- 1Department of Pediatric Dentistry, School of Dentistry, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 2Department of Pharmaceutics, School of Pharmacy, Nanotechnology Research Center, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 3Department of Food and Drug Control, Faculty of Pharmacy, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 4Department of Biostatistics and Epidemiology, School of Public Health, Alimentary Tract Research Center, Clinical Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 5Infectious and Tropical Diseases Research Center, Health Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Background: Children in mixed dentition are highly at risk for dental caries, which is a major health issue worldwide. Despite their effect in controlling dental caries, using probiotics can be challenging. Therefore, it has been advised to use their inanimate forms, called postbiotics. We hypothesize that postbiotics can enhance the oral immunity.
Methods: The aim of this triple-blind, randomized, placebo-controlled trial is to investigate the effect of postbiotic-toothpaste (Bifidobacterium animalis subsp. animalis) on salivary levels of Immunoglobulin A (IgA) and pH in children. Using comparing two means formula to calculate the sample size, for this trial 80 healthy 6- to 12-year-old children during mixed dentition with no cavitated dental caries will be selected by convenience sampling method and randomly allocated to two groups, postbiotic-toothpaste or placebo-toothpaste. Saliva samples will be gathered at baseline and four weeks after the intervention. The level of salivary IgA will be determined by ELISA and salivary pH will be measured using a pH meter. Data will be compared within and between groups using independent t-test and paired t-test, in case of normality, with a p < 0.05 as statistically significant.
Discussion: If postbiotics-toothpaste prove to be effective in improving the oral immunity, they can be used to prevent dental caries and other oral diseases. The result of this study can help researchers who are working on the immunomodulatory effects of postbiotics in children.
Trial registration number: Iranian Registry of Clinical Trials (IRCT), IRCT20191016045128N2. Registered on 7 March 2022.
Background
Background and rationale
Dental caries, the most prevalent oral disease, is still a major health issue worldwide (1). This multifactorial dynamic disease results in mineral loss of dental structure, is dental-biofilm-mediated, and can be modulated by diet (2). In children, it can affect their mastication function, alongside their smile and manner of speaking, and can be a significant blow to their oral health-related quality of life (3).
Children in mixed dentition, which usually begins at six years of age with the eruption of the first permanent tooth and continues until the exfoliation of the last primary tooth at around 12 years of age, are highly at risk for dental caries (4). This is mainly because teeth are most prone to caries in the first 2–4 years of eruption (5). Moreover, erupting teeth are out of alignment with their adjacent teeth and the gingiva in mixed dentition is sensitive and tender, making dental hygiene a challenge for children and their parents (4).
Contrary to the common belief that the rate of dental caries has declined in industrialized countries, this disease is still highly prevalent in these countries (6). It is believed that in European countries, 61% of 6- to 12-year-old children have experienced dental caries (7). A systematic review recently pointed out that the prevalence of dental caries has increased over the past few years in countries from the Middle East and North Africa region (6). So, it is evident that there is a great need for preventive interventions to decrease caries prevalence in children worldwide.
A rather novel preventive method is the usage of probiotics, which seems to be quite effective in controlling dental caries (8). Probiotics are “live microorganisms that confer a health benefit on the host when administered in adequate amounts” (9). Throughout the years, several studies have evaluated the effects of probiotics on oral health and stated that probiotics can reduce the numbers of pathogenic microorganisms, replace them (10, 11), and prevent or treat oral candidiasis, periodontitis, gingivitis, and halitosis (12, 13). Additionally, previous studies indicated the capability of probiotics to enhance the oral immune system and increase salivary Immunoglobulin A (IgA) secretion (14, 15).
Salivary IgA is an antimicrobial protein that is the first immune defense against Streptococcus mutans, the primary cariogenic bacteria. It inhibits bacterial adherence and reduces their colonization (16). A recent systematic review showed that, salivary IgA can have protective effects against dental caries (17).
Withal, it has recently been brought to attention that despite the aforementioned health benefits of probiotics, their usage can be quite challenging. Probiotics viability, storage stability, unfavorable effects in immunocompromised patients, antibiotic resistance, and transmission potential are a few of the issues (18, 19). To confront the side effects of probiotics, the usage of their inanimate form, also known as postbiotics, is increasingly gaining interest. According to the recent consensus statement of the International Scientific Association of Probiotics and Prebiotics (ISAPP), postbiotics are “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” (20). They have been shown to have an inhibitory influence on biofilm formation and alveolar bone loss (19), as well as antibacterial, antiviral, and antioxidant activities (18). Few studies, however, have evaluated the efficacy of postbiotics in enhancing oral immunity.
All in all, there seems to be a lack of enough data to fully support the hypothesis that postbiotics can elevate the salivary IgA concentration. In this protocol, a four-week triple-blind, placebo-controlled, randomized trial, investigating the effects of postbiotics on salivary IgA concentration in 6- to 12-year-old children is reported in detail.
Objectives
In a study of 80 children, we aim to investigate if daily usage of postbiotic-toothpaste for 30 days can affect salivary levels of IgA in healthy 6- to 12-year-old children. Our secondary aim is to examine the impact of the intervention on salivary pH levels of the children. We hypothesize that daily usage of the postbiotic-toothpaste can elevate the salivary IgA concentration and improve oral immunity.
Trial design
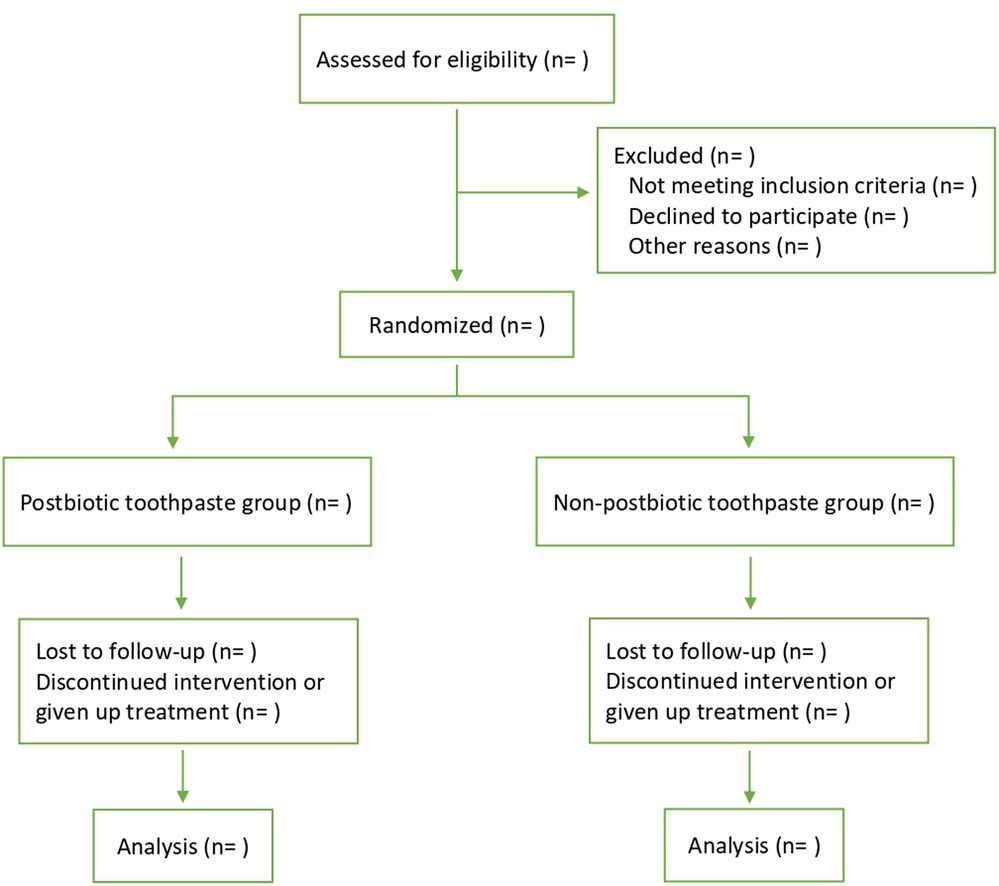
This will be a four-week, single-center, institutional-based, triple-blind, parallel, placebo-controlled, randomized clinical trial with a convenience sampling technique. Figure 1 shows the flow diagram of the study design from screening the patients to follow-up. This protocol is written according to the SPIRIT 2013 statement (21).
Methods
Study setting
The outpatients presenting to the Department of Pediatric Dentistry, School of Dentistry, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran will be recruited.
Eligibility criteria
The inclusion criteria will be as followed: healthy, 6- to 12-year-old children, during the mixed dentition, no active or untreated carious lesions, no severe crowding, no marked inflammation in oral cavity.
The exclusion criteria will be the presence of any inflammatory disease and use of any medications, supplements, or probiotic intake within the last two weeks or during the intervention. The patients who fail to follow-up will also be excluded from the study.
Study product and intervention
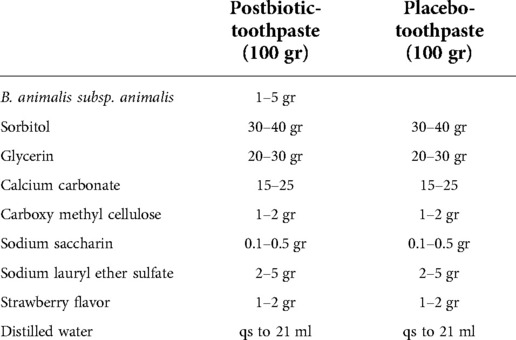
The postbiotic strain Bifidobacterium animalis subsp. animalis PTCC1631 will be used. The intervention will be in form of a postbiotic-toothpaste (B. animalis subsp. animalis, 109CFU/g (22)). The postbiotic strain was ordered from the Persian Type Culture Collection center, a subset of the Iranian Research Organization for Science and Technology. The postbiotic and placebo-toothpaste are similar in packaging, color, taste, and scent, and the only difference is the absence of the postbiotic ingredient in the placebo-toothpaste. Table 1 shows the ingredients of the toothpastes.
Eligible participants will be randomly allocated to two groups, intervention and control. The postbiotic-toothpaste will be given to the children in the intervention group and the children in the control group will receive the placebo-toothpaste. Children in both groups will be asked to brush their teeth twice a day (in the morning and at night) for two minutes, under the supervision of their parents, for four weeks. To eliminate any possible confounding effect of the method of tooth-brushing on the results, children and their parents will be instructed on how, when, and how long to brush their teeth at the beginning of the study.
In order to ensure participants adherence, parents will receive a message on a weekly basis to remind them the importance of tooth-brushing twice a day. Also, to motivate and encourage the children, they will be asked to record daily short videos of themselves while brushing their teeth and put it all together at the end of the four weeks.
Outcomes
The primary outcome of this study will be the salivary IgA levels of the children and will be reported in mg/100ml. The secondary outcome will be the pH levels of saliva in children. The primary and secondary outcomes will be assessed at the baseline, before any intervention, and after four weeks of intervention.
Sample size
To calculate the sample size, we used the following comparing two means formula (, significance; ) based on the available literature (23) with maximum 30% falls during the follow-up, the final sample size was 40 participants per arm, 80 in total.
The participants will be selected using the convenience non probability sampling method. So, from the beginning of the study, any patient who meets the inclusion criteria and does not have the exclusion criteria will be selected and this process will continue until the sample size is reached.
Randomization and blinding
To limit the potential confounding effect of the age of the children, they will be randomly allocated to two groups. Participants will be randomized according to the method of block randomization in blocks of six using a computer-generated random number sequence. This process will be done by an independent statistician who will also give the sealed envelope of the treatment codes to the pharmacist in charge of the production of toothpastes. Each of the eligible patients accepted into the study will be assigned a number from a consecutive range. This number will then be sent to the pharmacist.
During the study, the participants, the outcome assessor, and the data analyst will be blinded and will not have access to the nature of the toothpaste the subjects will be testing. The product will be divided into opaque sealed bags and the labels will not show the difference between the postbiotic and the placebo toothpastes. In case of a serious and adverse event, the blind will be broken and the pharmacist will have access to the sealed envelopes.
Data collection
At the beginning of the study, demographic information (age and gender), medical history, accompanying medication, and any usage of antibiotics and probiotics will be recorded. The eligible subjects will be told not to eat or drink anything for one hour before the saliva collection. Afterwards, one ml of the unstimulated whole saliva of children will be collected into sterilized plastic test tubes using the spitting method between 9.00 and 12.00 a.m. under controlled temperature (23°C). The saliva samples will be kept at −80°C until they are sent to laboratory, where the levels of salivary IgA will be determined with enzyme-linked immunosorbent assay (ELISA) previously described in detail by Sonesson et al. (24). The 96 well-plates will be coated overnight by anti-human α-chain-specific IgA. The next day, the wells will be washed with Phosphate-Buffered Saline (PBS) containing 0.05% Tween-20 and blocked by 1% Bovine Serum Albumin (BSA) at 37°C for 1 h. Diluted saliva samples and standards will be incubated in wells for 1 h at 37°C. The wells will be washed and biotinylated anti-human IgA antibody will be added. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin will be pipetted to the wells. The wells will again be washed, a TMB substrate solution will be added to the wells and color develops in proportion to the amount of IgA bound. The Stop Solution will change the color from blue to yellow, and the intensity of the color will be measured at 450 nm. The results will be plotted against a standard curve and multiplied with the dilution factor. Immediately after sample collection in order to prevent degradation of the saliva, pH analysis will be carried out using portable pH meter (Hanna Instruments®, HI98191, RI, United States) which will be calibrated with distilled water for each time of use. At the follow-up session, outcomes will be reevaluated accordingly. A trained pediatric dentist will collect all the data. Figure 2 presents schedule of enrolment, intervention, and assessments.
Statistical analysis
The analysis of the data will be based on the intention-to-treat analysis and all recruited participants will be considered. Data normality will be examined using the Kolmogorov-Smirnov test. The variables will be reported as mean ± SD or median. The percentage changes for each variable will be calculated using the following formula in which E and B stand for the end value and the baseline value of the variable, respectively.
In case of meeting the assumption of normality, the significant changes between the two groups will be evaluated using the independent t-test, otherwise, Mann-Whitney analysis will be used. Comparison of the mean of variables at baseline with that of post-intervention within each group will be conducted using paired t-test or Wilcoxon signed-rank test. In order to control any possible confounding variable, the analysis of covariance (ANCOVA) will be used when necessary.
All analyses will be performed using the Statistical Package for Social Sciences, version 22.0 (SPSS, Inc., Chicago, IL, United States) with statistical significance at p < 0.05.
Monitoring
To maintain the accuracy and quality of the trial, and to check its compliance with the protocol, it will be monitored regularly by research associates. The findings of the trial monitoring will be reviewed by the Data Monitoring Committee (DMC).
Adverse events
Since the study product will be in form of a toothpaste, minimal harm is expected. However, an adverse event (AE) has been defined as any unfavorable discomfort in the mouth during the study period. All the children and their parents will be advised to report any AE immediately and in case of AE, participant(s) will be excluded from the study.
Discussion
Existing literature indicates that postbiotics have advantageous effects on the human body. They have been proven to be effective in preventing diarrhea, acute gastroenteritis, pharyngitis, and laryngitis (24), and reducing respiratory tract infection in children (25). Numerous in vitro and in vivo studies have also shown the immunomodulatory effects of postbiotics, such as enhancing anti-inflammatory cytokines production (26), inhibiting tumor necrosis factor (TNF-α) secretion (27), and promoting T helper one immune response (28). In addition, probiotics and fermented foods supplementation significantly increased salivary IgA secretion rate (15). There have been few studies, however, that evaluated the influence of postbiotics on the secretion of salivary IgA.
Hishiki et al. in 2020 evaluated the effect of a heat-killed probiotic strain on viral infection of the respiratory tract in 3- to 6-year-old children and stated that after four months of intervention, the levels of secretory IgA in saliva were higher in the postbiotic group than that of the placebo group. Although, the results were not significant (29).
In another study in 2021, Lin et al. compared the effect of viable and heat-killed probiotic strains on oral immunity and found that after four weeks of viable and heat-killed probiotic administration, salivary IgA levels significantly increased in both groups (14).
In 2022, Lin et al. evaluated the effects of heat-killed probiotics and postbiotics on oral health and concluded that after four weeks of intervention, the levels of salivary IgA increased in both groups, and postbiotic supplementation can improve oral immunity (30).
Considering the shortage of clinical studies on the administration of postbiotics to increase oral immunity in children, through this randomized controlled trial, we propose to inspect the effect of postbiotics on children's oral immunity. If postbiotics-toothpaste prove to be effective in enhancing the oral immunity of children, they can be used as a new prophylactic method to prevent dental caries and other oral diseases. We expect the result of this study to be of great help to researchers working on the immunomodulatory effects of postbiotics in children.
Ethics statement
Based on the Helsinki Declaration this trial was approved by the Medical Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (ethical code: IR.AJUMS.REC.1400.676). It was also prospectively registered at the Iranian Registry of Clinical Trials (IRCT Id: IRCT20191016045128N2). A voluntary written informed consent will be obtained from children's parents or legal guardian prior to inclusion. The collected data will be held confidential.
Author contributions
L.B., E.M., A.S., and S.Kh. participated in the study design and protocol development. S.Kh. collected the data adhering to the study protocol. B.Ch. provided statistical advice and input. L.B., E.M., and B.Ch. participated in the preparation of the study protocol by providing comments on drafts written by S.Kh. All authors contributed to the article and approved the submitted version.
Funding
The Ahvaz Jundishapur University of Medical Sciences financially supported this project under the research grant no. 0054. The funding body will not have any involvement in any stage of the study.
Acknowledgment
The authors wish to express their gratitude to the pharmacist in charge of the making of the toothpastes.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IgA, immunoglobulin A; ISAPP, international scientific association of probiotics and prebiotics; TNF-α, tumor necrosis factor.
References
1. World Health Organization. Oral Health 2022, March 15 [Available at: https://www.who.int/news-room/fact-sheets/detail/oral-health
2. Machiulskiene V, Campus G, Carvalho JC, Dige I, Ekstrand KR, Jablonski-Momeni A, et al. Terminology of dental caries and dental caries management: consensus report of a workshop organized by ORCA and cariology research group of IADR. Caries Res. (2020) 54(1):7–14. doi: 10.1159/000503309
3. Mathur VP, Dhillon JK. Dental caries: a disease which needs attention. Indian J Pediatr. (2018) 85(3):202–6. doi: 10.1007/s12098-017-2381-6
4. Lynch RJ. The primary and mixed dentition, post-eruptive enamel maturation and dental caries: a review. Int Dent J. (2013) 63:3–13. doi: 10.1111/idj.12074
5. Carlos J, Gittelsohn A. Longitudinal studies of the natural history of caries—iI: a life-table study of caries incidence in the permanent teeth. Arch of Oral Biol. (1965) 10(5):739–51. doi: 10.1016/0003-9969(65)90127-5
6. Elamin A, Garemo M, Mulder A. Determinants of dental caries in children in the Middle East and north Africa region: a systematic review based on literature published from 2000 to 2019. BMC Oral Health. (2021) 21(1):1–30. doi: 10.1186/s12903-021-01482-7
7. Kazeminia M, Abdi A, Shohaimi S, Jalali R, Vaisi-Raygani A, Salari N, et al. Dental caries in primary and permanent teeth in children's Worldwide, 1995 to 2019: a systematic review and meta-analysis. Head Face Med. (2020) 16(1):1–21. doi: 10.1186/s13005-020-00237-z
8. Amargianitakis M, Antoniadou M, Rahiotis C, Varzakas T. Probiotics, prebiotics, synbiotics and dental caries. New perspectives, suggestions, and patient coaching approach for a cavity-free mouth. Appl Sci. (2021) 11(12):5472. doi: 10.3390/app11125472
9. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11(8):506–14. doi: 10.1038/nrgastro.2014.66
10. Sengupta P, Desai PD, Maity I, Mazumdar P, Biswas S, Choudhury SR, et al. Comparative evaluation of different probiotic products on salivary Streptococcus mutans and Lactobacillus level in caries risk population. J Conserv Dent. (2020) 23(6):619. doi: 10.4103/JCD.JCD_467_20
11. Voidarou C, Antoniadou M, Rozos G, Tzora A, Skoufos I, Varzakas T, et al. Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues. Foods. (2020) 10(1):69. doi: 10.3390/foods10010069
12. Ito R, Mine Y, Yumisashi Y, Yoshioka R, Hamaoka M, Taji T, et al. In vivo efficacy of lacticaseibacillus rhamnosus L8020 in a mouse model of oral candidiasis. J Fungi. (2021) 7(5):322. doi: 10.3390/jof7050322
13. Chugh P, Dutt R, Sharma A, Bhagat N, Dhar MS. A critical appraisal of the effects of probiotics on oral health. J Funct Foods. (2020) 70:103985. doi: 10.1016/j.jff.2020.103985
14. Lin W-Y, Kuo Y-W, Chen C-W, Huang Y-F, Hsu C-H, Lin J-H, et al. Viable and heat-killed probiotic strains improve oral immunity by elevating the IgA concentration in the oral Mucosa. Curr Microbiol. (2021) 78(9):3541–9. doi: 10.1007/s00284-021-02569-8
15. Kazemi A, Soltani S, Nasri F, Clark CC, Kolahdouz-Mohammadi R. The effect of probiotics, parabiotics, synbiotics, fermented foods and other microbial forms on immunoglobulin production: a systematic review and meta-analysis of clinical trials. Int J Food Sci Nutr. (2021) 72(5):632–49. doi: 10.1080/09637486.2020.1857710
16. Lo Giudice G, Nicita F, Militi A, Bertino R, Matarese M, Currò M, et al. Correlation of s-IgA and IL-6 salivary with caries disease and oral hygiene parameters in children. Dent J (Basel). (2019) 8(1):3. doi: 10.3390/dj8010003
17. Wu Z, Gong Y, Wang C, Lin J, Zhao J. Association between salivary s-IgA concentration and dental caries: an updated meta-analysis. Biosci Rep. (2020) 40(12):BSR20203208. doi: 10.1042/BSR20203208
18. Aghebati-Maleki L, Hasannezhad P, Abbasi A, Khani N. Antibacterial, antiviral, antioxidant, and anticancer activities of postbiotics: a review of mechanisms and therapeutic perspectives. Biointerface Res Appl Chem. (2022) 12(2):2629–45. doi: 10.33263/BRIAC122.26292645
19. Jung J-I, Baek S-M, Nguyen TH, Kim JW, Kang C-H, Kim S, et al. Effects of probiotic culture supernatant on cariogenic biofilm formation and RANKL-induced osteoclastogenesis in RAW 264.7 macrophages. Molecules. (2021) 26(3):733. doi: 10.3390/molecules26030733
20. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol & Hepatol. (2021) 18(9):649–67. doi: 10.1038/s41575-021-00440-6
21. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
22. Elgamily H, Safwat E, Soliman Z, Salama H, El-Sayed H, Anwar M. Antibacterial and remineralization efficacy of casein phosphopeptide, glycomacropeptide nanocomplex, and probiotics in experimental toothpastes: an in vitro comparative study. Eur J Dent. (2019) 13(03):391–8. doi: 10.1055/s-0039-1693748
23. Braathen G, Ingildsen V, Twetman S, Ericson D, Jørgensen M. Presence of Lactobacillus reuteri in saliva coincide with higher salivary IgA in young adults after intake of probiotic lozenges. Benef Microbes. (2017) 8(1):17–22. doi: 10.3920/BM2016.0081
24. Malagón-Rojas JN, Mantziari A, Salminen S, Szajewska H. Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients. (2020) 12(2).
25. Żółkiewicz J, Marzec A, Ruszczyński M, Feleszko W. Postbiotics-A step beyond Pre- and probiotics. Nutrients. (2020) 12(8):2189. doi: 10.3390/nu12082189
26. Jensen GS, Benson KF, Carter SG, Endres JR. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. (2010) 11:15. doi: 10.1186/1471-2172-11-15
27. Hoarau C, Martin L, Faugaret D, Baron C, Dauba A, Aubert-Jacquin C, et al. Supernatant from bifidobacterium differentially modulates transduction signaling pathways for biological functions of human dendritic cells. PLoS One. (2008) 3(7):e2753. doi: 10.1371/journal.pone.0002753
28. Ménard S, Laharie D, Asensio C, Vidal-Martinez T, Candalh C, Rullier A, et al. Bifidobacterium breve and Streptococcus thermophilus secretion products enhance T helper 1 immune response and intestinal barrier in mice. Exp Biol Med (Maywood). (2005) 230(10):749–56. doi: 10.1177/153537020523001008
29. Hishiki H, Kawashima T, Tsuji NM, Ikari N, Takemura R, Kido H, et al. A double-blind, randomized, placebo-controlled trial of heat-killed Pediococcus acidilactici K15 for prevention of respiratory tract infections among preschool children. Nutrients. (2020) 12(7):1989. doi: 10.3390/nu12071989
Keywords: probiotics, oral health, immunity, proteins, serum globulins, hydrogen-Ion concentration, saliva
Citation: Basir L, Moghimipour E, Saadatzadeh A, Cheraghian B and Khanehmasjedi S (2022) Effect of postbiotic-toothpaste on salivary levels of IgA in 6- to 12-year-old children: Study protocol for a randomized triple-blind placebo-controlled trial. Front. Pediatr. 10:1042973. doi: 10.3389/fped.2022.1042973
Received: 13 September 2022; Accepted: 14 November 2022;
Published: 12 December 2022.
Edited by:
Tatiana Fidalgo, Rio de Janeiro State University, BrazilReviewed by:
Lucianne Cople Maia, Federal University of Rio de Janeiro, BrazilPrathip Phantumvanit, Thammasat University, Thailand
Dorota Olczak-Kowalczyk, Medical University of Warsaw, Poland
© 2022 Basir, Moghimipour, Saadatzadeh, Cheraghian and Khanehmasjedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Samaneh Khanehmasjedi bWFzamVkaS5zYW1hbmVoQHlhaG9vLmNvbQ==
†ORCID Leila Basir orcid.org/0000-0002-1684-1774 Eskandar Moghimipour orcid.org/0000-0002-6686-2485 Afrooz Saadatzadeh orcid.org/0000-0002-3275-5374 Bahman Cheraghian orcid.org/0000-0001-5446-6998 Samaneh Khanehmasjedi orcid.org/0000-0001-7736-7773
Specialty section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Leila Basir1,†
Leila Basir1,† Samaneh Khanehmasjedi
Samaneh Khanehmasjedi