
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 09 November 2022
Sec. Pediatric Oncology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1046586
Twelve to 22% of pediatric acute myeloid leukemia (AML) patients present with hyperleukocytosis, which is one of the main risk factors of early death due to its clinical complications: leukostasis, causing pulmonary or central nervous system injuries, tumor lysis syndrome, and disseminated intravascular coagulation. Sorafenib is a multi-kinase inhibitor that blocks the Fms-Related Tyrosine Kinase 3 receptor (FLT3) in AML patients with a FLT3-internal tandem duplication (FLT3-ITD), leading to a reduction of proliferation. Here we report four de novo diagnosed or relapsed pediatric FLT3-ITD–positive AML patients with hyperleukocytosis, which were treated with sorafenib in combination with cytoreductive chemotherapy prior to the start of the induction phase. We observed a fast reduction of white blood cells in peripheral blood and bone marrow. This resulted in a rapid clinical stabilization of the patients. Adverse side effects—such as dermatologic toxicity, elevation of transaminases and hypertension—occurred but were mild and inductive chemotherapy could be started in parallel or subsequently. This implies sorafenib as a safe and effective treatment option in combination with chemotherapy during cytoreductive prephase for children with this life-threatening condition.
Twelve to 22% of pediatric acute myeloid leukemia (AML) patients present with hyperleukocytosis at diagnosis or relapse (1), defined as more than 100 × 109/L white blood cells (WBC). Even with best supportive care, hyperleukocytosis is one of the main risk factors for early death in AML due to leukostasis, causing pulmonary and central nervous system (CNS) injuries, or disseminated intravascular coagulation. Creutzig et al. reported that a WBC count higher than 200 × 109/L increases the risk of death within the first 2 weeks of therapy from 2.4% to 16.9% (2). Furthermore, the risk of nonresponse is higher. To prevent lethal complications, fast and effective leukoreduction is intended. However, tumor lysis syndrome in children (3, 4) as well as its clinical complications (renal dysfunction, cardiac arrhythmia, seizures), induced by too aggressive cytoreduction should be avoided as well. Therefore, intensive supportive care to prevent bleeding, leukostasis and kidney failure is essential. The use of hydroxyurea, low-dose chemotherapy or leukapheresis, could not substantially reduce early mortality in hyperleukocytosis (1), but represent to date the standard approaches. The BFM study group recommends besides intensive, intravenous hydration and hyperurecaemia prophylaxis the treatment with cytarabine (starting with 20–40 mg/m2 i.v./s.c. followed by continues infusion with 100 mg/m2/24 h). Leukapheresis or exchange transfusion is only recommended for patients with WBC of more than 200 × 109/L or AML FAB M4/M5 (5). Other patients do not seem to benefit from this procedure. Limitations of leukapheresis can often be the requirement of technical expertise and technical problems in small children (6).
One group of AML patients that has an enhanced risk of hyperleukocytosis, are patients with a mutation in the juxtamembrane domain, called internal tandem duplication, of Fms-Related Tyrosine Kinase 3 (FLT3-ITD) (7). This mutation causes an independent and constitutive activation of the FLT3-receptor due to autophosphorylation, leading to aberrant proliferation and block of apoptosis (8). 12% of pediatric AML patients carry this mutation, whereas the prevalence increases from infants to adolescents (9). In children with AML harboring a mutant FLT3-ITD, initial WBC is more often elevated above 50 × 109/L than in wild type (60.1% vs. 34.7%) (10). The rate of FLT3-ITD mutations in patients with hyperleukocytosis is high, ranging between 27% and 62% (7, 11, 12).
Sorafenib is a multi-tyrosine kinase inhibitor. One of the kinases being inhibited by sorafenib is FLT3 (13). In a murine AML cell line, Zhang et al. could show that the effect of sorafenib is 1,000- to 3,000-fold stronger against mutated than wild type FLT3 (14).
Several studies showed efficacy of sorafenib in the treatment of AML leading to fast remission in combination with chemotherapy as well as monotherapy (15–17). Therefore, sorafenib became part of the AML BFM 2012 trial for children with FLT3-ITD/TKD mutation during induction, consolidation and re-intensification (18). A tolerable toxicity profile was reported, consisting of fatigue, hand foot skin reaction, hypertension, elevated transaminases and mild myelosuppression (19). In cases, when sorafenib was used as treatment of relapsed or refractory AML, a fast and efficient leukoreduction has been reported (20–22), suggesting that it might be of clinical benefit in the management of hyperleukocytosis, even in cases where the FLT3-ITD status in unknown.
We conducted a retrospective analysis of four pediatric AML patients presenting with hyperleukocytosis that were reported to the AML-BFM study group and who were treated with sorafenib prior to the start of the induction phase based on the decision of the treating doctor or local hospital. Clinical condition, complete blood cell (CBC) counts, serum electrolytes and coagulation parameters and parameters of tumor lysis syndrome before, during and after treatment with sorafenib were analyzed. Response to treatment was evaluated depending on the reduction of WBC in peripheral blood after 72 h. To identify and analyze mutations in the FLT3 gene in AML patients, a combined NGS and a fragment assay that detects and quantifies selected mutations of the FLT3 gene was used.
The patients, diagnosed with AML and hyperleukocytosis, were reported to the AML-BFM study group. They were FLT3-ITD positive and presented with a hyperleukocytosis and were in a clinically critical state, whereas one of them was de novo diagnosed and three of them presented with relapsed or refractory AML. The FLT3-ITD status was not known at the start of treatment. In relapsed/refractory AML patients molecular genetics, including the FLT3-ITD status, was reassessed.
All four patients received sorafenib for 3–4 days during the cytoreductive phase. Either sorafenib was given as monotherapy or additionally at the start of chemotherapy at a dose of 2 × 84–2 × 200 mg/m2/day. The specific treatment was dependent on the patient's condition or pre-treatments. The cytoreductive treatment was accompanied by supportive care to control metabolic parameters. This included rasburicase or allopurinol, intensive, intravenous hydration, urine alkalization, correction of coagulopathy and treatment of fever and/or infection. Patients were monitored for tumor lysis syndrome using the Cairo-Bishop definition for children. Laboratory tumor lysis syndrome was considered when meeting two or more of the following criteria: hyperuricaemia (uric acid ≥476 µmol/L or 25% increase from baseline), hyperkaliaemia (potassium ≥6.0 mmol/L or 25% increase from baseline), hyperphosphatemia (phosphate ≥2.1 mmol/L or 25% increase from baseline), hypocalciemia (calcium ≤1.75 mmol/L or 25% decrease from baseline) (4). On occurrence of side effects continuation of sorafenib was reevaluated. Treatment or prophylaxis of hand foot skin reaction was at the discretion of the local physician.
Between December 2013 and April 2016 four patients with AML and hyperleukocytosis that were treated with sorafenib were reported to the AML-BFM study group. The patients were in a clinically critical state. One of them was de novo diagnosed and three of them presented with relapsed or refractory AML. The patients had hyperleukocytosis with an average WBC count of 131 × 109/L (range 80–170 × 109/L) and an average percentage of blasts of 94% (range 81%–100%) before start of therapy. Since the disease status differed between the patients, the course of treatment is presented per patient (Figure 1; Supplementary Table S1).
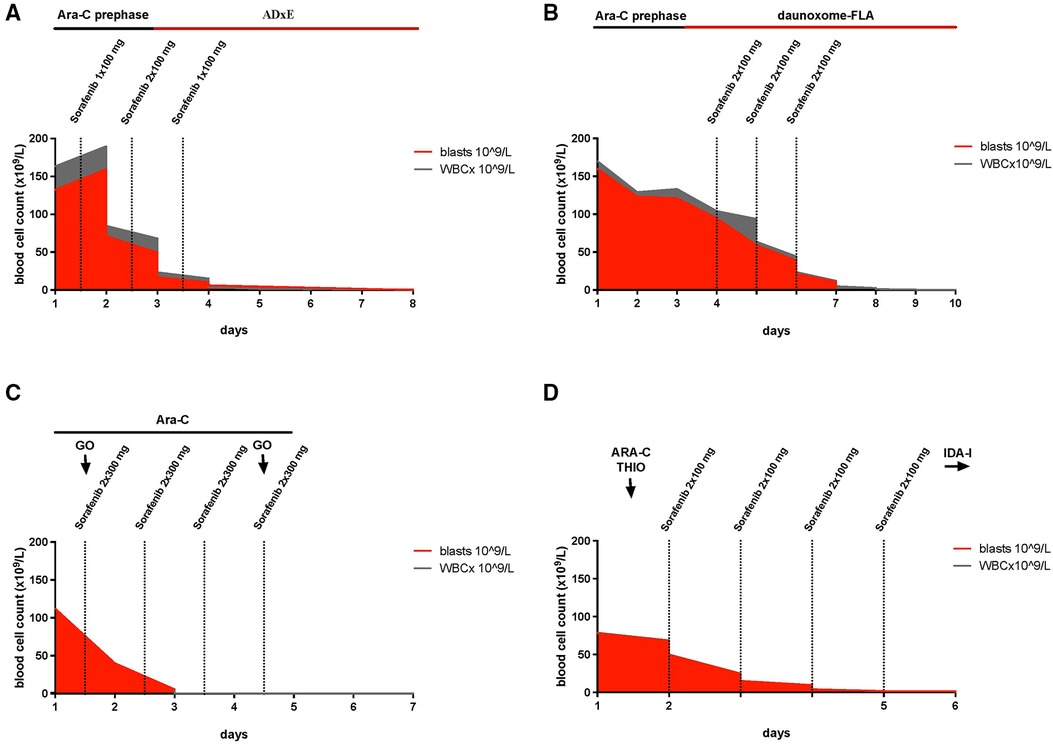
Figure 1. Timelines, illustrating the reduction of white blood cells during treatment with sorafenib and depicting the timing of chemotherapeutic treatment in relation to sorafenib. (A) Patient #1: Cytarabine (prephase Ara-C) was started together with sorafenib on day 1 with 1 × 100 mg, increasing the dose to 2 × 100 mg on day 2 and 1 × 200 mg on day 3, on day three induction with intravenous therapy with cytarabine, liposomal daunorubicin and etoposide and intrathecal therapy with cytarabine, methotrexate and prednisolone (ADxE). (B) Patient #2: Due to an inadequate response to the cytoreductive prephase with cytarabine (100 mg/m2/day continuous infusion) for 3 days (prephase Ara-C), sorafenib (2 × 100 mg/day) was administrated parallel to daunoxome-FLA consisting ofintravenous therapy with liposomal daunoxome, fludarabine, cytarabine and intrathecal therapy with cytarabine, methotrexate and prednisolone for 3 days. (C) Patient #3: For 4 days, sorafenib (2 × 300 mg/day) was administrated in combination with cytarabine, 500 mg/m2/day, (Ara-C), and gemtuzumab ozogamicin, 2 × 5 mg, (GO). (D) Patient #4: On day 2 of treatment with cytarabine, 37.5 mg/m2, (ARA-C) and thioguanine, 33 mg/m2, (THIO), the treatment was expanded by sorafenib (2 × 100 mg/day) for 4 days, followed by cytarabine without idarubicine (IDA-I).
The first patient was a 5-year-old boy, diagnosed with de novo AML FAB M5, FLT3-ITD positive with an FLT3-ITD allele ratio of 0.88, trisomy 8 and MYC mutation. At the time of diagnosis, he had a WBC count of 163 × 109/L with 81% blasts, isolated petechiae and a disseminated intravascular coagulation (DIC) score of 6 according to the modified criteria of the International Society on Thrombosis and Haemostasis (ISTH) (23, 24). Initially, the patient presented with hepatosplenomegaly, elevated lactate dehydrogenase (LDH) (1,226 UI/L) and aspartate aminotransferase (AST) (113 UI/L). CNS status was negative. Prephase with cytarabine (starting with single doses of 20 and 40 mg/m2, then continuous infusion of 100 mg/m2/24 h for 48 h) was initiated. One the same day and before receiving the results of molecular diagnostics, sorafenib was administered for 72 h starting at a dose of 100 mg/12 h for 48 h followed by 200 mg/24 h for the following. After 72 h, WBC declined to 3.5 × 109/L. Cytoreductive prephase was followed by the induction protocol ADxE according to the recommendations of the AML-BFM 2012 registry (intravenous therapy with cytarabine, liposomal daunorubicin and etoposide and intrathecal therapy with cytarabine, methotrexate and prednisolone). The first dose of liposomal daunorubicin overlapped with the sorafenib treatment. When WBC normalized sorafenib was terminated. On the day of admission, he was diagnosed with influenza B (by polymerase chain reaction). He showed fever but no other clinical signs of infection. On the same day, treatment with oseltamivir was started as well as an intravenous antibiotic treatment with piperacillin, tazobactam and tobramycin. During the cytoreductive prephase, the patient developed a transient hypocalcemia with a minimum serum calcium of 1.74 mmol/L, hyperphosphatemia (2.18 mmol/L), and 25% increase of the potassium level over baseline (max. 5.2 mmol/L), which just barely meets the criteria of a laboratory tumor lysis syndrome in children (4, 25) (Supplementary Table S1). With supportive measures, the laboratory changes improved within 1 day and no clinical symptoms of a tumor lysis syndrome were reported. Renal parameters remained stable, a decline of WBC to less than 0.5 × 109/L was achieved (Figure 1A). The fever persisted spontaneously and LDH and transaminases declined. Subsequent to the treatment with sorafenib, an echocardiography showed no abnormalities in cardiac function.
The second patient, a 16-years-old male, was diagnosed with AML FAB M1 with Auer rods, mutation in WT1 and FLT3-ITD, trisomy 8 and a deletion in 2p. He had a relapse 1 year after allogeneic hematopoietic stem cell transplantation (HSCT) with 170 × 109/L WBC and 94% blasts in the peripheral blood. He showed laboratory findings of DIC (DIC-score of 6) as well as petechiae at his legs but without complications of bleeding. Due to an inadequate response (WBC 135 × 109/L) to the cytoreductive prephase with cytarabine (100 mg/m2/day continuous infusion) for 3 days and coagulopathy (D-dimere >30 mg/L, fibrinogen 2.67 g/L), sorafenib with a dose of 1 × 200 mg/day for 3 days was started together with the reinduction therapy daunoxome-FLA of the Relapsed AML 2009 registry (intravenous therapy with liposomal daunoxome, fludarabine, cytarabine and intrathecal therapy with cytarabine, methotrexate and prednisolone). 72 h after the start of sorafenib, WBC decreased to 0.2 × 109/L (Figure 1B). At diagnosis, he presented with fever and a CRP of 69.7 mg/L without a focus of infection. When fever continued, antibiotic and antifungal therapies were escalated and oxygen therapy with up to 4 L/min was required. Later on, respiratory syncytial virus was detected and he was treated with ribavirin. With a history of transposition of the great arteries following arterial switch operation, cardiac function was controlled by echocardiography and showed a sufficient cardiac output. On the second day of treatment with sorafenib, LDH increased to a maximum of 2,997 UI/L, AST was slightly elevated (77 UI/L) and an abdominal ultrasound showed no enlargement of the liver and only marginal increase in spleen size. During administration of sorafenib, the patient barely met the criteria of laboratory tumor lysis syndrome with an increase of potassium as well as urine acid of more than 25% of baseline to a maximum of 5.0 mmol/L and 132 µmol/L, respectively, which is still in the limit of normal (Supplementary Table S1). However, no clinical features of a tumor lysis syndrome were observed. Values decreased during intensified hydration and treatment with rasburicase, later with allopurinol. On the last day of the treatment with sorafenib, the patient developed exanthema on the hands and feet corresponding to hand foot skin reaction. During the following days, the skin reactions improved and only got worse later during the therapy, when sorafenib was restarted between the first and the second course of chemotherapy.
The third patient was a 17-year-old female, with refractory AML FAB M1/2, FLT3-ITD, 8 months after initial diagnosis. She presented with hyperleukocytosis of 112 × 109/L WBC with 100% blasts. In addition to the treatment with cytarabine (500 mg/m2/day) for 4 days and gemtuzumab ozogamicin (5 mg on day 1 and 4) she received sorafenib 2 × 300 mg/day. WBC decreased to 0.3 × 109/L within 72 h (Figure 1C). LDH rose to a maximum of 2,060 UI/L on the first day of administration with sorafenib. The criteria for laboratory tumor lysis syndrome were not fulfilled (Supplementary Table S1). Under antiemetic therapy with ondansetron and dimenhydrinate, chemotherapy was tolerated well. Having fever already before admission, she developed pneumonia, which required anti-infectious treatment and oxygen therapy with 5 L/min. During the cytoreductive phase, hypertension was treated with amlodipine and hydrochlorothiazide as well nifedipine and dihydralazine as needed. Cardiac function after termination of cytoreductive therapy was normal. A hematoma, resulting from complications during the implantation of a central venous catheter, aggravated the decrease of hemoglobin (hemoglobin 6.2 mg/dl) and required several transfusions of red blood cells, thrombocytes and fresh frozen plasma. Two weeks after cytoreductive therapy had been started, bone marrow aspiration showed a reduction of blasts from 94% to 15%. A third dose of gemtuzumab ozogamicin was administered. Sorafenib orally 2 × 300 mg was given until conditioning regimen for stem cell transplantation was started.
The fourth patient was an 11-year-old boy with AML FAB M4, mutations in WT1 and FLT3-ITD (allele ratio of 0.54), who relapsed during first-line therapy with 50% blasts in peripheral blood before the last of five courses (AML-BFM 98 trial). The therapy of the relapse was induced with a course of idarubicin, fludarabine, high dosage cytarabine and G-CSF (IDA-FLAG), to which he did not respond, presenting a WBC count of 80 × 109/L and 99% blasts in peripheral blood. An off study treatment to achieve cytoreduction was started. On the first day, he received cytarabine (37.5 mg/m2) and thioguanine (33 mg/m2). On the second day, sorafenib with a dose of 2 × 100 mg/day was added for 4 days due to inadequate response. The WBC decreased to 1.9 × 109/L, 72 h later (Figure 1D). On the third day LDH increased to 2,243 IU/L and alanine transaminase (ALT) to 416 IU/L but declined the next day, when WBCs fell below the level of normal. The level of potassium and phosphate where within the norm (Supplementary Table S1). Under treatment with rasburicase urin acid showed an increase over 25% but maximum level of 89 µmol/L. Parallel to cytoreduction, intrathecal therapy with cytarabine, prednisolone and methotrexate was initiated. Even though cytoreductive treatment of hyperleukocytosis was effective, the subsequent antileukemic treatment could not achieve a remission and the patient succumbed 3 months later.
We present four different pediatric cases of FLT3-ITD-positive AML and hyperleukocytosis that were treated with sorafenib during their cytoreductive prephase or—in one case—after inadequate response to the cytoreductive prephase in parallel to induction. In all four patients, WBC fell within 72 h after start of sorafenib administration to or below the level of normal (Figure 1). The patients had no signs of neurologic or renal toxicity or liver failure. But all patients experienced anemia and thrombocytopenia, without developing massive bleeding but making transfusion of blood components inevitable. The changes in electrolytes—despite massive cell reduction—were mild and only two patients just about met the criteria of a laboratory tumor lysis syndrome, which normalized under intensified supportive care. One patient developed hand foot skin reaction, which is very common during sorafenib treatment. This suggests that sorafenib administration for several days as cytoreductive prephase in children with AML and hyperleukocytosis may be effective and well-tolerated, even if administered in combination with cytarabine, liposomal daunorubucin or gemtuzumab ozogamicin. However, prospective clinical trials are required to determine the role of sorafenib in the treatment of hyperleukocytosis in AML.
A relevant factor in considering a treatment with sorafenib is the high incidence of adverse events that occur during hyperleukocytosis. On the other hand, pediatric patients suffer more often from toxicity due to sorafenib, probably due to the higher conversion to active n-oxide metabolites (16). Still the short treatment interval and the life-threating condition counterbalance this risk and seem to rectify the inclusion of sorafenib to the cytoreductive if FLT3-ITD-positive AML with hyperleukocytosis. One of the most common dose-dependent adverse effects of sorafenib is dermatologic toxicity, especially when used in combination with chemotherapy (15, 16, 19). In fact, hand foot skin reaction was observed in one of four patients. However, as the patient simultaneously received high dose cytarabine, it is unknown which of the two drugs caused this reaction. Still, the symptoms aggravated again, when sorafenib was restarted, implying that sorafenib at least influenced the erythema. Elevation of transaminases is another common adverse event (16, 19), which occurred in two of our patients. Only in one patient, transaminase elevation was classified as grade 3 (NCI CTCAEv3.0) (26). As ALT decreased the following day, no discontinuation of drug administration became necessary (19). The first patient showed a 2-fold elevated AST already at diagnosis, suggesting that this was related to the leukemia itself. The third patient showed manageable hypertension under administration of sorafenib, so that discontinuation of sorafenib was not necessary. Hypertension is an infrequently reported side effect of sorafenib in children (16, 19) even though it is common in adults (27) and mostly manageable with the use of angiotensin-converting enzyme inhibitors and beta-blockers. Tarlock et al. reported two patients, who experienced adverse cardiac events during treatment with sorafenib, whereas one of them received chemotherapeutics causing cardiac side effects in parallel to administration of sorafenib (15). Showing an elevated incidence for cardiac ischemia in adults as well (27), cardiac function of patients, presenting with a history of cardiac diseases or receiving drugs that can cause cardiac dysfunction, should be controlled regularly. Our patients showed no abnormalities in cardiac function. Targeted therapies—including sorafenib—in the initial phase of treatment have the potential to increase the incidence of a tumor lysis syndrome (28–31). In our cohort, the patients received preventive, supportive care, consisting of intensified intravenous hydration, hypouricemic agents and urinary alkalization, which precented massive or clinically relevant tumor lysis syndrome. We anticipate that this can be attributed to a stronger effect of sorafenib on cell proliferation than on apoptosis in AML with hyperleukocytosis (14, 32).
Our patients were treated with a dose of 2 × 84–2 × 200 mg/m2/day. Based on a phase I study of the Children's Oncology Group, the recommended dose of sorafenib for children with leukemia is 150 mg/m2 every 12 h (19). Inaba et al. recommended the identical dose for the use in combination with cytarabine or clofarabine (16). If sorafenib is considered as a treatment option in future cases of hyperleukocytosis this could serve as a reference dosage. The reported patients presented with different disease status (initial diagnosis or relapse), risk profile or pre-treatment. Therefore, combinations of sorafenib with the different chemotherapeutic agents were required. All combinations showed an effective leukoreduction. Since several studies show a limited single-agent activity (33–35) our study does recommend sorafenib in combination with chemotherapy for effective and safe cytoreduction in case of hyperleukocytosis. Following that, an intensive multiagent induction chemotherapy is indispensable to achieve remission.
Sorafenib is less effective in FLT3 wild type (WT) compared to FTL3-ITD (14, 17). In contrast to FLT3-WT, FLT3-ITD enhances the interactions of sorafenib at the ligand binding site (14). Furthermore, AML cell lines with wild type FLT3 show a paradox phosphorylation and thereby activation of the ERK2 (MAPK1) pathway, possibly explaining the resistance of sorafenib in FLT3-WT AML cell lines (36). These findings make effective treatment of hyperleukocytosis with sorafenib more likely in FLT3-ITD than in FLT3-WT. However, results of mutation analysis are not always available in the first hours after diagnosis. Largeaud et al. recently analyzed the genomic landscape of adult AML patients with hyperleukocytosis. The study revealed an average of four mutations per patient, whereby “drug-actionable” mutations were detected in 113 of 154 patients; 96 of them carried mutations in FLT3 (62.3%) (12). Thus, seeing hyperleukocytosis as a severe life-threatening condition in the progression of AML with a high frequency of activating mutations in signaling cascades, particularly in FTL3 (12), and showing a favorable toxicity profile, addition of sorafenib should be considered early during hyperleukocytosis and—if necessary—already before receiving the mutational analysis. Our patients were treated with sorafenib for cytoreduction as it was already part of the AML BFM 2012 trial for children with FLT3-ITD/TKD mutation during induction, consolidation and re-intensification (18). However, with gilterinib and midostaurin two drugs were recently approved for the treatment of FLT3-mutated AML (37, 38). Both are under investigation evaluation in children as a single agent or in combination with chemotherapy in newly diagnosed or relapsed/refractory AML (NCT04240002, NCT03591510 and NCT00866281). In a prospective trial of Stone et al., midostaurin showed a blood blast reduction in 70%, and a bone marrow blast reduction of over 50% in 6 of 20 patients. Nevertheless no complete remission was achieved by monotherapy with midostaurin (39). Still, both substances could potentially be used as an alternative to sorafenib in children with AML and hyperleukocytosis.
In summary, on the basis of our here presented experience, we think that sorafenib—or other FLT3 inhibitors—in combination with chemotherapy is an adequate treatment option for cytoreduction in AML FLT3-ITD hyperleukocytosis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by University Hospital Münster and Hannover Medical School. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Conception of the analysis was done by DR and J-HK. DR, J-HK, CN and ME oversaw the clinical aspects of the study. ME and CN provided clinical data and information. Data collection, data analysis and interpretation were done by FS. FS and J-HK wrote the manuscript. All authors contributed to the article and approved the submitted version.
The AML-BFM trials are supported by the German Cancer Aid (DKH). J-HK receives funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No 714226).
We would like to thank all medical and healthcare professionals, who were involved in the care and treatment of our patients. Special gratitude goes to the patients of the study and their parents.
DR has advisory roles for Celgene Corporation, Novartis, Bluebird Bio, Janssen, and receives research funding from CLS Behring and Roche. J-HK has advisory roles for Bluebird Bio, Novartis, Roche and Jazz Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1046586/full#supplementary-material.
1. Oberei S, Lehrbücher T, Phillips B, Hitzler J, Ethiker M, Bekenne J, et al. Leukapheresis and low-dose chemotherapy do not reduce early mortality in acute myeloid leukemia hyperleukocytosis: a systematic review and meta-analysis. Leuk Res. (2014) 38(4):460–8. doi: 10.1016/j.leukres.2014.01.004
2. Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T. Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98. J Clin Oncol. (2004) 22:4384–93. doi: 10.1200/JCO.2004.01.191
3. Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. (2004) 127(1):3–11. doi: 10.1111/j.1365-2141.2004.05094.x
4. Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. J Clin Oncol. (2008) 26(16):2767–78. doi: 10.1200/JCO.2007.15.0177
5. Creutzig U, Rossig C, Dworzak M, Stary J, von Stackelberg A, Wossmann W, et al. Exchange transfusion and leukapheresis in pediatric patients with AML with high risk of early death by bleeding and leukostasis. Pediatr Blood Cancer. (2016) 63(4):640–5. doi: 10.1002/pbc.25855
6. Jain R, Bansal D, Marwaha RK. Hyperleukocytosis: emergency management. Indian J Pediatr. (2013) 80(2):144–8. doi: 10.1007/s12098-012-0917-3
7. Sung L, Aplenc R, Alonzo TA, Gerbing RB, Gamis AS, AAML0531/PHIS Group. Predictors and short-term outcomes of hyperleukocytosis in children with acute myeloid leukemia: a report from the children's oncology group. Haematologica. (2012) 97(11):1770–3. doi: 10.3324/haematol.2012.065490
8. Zheng R, Small D. Mutant FLT3 signaling contributes to a block in myeloid differentiation. Leuk Lymphoma. (2005) 46(12):1679–87. doi: 10.1080/10428190500261740
9. Meshinchi S, Alonzo TA, Stirewalt DL, Zwaan M, Zimmerman M, Reinhardt D, et al. Clinical implications of FLT3 mutations in pediatric AML. Blood. (2006) 108(12):3654–61. doi: 10.1182/blood-2006-03-009233
10. Qiu KY, Liao XY, Liu Y, Huang K, Li Y, Fang JP, et al. Poor outcome of pediatric patients with acute myeloid leukemia harboring high FLT3/ITD allelic ratios. Nat Commun. (2022) 13(1):3679. doi: 10.1038/s41467-022-31489-9
11. Xu LH, Wang JW, Wang Y, Yang FY. Hyperleukocytosis predicts inferior clinical outcome in pediatric acute myeloid leukemia. Hematology. (2020) 25(1):507–14. doi: 10.1080/16078454.2020.1859169
12. Largeaud L, Bertoli S, Berard E, Tavitian S, Picard M, Dufrechou S, et al. Genomic landscape of hyperleukocytic acute myeloid leukemia. Blood Cancer J. (2022) 12(1):4. doi: 10.1038/s41408-021-00601-5
13. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. (2004) 64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443
14. Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. (2008) 100(3):184–98. doi: 10.1093/jnci/djm328
15. Tarlock K, Chang B, Cooper T, Gross T, Gupta S, Neudorf S, et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr Blood Cancer. (2015) 62(6):1048–54. doi: 10.1002/pbc.25437
16. Inaba H, Rubnitz JE, Coustan-Smith E, Li L, Furmanski BD, Mascara GP, et al. Phase I pharmacokinetic and pharmacodynamic study of the multikinase inhibitor sorafenib in combination with clofarabine and cytarabine in pediatric relapsed/refractory leukemia. J Clin Oncol. (2011) 29(24):3293–300. doi: 10.1200/JCO.2011.34.7427
17. Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Gotze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. (2012) 26(11):2353–9. doi: 10.1038/leu.2012.105
18. AML-BFM 2012: clinical trial for the treatment of acute myeloid leukemia in children and adolescents.
19. Widemann BC, Kim A, Fox E, Baruchel S, Adamson PC, Ingle AM, et al. A phase I trial and pharmacokinetic study of sorafenib in children with refractory solid tumors or leukemias: a children's oncology group phase I consortium report. Clin Cancer Res. (2012) 18(21):6011–22. doi: 10.1158/1078-0432.CCR-11-3284
20. Fontanelli G, Rocco M, Caracciolo F, Benedetti E, Buda G, Orciuolo E, et al. Sorafenib as monotherapy or in association with cytarabine and clofarabine for the treatment of relapsed/refractory FLT3 ITD-positive advanced acute myeloid leukemia. Clin Lymphoma Myeloma Leuk. (2014) 14(1):e13–7. doi: 10.1016/j.clml.2013.08.005
21. Metzelder S, Wang Y, Wollmer E, Wanzel M, Teichler S, Chaturvedi A, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood. (2009) 113(26):6567–71. doi: 10.1182/blood-2009-03-208298
22. Ernst J, Schafer V, Rinke J, Wittig S, Beck JF, Ernst T, et al. Continuous molecular remission and regression of side effects after discontinuation of salvage therapy with sorafenib and donor lymphocyte infusions in a young patient with relapsed AML. Ann Hematol. (2016) 95(6):1027–30. doi: 10.1007/s00277-016-2637-7
23. Taylor F, Tot C Jr, Hoots W, Wada H, Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. (2001) 86(5):1327–30. doi: 10.1055/s-0037-1616068
24. Dempfle C. Determination of D-dimer antigen in clinical routine. Dtsch Arztebl. (2005) 102(7):428–32.
25. Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. (2010) 149(4):578–86. doi: 10.1111/j.1365-2141.2010.08143.x
26. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v3.0 (CTCAE) (2006).
27. Li Y, Gao ZH, Qu XJ. The adverse effects of sorafenib in patients with advanced cancers. Basic Clin Pharmacol Toxicol. (2015) 116(3):216–21. doi: 10.1111/bcpt.12365
28. McBride A, Trifilio S, Baxter N, Gregory TK, Howard SC. Managing tumor lysis syndrome in the era of novel cancer therapies. J Adv Pract Oncol. (2017) 8(7):705–20. doi: 10.6004/jadpro.2017.8.7.4
29. Bose P, Qubaiah O. A review of tumour lysis syndrome with targeted therapies and the role of rasburicase. J Clin Pharm Ther. (2011) 36(3):299–326. doi: 10.1111/j.1365-2710.2011.01260.x
30. Huang WS, Yang CH. Sorafenib induced tumor lysis syndrome in an advanced hepatocellular carcinoma patient. World J Gastroenterol. (2009) 15(35):4464–6. doi: 10.3748/wjg.15.4464
31. Imam SZ, Zahid MF, Maqbool MA. Sorafenib-induced tumor lysis syndrome in a patient with metastatic hepatocellular carcinoma. Hematol Oncol Stem Cell Ther. (2020) 13(3):168–70. doi: 10.1016/j.hemonc.2018.03.004
32. Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, et al. Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia. (2007) 21(3):439–45. doi: 10.1038/sj.leu.2404508
33. Pratz KW, Cho E, Levis MJ, Karp JE, Gore SD, McDevitt M, et al. A pharmacodynamic study of sorafenib in patients with relapsed and refractory acute leukemias. Leukemia. (2010) 24(8):1437–44. doi: 10.1038/leu.2010.132
34. Crump M, Hedley D, Kamel-Reid S, Leber B, Wells R, Brandwein J, et al. A randomized phase I clinical and biologic study of two schedules of sorafenib in patients with myelodysplastic syndrome or acute myeloid leukemia: a NCIC (national cancer institute of Canada) clinical trials group study. Leuk Lymphoma. (2010) 51(2):252–60. doi: 10.3109/10428190903585286
35. Feldmann F, Schenk B, Martens S, Vandenabeele P, Fulda S. Sorafenib inhibits therapeutic induction of necroptosis in acute leukemia cells. Oncotarget. (2017) 8(40):68208–20. doi: 10.18632/oncotarget.19919
36. Fouladi F, Jehn LB, Metzelder SK, Hub F, Henkenius K, Burchert A, et al. Sorafenib induces paradoxical phosphorylation of the extracellular signal-regulated kinase pathway in acute myeloid leukemia cells lacking FLT3-ITD mutation. Leuk Lymphoma. (2015) 56(9):2690–8. doi: 10.3109/10428194.2014.1003055
37. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. (2019) 381(18):1728–40. doi: 10.1056/NEJMoa1902688
38. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. (2017) 377(5):454–64. doi: 10.1056/NEJMoa1614359
Keywords: hyperleukocytosis, AML, FLT3-ITD, sorafenib, leukostasis, oncologic emergencies
Citation: Schmidt F, Erlacher M, Niemeyer C, Reinhardt D and Klusmann J (2022) Leukoreductive response to the combination of sorafenib and chemotherapy in hyperleukocytosis of FLT3-ITD mutated pediatric AML. Front. Pediatr. 10:1046586. doi: 10.3389/fped.2022.1046586
Received: 16 September 2022; Accepted: 24 October 2022;
Published: 9 November 2022.
Edited by:
Simone Cesaro, Integrated University Hospital Verona, ItalyReviewed by:
Jan Stary, University Hospital in Motol, Czechia© 2022 Schmidt, Erlacher, Niemeyer, Reinhardt and Klusmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan-Henning Klusmann a2tqbS1kaXJla3RvckBrZ3UuZGU=
†These authors have contributed equally to this work and share last authorship
Specialty Section: This article was submitted to Pediatric Oncology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.