
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 29 November 2022
Sec. Pediatric Urology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1045936
This article is part of the Research TopicCase Reports in Pediatric Urology 2022View all 6 articles
Cases of patients with cystic partially differentiated nephroblastoma (CPDN) have been reported to date, which presented as polycystic renal tumor in all of them. It is a special pathological type of nephroblastoma. Here, we report the first case of a unicystic CPDN in a child. The patient was diagnosed with a simple renal cyst and underwent laparoscopic decortication. The naive nephron was found in the pathological section, and the diagnosis of CPDN was confirmed. The patient then underwent a radical nephrectomy and six cycles of postoperative chemotherapy. There was no recurrence or metastasis after 2 years of follow-up. Pediatric CPDN presenting as a unicystic renal tumor poses a new challenge to the diagnosis, differential diagnosis, and treatment of unicystic renal tumor.
Cystic partially differentiated nephroblastoma (CPDN) usually occurs in children under 2 years old, with a male-to-female ratio of about 2:1 (1, 2). It is a rare type of nephroblastoma, which is recognized as a tumor with low malignant potential (3). More than 120 cases of patients with CPDN have been reported to date, and all of them had the gross/macroscopic appearance of a polycystic renal tumor, previously considered to be the only pathological presentation. Clinical features and image studies have failed to discriminate CPDN from cystic nephroma (CN) and cystic Wilms tumor (CWT) (4). Here, we present the first case of pediatric CPDN presenting as a unicystic renal tumor and propose new insights into the differential diagnosis and treatment of unicystic renal tumor.
A 3-year-old girl was diagnosed with a cystic tumor on the left kidney by abdominal ultrasound in another hospital 2 weeks prior after she experienced occasional abdominal pain, but she had no abdominal pain when she presented to our clinic. The reexamined ultrasound showed a cystic tumor inside the left kidney, with a size of 4.6 × 3.9 cm, with a clear boundary, thin wall, and uniform internal sound transmission (Figure 1, yellow arrow). An abdominal enhanced CT showed a cystic low-density lesion inside the left kidney with a clear boundary, about 4.0 × 3.8 × 3.5 cm in size, and without contrast filling. The adjacent renal pelvis was dilated with a diameter of about 1.9 cm, and there was contrast filling in the renal pelvis (Figure 2).
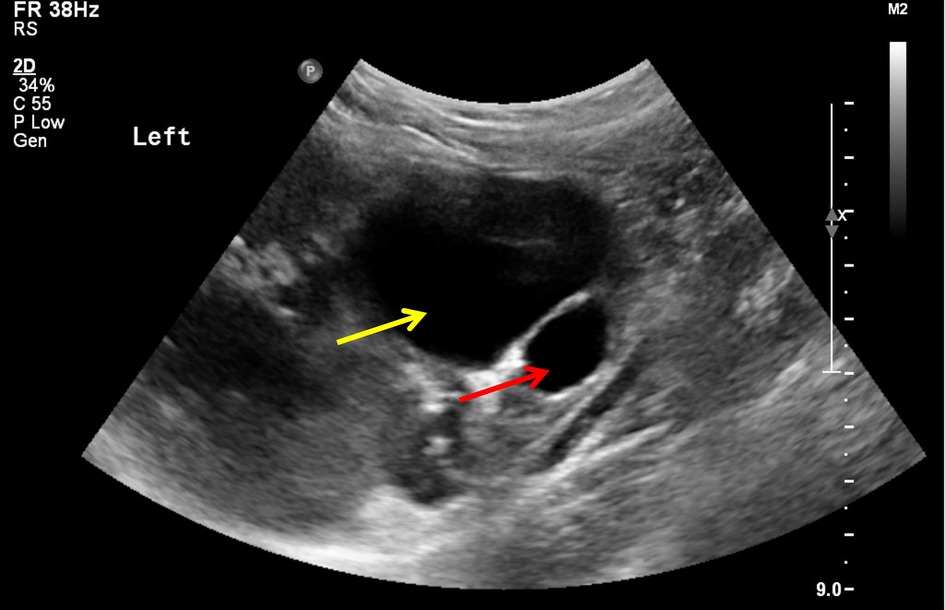
Figure 1. Image of ultrasound: unicystic tumor (yellow arrow) in the left kidney with a clear boundary and uniform internal sound transmission, accompanied by a dilatation of the renal pelvis (red arrow).
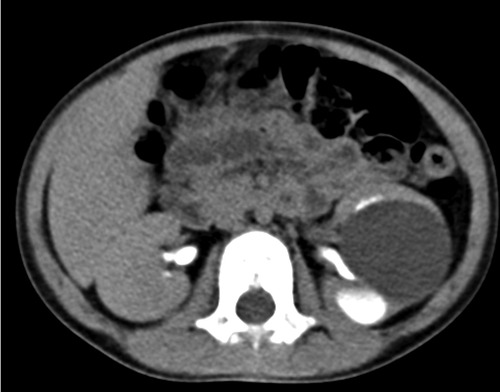
Figure 2. Image of an abdominal enhanced CT: unicystic tumor in the left kidney with a clear boundary but without contrast filling. There was contrast filling in the renal pelvis.
A measurement of the patient’s kidney showed a creatinine level of 33 µmol/L and a urea nitrogen level of 4.6 mmol/L. Tumor marker tests showed carcinoembryonic antigen (CEA) <0.30 ng/ml, alpha fetoprotein (AFP) 2.15 IU/ml, carbohydrate antigen 125 (CA-125) 17.0 U/ml, carbohydrate antigen 19-9 (CA19-9) 9.42 U/ml, β-human chorionic gonadotropin (β-HCG) < 0.20 mIU/ml, neuron-specific enolase (NSE) 11.6 ng/ml, and cytokeratin 19 fragment (CK-19) 1.8 ng/ml. However, the girl’s routine blood test and urinalysis were normal.
The patient was diagnosed with a simple left renal cyst, and she underwent a laparoscopic decortication of the renal cyst. During the operation, a unicystic tumor in the middle and upper pole of the left kidney was revealed, the inner wall of the cyst was smooth, the cystic fluid was clear, and there was no obvious connection between the cyst and the renal pelvis (Figure 3). The cyst wall with an area of 3 × 3 cm was resected. Immature nephrons of different sizes/stages of differentiation were found in the histological sections (Figure 4). The diagnosis of CPDN was confirmed.

Figure 3. Image of a laparoscopic decortication of the renal cyst. After incision of the cyst wall, the inner wall of the cyst became smooth, and the cyst became unicystic.
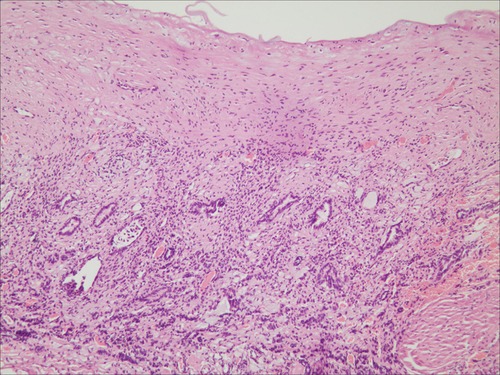
Figure 4. Image of histopathological examination (HE, ×100). The naive nephron was found in the pathological section.
In the second operation, a radical nephrectomy was performed on the left kidney, and the operated area was rinsed with distilled water. No abnormal lymph nodes were found. The second histopathological examination also showed CPDN, with the immunohistochemistry showing vimentin (+), Wilms tumor protein (+), epithelial membrane antigen (+), acidic calcium-binding protein S-100 (−), neuron-specific enolase (−), and a protein Ki-67 index of 17%.
Six cycles of chemotherapy were performed after the operation, and the chemotherapy protocol was vincristine combined with dactinomycin. There was no recurrence of the tumor and no occurrence of metastasis after 2 years of follow-up.
Brown standardized the naming of CPDN for the first time in 1975 (5). He suggested that CNPD was a low-grade malignant or malignant renal cystic tumor and that WT could undergo spontaneous maturation to a fully differentiated, benign, and cystic tumor. Previous studies have suggested that WT, CPDN and CN represent different degrees of differentiation in the same differentiation spectrum, with WT being the least differentiated and CN being the most differentiated ends of the spectrum and CPDN being the transition between the two (6).
A complete search of the PubMed, EMBASE, and Wanfang databases was performed in the period between January 1975 and May 2022 to identify all reports describing pediatric CPDN. We also included articles that described CPDN, as this is the most important differential diagnosis in children with CWT and CN. A cross-reference check was used to identify potential additional reports. For multiple case reports from the same author or institution, we checked each case carefully to avoid the same case being repeatedly included in the study. Cases with a high probability of repetition were also excluded from the study. The inclusion criteria of the articles included the following conditions: First, the article had to report well-described pediatric patients with CPDN. Second, the article had to be an original one. Third, the article had to be available as a full text or a well-described abstract. Fourth, the article had to be written in English, Spanish, Chinese, or Dutch. All manuscripts were screened by two independent reviewers (Liu Chao and Li Xiang). A total of 121 cases of pediatric CPDN were retrieved from 48 articles. In all cases, the tumors were polycystic, as described by surgical specimen pictures or literature. A polycystic renal tumor was considered the only manifestation of CPDN (7). However, the case that we report in this study is a patient with an unicystic renal tumor, which is the first of its kind.
CPDN has no specific clinical characteristics, and most patients show only a painless abdominal mass found unintentionally or a renal tumor found by abdominal ultrasound or CT (8). In the statistical literature, abdominal mass accounted for 87.1% (54/62) of cases. Our present case is a patient with a unicystic renal tumor inadvertently found by ultrasonography.
At present, establishing the correct diagnosis of CPDN requires a careful histopathological examination of the resected tumor because imaging studies have failed to discriminate among CPDN, CN, and CWT (9). In 1998, Eble and Bonsib proposed a complete diagnostic method for CPDN (1). The patients in their study were mainly children under 2 years old. The tumor is an expansive mass surrounded by a fibrous pseudocapsule, and the interior of the tumor is fully composed of cysts and septa, without expansive solid nodules. The cyst is lined by a flattened, hobnail, or cuboidal epithelium. The septa may contain epithelial structures resembling mature renal tubules and may also contain blastema, with or without embryonic stromal or epithelial elements.
Our patient was initially misdiagnosed as having a simple renal cyst because of the unicystic manifestation of the tumor, but the final histopathological examination confirmed the diagnosis of CPDN. The unicystic manifestation of CPDN not only enriches our understanding of CPDN but also poses a new challenge to the diagnosis and differential diagnosis of unicystic renal tumor. We consider that some of the diagnostic criteria proposed by Eble and Bonsib should be further improved. The tumor is composed of single or multiple cysts, without solid expandable nodules, with or without septum. The septum or cyst wall may contain epithelial structures that are immature or resemble mature renal tubules.
For polycystic renal tumors such as CN, CPDN, and CWT, tumorectomy or nephrectomy is the most common surgical method, and biopsies to confirm the diagnosis are not encouraged (10–12). Our study involved an examination of the kidneys of 125 patients, of which 119had polycystic CPDN, and out of the 125, 86 patients underwent nephrectomy, 15 underwent partial nephrectomy, and information on the remaining 24 was not available. However, these surgical methods may not be completely applicable to unicystic renal tumor because the incidence of simple renal cysts is much higher than that of unicystic CPDN, and simple renal cysts are usually operated upon by a decortication of the renal cyst (13).
We considered that partial tumorectomy or a decortication of the renal cyst can still be used preferentially for unicystic renal tumors, without excluding the possibility of CPDN, but the role of the intraoperative frozen section during surgery should be emphasized (14). Although tumorectomy or decortication of the renal cyst may lead to a rupture of CPDN, resulting in a change in the tumor stage from stage I to stage III, we can further treat it by washing the operated area with distilled water and postoperative chemotherapy. The patient we reported received intraoperative distilled water washing and six cycles of chemotherapy after the operation, and there was no recurrence of the tumor or occurrence of metastasis after 2 years of follow-up, which may be related to the low malignancy and low invasiveness of CPDN.
According to the conclusions provided by the International Society of Pediatric Oncology (SIOP), preoperative chemotherapy cannot effectively reduce the size of a CPDN tumor (9). The 10 CPDN patients examined in the study were treated with preoperative chemotherapy, which in all cases did not reduce the size of the tumor. As recommended by the National Wilms Tumor Study Group (NWTSG), for stage I CPDN, only surgical resection is required. However, for stage II and stage III CPDN, the tumor cannot be resected completely, and postoperative chemotherapy is considered effective (15). In the statistical literature, it has been reported that 47 patients received postoperative chemotherapy. Although a few patients had different manifestations of chemotherapy complications, there was no death directly related to chemotherapy. Therefore, postoperative chemotherapy is recommended for intraoperative tumor rupture caused by a misdiagnosis of CPDN as a simple renal cyst or other benign tumors.
The postoperative recurrence rate of CPDN was low (4). The 92 patients examined in the study were reported for follow-up, including 3 patients with local recurrence and 1 with CWT recurrence after undergoing partial nephrectomy for bilateral CPDN. Chemotherapy was given after recurrence for these 4 patients, and the survival rate was good during the follow-up period. There were no deaths directly related to CPDN or recurrence (4).
In summary, CPDN is rarely reported in the clinic and can be easily confused with CN and CWT. In this study, a unicystic pediatric CPDN is reported for the first time, which poses a new challenge to the diagnosis and treatment of renal cystic tumor. There are no specific clinical characteristics of pediatric CPDN. Surgery is the first choice of treatment, and histopathological examination is the only means of diagnosis. For patients with stage II CPDN or above, postoperative chemotherapy is recommended. The recurrence rate of CPDN is low, and the overall prognosis is good.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Qilu Hospital of Shandong University (Qingdao) (KYLL-KS-2021044). Written informed consent to participate in this study was provided by the participant's legal guardian/next of kin.
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
LC edited the manuscript. LX and ZQ contributed to the sample collection. LC and ZL revised the paper. All authors contributed to the article and approved the submitted version.
LC was supported by the Qingdao Medical and Health Research Program (2021-WJZD214) and the Research Foundation of Qilu Hospital of Shandong University (Qingdao) (QDKY2021QN04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Luithle T, Szavay P, Furtwängler R, Graf N, Fuchs J. Treatment of cystic nephroma and cystic partially differentiated nephroblastoma – a report from the SIOP/GPOH study group. J Urol. (2007) 177(1):294–6. doi: 10.1016/j.juro.2006.09.011
2. Ben Hammouda S, Ben Abdeljelil N. A rare pediatric renal tumor: cystic partially differentiated nephroblastoma. Clin Case Rep. (2021) 9(10):e04929. doi: 10.1002/ccr3.4929
3. Tiryaki T, Hücümenoğlu S, Livanelioğlu Z, Atayurt H. Cystic partially differentiated nephroblastoma. A case report. Urol Int. (2003) 70(3):223–6. doi: 10.1159/000068754
4. van Peer SE, Pleijte CJH, de Krijger RR, Jongmans MCJ, Kuiper RP, Lilien MR, et al. Clinical and molecular characteristics and outcome of cystic partially differentiated nephroblastoma and cystic nephroma: a narrative review of the literature. Cancers (Basel). (2021) 13(5):997. doi: 10.3390/cancers13050997
5. Brown JM. Cystic partially differentiated nephroblastoma. J Pathol. (1975) 115(3):175–8. doi: 10.1002/path.1711150307
6. Cordeiro LPV, Carvalho ACM, Silva IM, Martins FP, Amaro AP, Carvalho EM. Cystic partially differentiated nephroblastoma: a rare pediatric renal tumor-case report. Radiol Case Rep. (2020) 15(8):1133–7. doi: 10.1016/j.radcr.2020.05.001
7. van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, van Tinteren H, Furtwängler R, Verschuur AC, et al. Position paper: rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol. (2017) 14(12):743–52. doi: 10.1038/nrurol.2017.163
8. Roy P, van Peer SE, de Witte MM, Tytgat GAM, Karim-Kos HE, van Grotel M, et al. Characteristics and outcome of children with renal tumors in the Netherlands: the first five-year's experience of national centralization. PLoS One. (2022) 17(1):e0261729. doi: 10.1371/journal.pone.0261729
9. van den Hoek J, de Krijger R, van de Ven K, Lequin M, van den Heuvel-Eibrink MM. Cystic nephroma, cystic partially differentiated nephroblastoma and cystic Wilms' tumor in children: a spectrum with therapeutic dilemmas. Urol Int. (2009) 82(1):65–70. doi: 10.1159/000176028
10. Vujanić GM, Gessler M, Ooms AHAG, Collini P, Coulomb-l'Hermine A, D'Hooghe E, et al. The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol [published correction appears in Nat Rev Urol. 2019 Sep;16(9):563]. Nat Rev Urol. (2018) 15(11):693–701. doi: 10.1038/s41585-018-0100-3
11. De Souza LMF, De Q Turíbio DDC, De Souza JPF, Oliveira RS, Lopes IC, De C Dantas AK, et al. Cystic nephroma in pediatrics. Andes Pediatr. (2021) 92(5):754–9. doi: 10.32641/andespediatr.v92i5.2622
12. Jain P, Prasad A, Jain S, Rahul KM. Cystic nephroma and its varied management scenarios: a report of two cases. J Indian Assoc Pediatr Surg. (2021) 26(4):268–70. doi: 10.4103/jiaps.JIAPS_152_20
13. Hong Y, Chen X, Wu M, Xi H, Hu J. Percutaneous vs laparoscopic treatment for simple renal cysts: a meta-analysis. J Endourol. (2021) 35(12):1793–800. doi: 10.1089/end.2021.0264
14. Jütte H, Tannapfel A. Intraoperativer Schnellschnitt – wann sinnvoll, wann notwendig? [Intraoperative rapid frozen section – when meaningful, when necessary?]. Chirurg. (2020) 91(6):456–60. doi: 10.1007/s00104-020-01115-9
Keywords: case report, pediatric, unicystic, cystic partially differentiated nephroblastoma, first
Citation: Chao L, Lei Z, Xiang L and Qi Z (2022) Pediatric unicystic cystic partially differentiated nephroblastoma: The first case report. Front. Pediatr. 10:1045936. doi: 10.3389/fped.2022.1045936
Received: 16 September 2022; Accepted: 8 November 2022;
Published: 29 November 2022.
Edited by:
Walid Farhat, University of Wisconsin–Madison, United StatesReviewed by:
Clecio Picarro, Federal University of Minas Gerais, Brazil© 2022 Chao, Lei, Xiang and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhang Lei cWx5eXFkZXdrMTFAMTYzLmNvbQ==
Specialty Section: This article was submitted to Pediatric Urology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.