (33). Disease-modifying interventions which act on the pathophysiology of AD may be used as tools to prevent exacerbation of disease or progression of the atopic march, when used adequately and in the right patients.
This paper aims to provide a review of the current role of AIT in the management of AD, discussing the most recent evidence on the safety and efficacy of AIT in AD treatment and summarizing the latest international recommendations on this topic, with a focus on pediatric AD.
Materials and methods
We performed a literature search in Medline through PubMed using default keywords related to pediatric Atopic Dermatitis and Allergen Immunotherapy. Original studies and review articles, with a focus on meta-analyses and randomized controlled trials (RCTs) in English, were identified up to May 1st, 2022.
Results
Among the most recent meta-analyses, Bae et al. performed a systematic review and meta-analysis in 2013 to assess the efficacy of AIT for AD patients (34). The analysis included 8 studies, 7 placebo-controlled RCTs and one quasi-RCT, including a total of 385 subjects. The characteristics of the studies are summarized in Table 1A. One of the studies included a population of adults only.
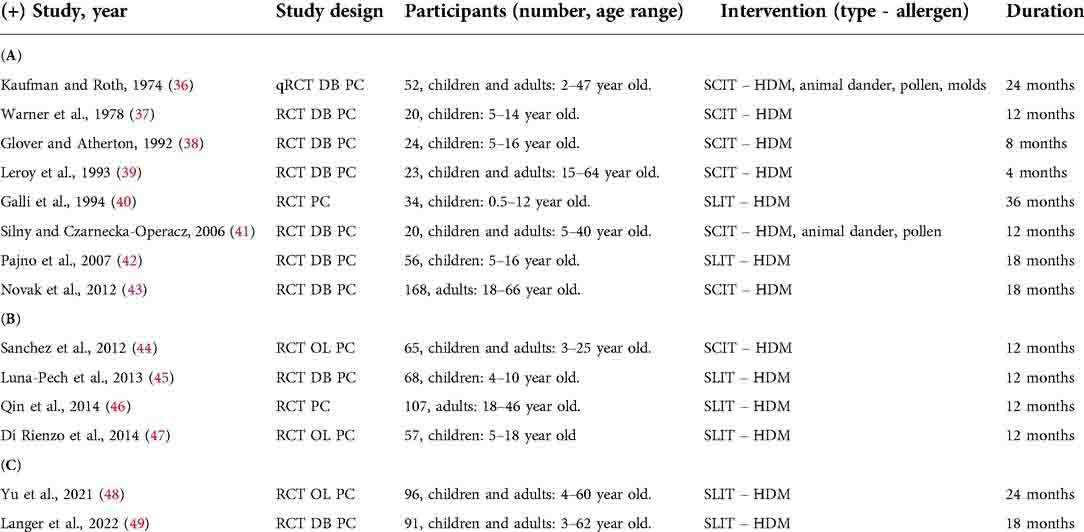
Table 1. Summary of the studies included in systematic review and meta-analysis, (A) adapted from Bae et al. (34) and (B) adapted from Tam et al. (35), and (C) of the two latest RCTs, including a pediatric population. DB, double-blind; HDM, house dust mites; OL, open label; PC, placebo-controlled; SCIT, subcutaneous immunotherapy; RCT, randomized controlled trial; SLIT, sublingual immunotherapy; y.o, years old.
Overall, the work demonstrated moderate-level evidence for the efficacy of AIT for AD patients, compared with placebo. Stratifying by type of intervention (SCIT vs. SLIT), a subgroup analysis study showed a conserved significant positive effect of SCIT on AD, while that of SLIT studies did not. In the subgroup analysis by age (adults vs. children), AIT did not prove significant efficacy in children. The other subgroup analyses (short-term vs. long-term treatment, mild vs. severe AD) showed significant efficacy of AIT in the six long-term treatment studies (more than one year) and in the five severe AD studies.
Comparable proportions of systemic (from 0.0% to 8.0% vs. from 0.0% to 10.7%, respectively) and local adverse reactions (from 6.3% to 80.0% vs. from 0.0% to 60.0%, respectively) were reported in the AIT and placebo groups, without fatal or near-fatal adverse events.
In conclusion, this meta-analysis provided moderate-level evidence for AIT efficacy in AD, in the general population but not in the children subgroup. However, these findings are based on a small number of RCTs, with considerable heterogeneity in study design, different allergens type and dose, age groups, administration schedules, duration of treatment and outcomes studied. Moreover, the authors do not further specify patient characteristics, and some of the trials also include patients who received systemic corticosteroids to control disease.
A second work was carried out by Tam et al. (35), who performed a Cochrane systematic review and meta-analysis in 2016 to assess the effect of AIT compared to placebo or standard treatment in AD. In this analysis, 12 RCTs were included with a total number of 733 participants. Eight of the included studies were in common with the meta-analysis performed by Bae et al. (34) and four were newly published studies, whose characteristics are summarized in Table 1B. One of the new studies was on an entirely adult population.
In this Cochrane, many different physician-assessed and patient-reported outcomes were analyzed. A significant improvement in disease severity based upon investigator- or physician-rated global assessment of SCORAD was shown. However, no significant difference in patient or parent-reported clinical manifestations of itching or sleep disturbance as assessed by SCORAD part C was observed. The overall quality of the evidence was low, mainly due to the differing results between studies and the lack of blinding in some studies. Moreover, subgroup analyses for allergen-type, patient age and disease severity could not be performed, because of the small number of trials that contributed with data to the analyses.
No statistically significant increase in the risk of local reactions between AIT and control groups was found in 484 participants. In a total of 492 participants, no statistically significant increase in the risk of systemic reactions was observed, with 18 events in the AIT group and 15 in the control group.
After the two above-mentioned meta-analyses, three new RCTs have been published, one of them on an entirely adult population (50) and two including children (48, 49), both investigating the efficacy of SLIT in subjects with AD and sensitized to house dust mites (HDM). The characteristics of the two latest RCTs, including a pediatric population, are summarized in Table 1C.
Among the two studies including children, in 2021 a RCT was published by Yu et al., on 96 HDM sensitized subjects (age 4–60 years, mean age 27 years). Seventy-seven subjects completed a full study period of 24 months, 38 receiving only standard treatment (oral antihistamines and/or topical steroid) and 39 receiving HDM SLIT (48). The patients in the treatment group showed a significant decrease from baseline SCORAD, Visual Analogue Scale (VAS) and rescue medication score from 12 months of treatment on, compared with the control group, without severe adverse events during the therapy. However, this was an unblinded study on a relatively small cohort of patients.
The latest RCT was published in February 2022 by Langer et al. and was a double-blind, placebo-controlled study on 91 patients (3–62 years, 40% < 12 years, distributed in the study arms), with SCORAD score greater than or equal to 15 and positive skin test result and/or IgE to Dermatophagoides pteronyssinus (49). Sixty-six patients (31 in the placebo group) completed the study and received placebo or HDM drops for 18 months. The work demonstrated a statistically significant difference in the decrease in mean SCORAD score from baseline to 18 months. There were similar reductions in objective SCORAD and an higher proportion of patients with Investigator Global Assessment 1/0, with the absence of severe adverse events. On the other hand, no difference in EASI and DLQI scores, VAS for symptoms, and pruritus scores was observed. Whilst this study demonstrates the safety and the potential efficacy of AIT in AD patients, there was a high drop-out rate (27%), according to other previous studies (20% in the above-mentioned Yu et al. paper) (48). Moreover, the results were not consistent across the different outcome measures, lending uncertainty to their interpretation.
Discussion
The American Academy of Dermatology published its guidelines for the management of AD in 2014 (51). In their opinion, AIT could be considered as a possible adjunct to conventional therapy but, due to the small number of published studies and the conflicting evidence, AIT was not recommended for the management of disease in the general AD population.
In 2017, the Italian Society of Pediatric Allergy and Immunology summarized the evidence in their clinical practice recommendations for AIT in children, stating that existing studies were often uncontrolled and overall results were controversial (52). They acknowledged the clinical efficacy of AIT in extrinsic AD, but they were unable to state clear recommendations. They concluded that the use of AIT in AD was still largely experimental, and the indications were still limited to those AD patients with co-existing allergic rhinitis or asthma.
In 2019, the European Academy of Allergy and Clinical Immunology (EAACI) was involved in the consensus-based European guidelines for the treatment of AD, where it was stated that the evidence regarding the use of AIT in AD treatment was conflicting, with more recent literature being more favorable towards therapy, which may have positive effects in chosen, highly sensitized patients (53). In these guidelines, AIT was not recommended as a general treatment option for AD, but its use was potentially suggested in selected cases, such as in patients with house dust mite, birch or grass pollen sensitization, with severe AD, and with a history of clinical exacerbation after exposure to the causative allergen or a positive corresponding atopy patch test. Moreover, it was specified that AIT was not contraindicated in patients with concomitant respiratory allergic diseases (mild allergic bronchial asthma or allergic rhinoconjunctivitis) and AD. The European Task Force on Atopic Dermatitis (ETFAD) of the European Academy of Dermatology and Venerology (EADV) 2020 position paper reinforced the same concepts of patient selection and underlined the fact that AIT may be used in AD when approved indications for this treatment exist in the same patient, as in the case of concomitant respiratory allergic diseases (26). Finally, the most recent European Dermatology Forum in the European guidelines (EuroGuiDerm) 2022 on atopic eczema treatment recommended once more against the use of AIT as routine treatment, but suggested to consider it for selected patients with house dust mite, birch or grass pollen sensitization, and a history of clinical exacerbation after exposure to the causative allergen or a positive corresponding atopy patch test, as mentioned above (54).
In conclusion, AIT is still not usually recommended as a treatment option for AD, and its employment remains empirical in clinical practice. Recent data suggest that AIT prescription may be considered but should be individualized for each patient, evaluating the risk-benefit ratio and after discussion with the patients/parents. A more precise selection of clinical phenotypes may help identify those patients with AD who could benefit from a tailored approach to AIT, such as patients with a proven sensitization to aeroallergens, particularly house dust mites, patients who present flare-ups induced by exposure to aeroallergens, and concomitant allergic rhinitis or asthma. In the pediatric subgroup, it may be relevant to consider which patients have a lower chance of spontaneous resolution before adulthood. There is a need for more well designed and adequately powered RCTs, with standardized outcomes, to better inform recommendations for AIT use in clinical practice for patients with AD. This is particularly true in the pediatric subgroup, for which an even greater lack of studies and recommendations is observed.
Author contributions
MG and EN conceptualized the work. BP and MG collected the data and drafted the manuscript. BP, MG, FM, GDC, EN, SC, CF and GDT analyzed the data. BP, MG, FM, GDC, EN, SC, CF and GDT critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chan LN, Magyari A, Ye M, Al-Alusi NA, Langan SM, Margolis D, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. (2021) 16(10 October):1–14. doi: 10.1371/journal.pone.0258219
2. Deckers IAG, McLean S, Linssen S, Mommers M, van Schayck CP, Sheikh A. Investigating international time trends in the incidence and prevalence of atopic eczema 1990–2010: a systematic review of epidemiological studies. PLoS One. (2012) 7(7):e39803. doi: 10.1371/journal.pone.0039803
3. Illi S, Von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, et al. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. (2004) 113(5):925–31. doi: 10.1016/j.jaci.2004.01.778
4. Garmhausen D, Hagemann T, Bieber T, Dimitriou I, Fimmers R, Diepgen T, et al. Characterization of different courses of atopic dermatitis in adolescent and adult patients. Allergy Eur J Allergy Clin Immunol. (2013) 68(4):498–506. doi: 10.1111/all.12112
5. Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. (2013) 24(5):476–86. doi: 10.1111/pai.12095
6. Carlsten C, Dimich-Ward H, Ferguson A, Watson W, Rousseau R, Dybuncio A, et al. Atopic dermatitis in a high-risk cohort: natural history, associated allergic outcomes, and risk factors. Ann Allergy Asthma Immunol. (2013) 110(1):24–8. doi: 10.1016/j.anai.2012.10.005
7. Dharmage SC, Lowe AJ, Matheson MC, Burgess JA, Allen KJ, Abramson MJ. Atopic dermatitis and the atopic march revisited. Allergy. (2014) 69(1):17–27. doi: 10.1111/all.12268
8. Hill DA, Grundmeier RW, Ramos M, Spergel JM. Eosinophilic esophagitis is a late manifestation of the allergic march. J Allergy Clin Immunol Pract. (2018) 6(5):1528–33. doi: 10.1016/j.jaip.2018.05.010
9. Nomura T, Wu J, Kabashima K, Guttman-Yassky E. Endophenotypic variations of atopic dermatitis by age, Race, and Ethnicity. J Allergy Clin Immunol Pract. (2020) 8(6):1840–52. doi: 10.1016/j.jaip.2020.02.022
10. Yaghmaie P, Koudelka CW, Simpson EL. Mental health comorbidity in patients with atopic dermatitis. J Allergy Clin Immunol. (2013) 131(2):428–33. doi: 10.1016/j.jaci.2012.10.041
11. Wan J, Takeshita J, Shin DB, Gelfand JM. Mental health impairment among children with atopic dermatitis: a United States population-based cross-sectional study of the 2013–2017 national health interview survey. J Am Acad Dermatol. (2020) 82(6):1368–75. doi: 10.1016/j.jaad.2019.10.019
12. Xie QW, Xiaolu D, Tang X, Chan CHY, Chan CLW. Risk of mental disorders in children and adolescents with atopic dermatitis: a systematic review and meta analysis. Front Psychol. (2019) 10(July):1–12. doi: 10.3389/fpsyg.2019.01773
13. Hanifin J, Rajka G. Diagnostic features of atopic eczema. Acta Dermatol Venereol. (1980) Suppl 92: 44–47. doi: 10.2340/00015555924447
14. Vakharia PP, Chopra R, Silverberg JI. Systematic review of diagnostic criteria used in atopic dermatitis randomized controlled trials. Am J Clin Dermatol. (2018) 19(1):15–22. doi: 10.1007/s40257-017-0299-4
15. Williams HC, Jburney PG, Hay RJ, Archer CB, Shipley MJ, Ahunter JJ, et al. The U.K. Working Party's Diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. Br J Dermatol. (1994) 131(3):383–96. doi: 10.1111/j.1365-2133.1994.tb08530.x
16. Geat D, Giovannini M, Barlocco G, Pertile R, Pace M, Mori F, et al. Assessing patients’ characteristics and treatment patterns among children with atopic dermatitis. Ital J Pediatr. (2021) 47(1):1–6. doi: 10.1186/s13052-020-00935-z
17. Schmitt J, Langan S, Williams HC. What are the best outcome measurements for atopic eczema? A systematic review. J Allergy Clin Immunol. (2007) 120(6):1389–98. doi: 10.1016/j.jaci.2007.08.011
18. Schmitt J, Langan S, Deckert S, Svensson A, Von Kobyletzki L, Thomas K, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. (2013) 132(6):1337–47. doi: 10.1016/j.jaci.2013.07.008
19. Harmonising Outcome Measures for Eczema (HOME). Available at: http://www.homeforeczema.org/ (Accessed October 3, 2022).
20. Sator PG, Schmidt JB, Hönigsmann H. Comparison of epidermal hydration and skin surface lipids in healthy individuals and in patients with atopic dermatitis. J Am Acad Dermatol. (2003) 48(3):352–8. doi: 10.1067/mjd.2003.105
21. Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, et al. Cellular and molecular immunologic mechanisms in patients with atopic dermatitis. J Allergy Clin Immunol. (2016) 138(2):336–49. doi: 10.1016/j.jaci.2016.06.010
22. Kim J, Kim H. Microbiome of the skin and gut in atopic dermatitis (AD): understanding the pathophysiology and finding novel management strategies. J Clin Med. (2019) 8(4):444. doi: 10.3390/jcm8040444
23. Novak N, Simon D. Atopic dermatitis - From new pathophysiologic insights to individualized therapy. Allergy. (2011) 66(7):830–9. doi: 10.1111/j.1398-9995.2011.02571.x
24. Bieber T. Atopic dermatitis 2.0: from the clinical phenotype to the molecular taxonomy and stratified medicine. (2012) 67(12):1475–82. doi: 10.1111/all.12049
25. Hui-Beckman JW, Goleva E, Berdyshev E, Leung DYM. Endotypes of atopic dermatitis and food allergy. J Allergy Clin Immunol. (2022) S0091-6749(22)01048-X. doi: 10.1016/j.jaci.2022.07.021.
26. Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. ETFAD/EADV eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. (2020) 34(12):2717–44. doi: 10.1111/jdv.16892
27. Ginsberg DN, Eichenfield LF. Debates in allergy medicine: specific immunotherapy in children with atopic dermatitis, the “con” view. World Allergy Organ J. (2016) 9(1):1–5. doi: 10.1186/s40413-016-0107-2
28. Cox L, Calderon MA. Allergen immunotherapy for atopic dermatitis: is there room for debate? J Allergy Clin Immunol Pract. (2016) 4(3):435–44. doi: 10.1016/j.jaip.2015.12.018
29. Passalacqua G, Bagnasco D, Canonica GW. 30 Years of sublingual immunotherapy. Allergy. (2020) 75(5):1107–20. doi: 10.1111/all.14113
30. Campana R, Moritz K, Neubauer A, Huber H, Henning R, Brodie TM, et al. Epicutaneous allergen application preferentially boosts specific T cell responses in sensitized patients. Sci Rep. (2017) 7(1):11657. doi: 10.1038/s41598-017-10278-1
31. van de Veen W, Stanic B, Wirz OF, Jansen K, Globinska A, Akdis M. Role of regulatory B cells in immune tolerance to allergens and beyond. J Allergy Clin Immunol. (2016) 138(3):654–65. doi: 10.1016/j.jaci.2016.07.006
32. Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J Allergy Clin Immunol. (2014) 133(3):621–31. doi: 10.1016/j.jaci.2013.12.1088
33. Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. (2017) 140(6):1485–98. doi: 10.1016/j.jaci.2017.10.010
34. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. (2013) 132(1):110–7. doi: 10.1016/j.jaci.2013.02.044
35. Tam H, Calderon MA, Manikam L, Nankervis H, García Núñez I, Williams HC, et al. Specific allergen immunotherapy for the treatment of atopic eczema: a cochrane database of systematic reviews. Cochrane Database Syst Rev. (2016) 2(2):CD008774. doi: 10.1002/14651858.CD008774.pub2
36. Kaufman HS, Roth HL. Hyposensitization with alum precipitated extracts in atopic dermatitis: a placebo controlled study. Ann Allergy. (1974) 32(6):321–30.4597822
37. Warmer JO, Soothill JF, Price JF, Hey EN. Controlled trial of hyposensitisation to dermatophagoides pteronyssinus in children with asthma. Lancet. (1978) 2(8096):912–5. doi: 10.1016/s0140-6736(78)91630-638
38. Glover MT, Atherton DJ. A double-blind controlled trial of hyposensitization to dermatophagoides pteronyssinus in children with atopic eczema. Clin Exp Allergy. (1992) 22(4):440–6. doi: 10.1111/j.1365-2222.1992.tb00145.x
39. Leroy BP, Boden G, Lachapelle JM, Jacquemin MG, Saint-Remy JMR. A novel therapy for atopic dermatitis with allergen-antibody complexes: a double-blind, placebo-controlled study. J Am Acad Dermatol. (1993) 28(2 Pt 1):232–9. doi: 10.1016/0190-9622(93)70033-p
40. Galli E, Chini L, Nardi S, Benincori N, Panei P, Fraioli G, et al. Use of a specific oral hyposensitization therapy to dermatophagoides pteronyssinus in children with atopic dermatitis. Allergol Immunopathol (Madr). (1994) 22(1):18–22.8030579
41. Silny W, Czarnecka-Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis - results of a double-blind, placebo-controlled study. Allergologie. (2006) 21(126):558–65.
42. Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. (2007) 120(1):164–70. doi: 10.1016/j.jaci.2007.04.008
43. Novak N, Bieber T, Hoffmann M, Folster-Holst R, Homey B, Werfel T, et al. Efficacy and safety of subcutaneous allergen-specific immunotherapy with depigmented polymerized mite extract in atopic dermatitis. J Allergy Clin Immunol. (2012) 130(4):925–31.e4. doi: 10.1016/j.jaci.2012.08.004
44. Sánchez Caraballo JM, Cardona Villa R. Clinical and immunological changes of immunotherapy in patients with atopic dermatitis: randomized controlled trial. ISRN Allergy. (2012) 2012:183983. doi: 10.5402/2012/183983
45. Luna-Pech JA, Newton-Sanchez OA, Torres-Mendoza BM, Garcia-Cobas CY. Efficacy of sublingual immunotherapy in the severity of atopic dermatitis in children with allergic sensitization to dermatophagoides pteronyssinus. Ann Allergy Asthma Immunol. (2013) 111(5 Suppl 1):A8.
46. Qin YE, Mao JR, Sang YC, Li WX. Clinical efficacy and compliance of sublingual immunotherapy with dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. (2014) 53(5):650–5. doi: 10.1111/ijd.12302
47. Di Rienzo V, Cadario G, Grieco T, Galluccio AG, Caffarelli C, Liotta G, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, open, parallel-group study. Ann Allergy Asthma Immunol. (2014) 113(6):671–673.e1. doi: 10.1016/j.anai.2014.09.009
48. Yu N, Luo H, Liang D, Lu N. Sublingual immunotherapy in mite-sensitized patients with atopic dermatitis: a randomized controlled study. Postepy DermatolAlergol. (2021) 38(1):69–74. doi: 10.5114/ada.2021.104281
49. Langer SS, Cardili RN, Melo JML, Ferriani MPL, Moreno AS, Dias MM, et al. Efficacy of house dust Mite sublingual immunotherapy in patients with atopic dermatitis: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol Pract. (2022) 10(2):539–549.e7. doi: 10.1016/j.jaip.2021.10.060
50. Liu L, Chen J, Xu J, Yang Q, Gu C, Ni C, et al. Sublingual immunotherapy of atopic dermatitis in mite-sensitized patients: a multi-centre, randomized, double-blind, placebo-controlled study. Artif Cells Nanomed Biotechnol. (2019) 47(1):3540–7. doi: 10.1080/21691401.2019.1640709
51. Sidbury R, Tom WL, Bergman JN, Cooper KD, Silverman RA, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches work group. J Am Acad Dermatol. (2014) 71(6):1218–33. doi: 10.1016/j.jaad.2014.08.038
52. Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. (2017) 43:1–18. doi: 10.1186/s13052-016-0315-y
53. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. (2018) 32(5):657–82. doi: 10.1111/jdv.14891
Keywords: atopic dermatitis, allergen immunotherapy, house dust mites, precision medicine, pediatrics
Citation: Pessina B, Giovannini M, Mori F, Di Cara G, Novembre E, Chan S, Flohr C and du Toit G (2022) Is there room for allergen immunotherapy for the treatment of atopic dermatitis in the precision medicine era?. Front. Pediatr. 10:1050560. doi: 10.3389/fped.2022.1050560
Received: 21 September 2022; Accepted: 4 October 2022;
Published: 2 November 2022.
Edited by:
Peck Y. Ong, Children’s Hospital of Los Angeles, United StatesReviewed by:
Linda Cox, Consultant, United States© 2022 Pessina, Giovannini, Mori, Di Cara, Novembre, Chan, Flohr and du Toit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mattia Giovannini bWF0dGlhZzg4QGhvdG1haWwuaXQ=
†These authors share first authorship
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Benedetta Pessina
Benedetta Pessina Mattia Giovannini
Mattia Giovannini Francesca Mori
Francesca Mori Giuseppe Di Cara
Giuseppe Di Cara Elio Novembre
Elio Novembre Susan Chan3,5,6
Susan Chan3,5,6