
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 18 November 2022
Sec. Pediatric Endocrinology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1038345
 Raeesha Rajan1,2,3
Raeesha Rajan1,2,3 Uma Athale1,4
Uma Athale1,4 Joycelyne Efua Ewusie5
Joycelyne Efua Ewusie5 Karen McAssey1,2
Karen McAssey1,2 Lehana Thabane3,5,6,7
Lehana Thabane3,5,6,7 M. Constantine Samaan1,2,3*
M. Constantine Samaan1,2,3*
Background: The COVID-19 pandemic led to substantial shifts in pediatric diabetes care delivery to virtual and hybrid models. It is unclear if these changes in care delivery impacted short-term patient outcomes.
Objectives: We aimed to explore glycemic control and other diabetes-related outcomes in children living with Type 1 Diabetes Mellitus (T1DM) during the first year of the COVID-19 pandemic at a tertiary pediatric academic center in Canada.
Subjects: Patients <18 years of age with a confirmed diagnosis of T1DM for at least one year were included.
Methods: This was a retrospective chart review. We compared data from two years pre-pandemic (March 15, 2018–March 14, 2020) to the first year of the pandemic (March 15, 2020–March 14, 2021). The data assessed included glycemic control [Hemoglobin A1c (HbA1c)], diabetic ketoacidosis (DKA), hospital attendance and hospitalizations, hypoglycemia, and hyperglycemia. The generalized estimating equation (GEE) analysis was used to model potential factors affecting the HbA1c and diabetes-related morbidities. Multiple imputations were conducted as a sensitivity analysis.
Results: There were 346 eligible patients included in the study. The HbA1c remained stable during the pandemic compared to the pre-pandemic phase (MD-0.14, 95% CI, −0.28, 0.01; p = 0.058). The pandemic saw an increase in the number of newly diagnosed patients (X2 = 16.52, p < 0.001) and a higher number of newly diagnosed patients presenting in DKA (X2 = 12.94, p < 0.001). In patients with established diabetes, there was an increase in hyperglycemia (OR1.38, 95% CI, 1.12,1.71; p = 0.003) and reduced DKA (OR 0.30, 95% CI, 0.12,0.73; p = 0.009) during the pandemic compared to the pre-pandemic phase. Stable rates of hospitalization (OR0.57, 95% CI, 0.31,1.04, p = 0.068) and hypoglycemia (OR1.11, 95% CI, 0.83,1.49; p = 0.484) were noted. These results were retained in the sensitivity analysis.
Conclusions: Glycemic control in children with T1DM remained stable during the first year of the pandemic. There were more newly diagnosed patients during the pandemic compared to the pre-pandemic phase, and more of these new patients presented in DKA. The latter presentation was reduced in those with established diabetes during the same period.
Further studies are needed to assess the ongoing impact of the COVID-19 pandemic on T1DM care pathways and outcomes to allow children, families, and diabetes teams to personalize choices of care models.
In March 2020, the World Health Organization declared COVID-19 a global pandemic (1, 2). The then-novel SARS-CoV-2, the virus responsible for the pandemic, led to global lockdowns and social distancing measures to curb viral spread and to help manage finite healthcare resources (3–5). For most children, in-person schooling and organized activities were cancelled or moved to virtual platforms (6, 7). The resurgent waves of the virus variants continue to disrupt access to healthcare services, care delivery, schooling, social activities, work, and global economic activities (8).
During the pandemic, pediatric outpatient services for chronic conditions, including Type 1 diabetes mellitus (T1DM), pivoted to virtual care models including video and telephone consultations (9–11). Even with the up-scaling of vaccinations and relaxation of social distancing measures, in-person clinical services continued to be disrupted, and hybrid models of care have become commonplace (10, 12).
In-person visits were the norm for providing diabetes care pre-pandemic (13–15), and virtual consultations occurred infrequently (16, 17). The rapid adoption of virtual care demonstrated resilience in care delivery to support patients to maintain health outcomes during the pandemic (18). The effect of the pandemic on health outcomes, diabetes technology use, and care delivery patterns in children with T1DM is only emerging, and it will take time before the impact of the pandemic is fully evaluated, but the pre-pandemic limited evidence for the value of virtual care in T1DM was promising (18–26).
Emerging evidence suggests that there may be a rise in the number of children newly diagnosed with T1DM during the pandemic compared to the pre-pandemic phase, although this association has not been universal (27–33). The data so far also suggest an increase in the number of children presenting in diabetic ketoacidosis (DKA) (26, 34–36).
This study aimed to compare T1DM outcomes during the pandemic to the pre-pandemic phase. We tested the hypothesis that in children with T1DM, glycemic control and diabetes-related morbidities will worsen during the COVID-19 pandemic compared to the pre-pandemic phase.
This study was a retrospective chart review that utilized data from the study of COVID-19 Effects on Glycemic Control in Children Living with Diabetes (CGC study).
We included boys and girls aged 2- < 18 years who attended the Pediatric Diabetes Program at McMaster Children's Hospital, a tertiary pediatric academic center in Ontario, Canada.
The study included patients who were diagnosed for at least one year with T1DM by the end of 2018 and had longitudinal follow-up data. The T1DM diagnosis ascertainment was based on standard criteria (13, 14). We excluded patients with cystic fibrosis-related diabetes, monogenic diabetes, medication-induced hyperglycemia, and type 2 diabetes. Patients less than 2 years of age were also excluded to avoid the potential inclusion of neonatal and genetic forms of diabetes.
We collected data for the two years pre-pandemic (March 15, 2018–March 14, 2020), and for the first year of the pandemic (March 15, 2020–March 14, 2021). At our center, clinical care was almost completely virtual from mid-March to September 2020. Then, a limited hybrid care delivery model was enacted with some in-person visits from October to December 2020. From January to March 2021, care returned to virtual platforms due to a new wave of the virus. The data from the pandemic phase, especially the Hemoglobin A1c (HbA1c), were limited by restrictions of patient access to local laboratory and clinic-based point-of-care testing. We included patients who had at least one HbA1c during the first year of the pandemic. We collected data including the number of newly diagnosed T1DM patients in the first year of the pandemic, age at diagnosis, diabetes duration, sex, visit date, treatment details including Multiple Daily Injections (MDI) or insulin pump therapy data, continuous glucose monitor (CGM) use, DKA, diabetes-related hospital attendance and hospitalizations, hypoglycemic events, and hyperglycemia.
We also collected available anthropometric data including weight, weight percentile, height, height percentile, and Body Mass Index (BMI) z-score (37). Of note, only a subgroup of patients had hospital-based height measured during the pandemic (n = 110, 31.80%), and weights included home-reported (n = 189, 61.00%) and hospital-measured (n = 122, 39.00%) data. We only included the BMI z-score data from the subset of patients who had weights and heights measured at the hospital to provide a conservative yet potentially more accurate data.
The main outcome of the study was the comparison of HbA1c change during the pandemic when compared to the pre-pandemic phase. Other outcomes included comparisons of the number of newly diagnosed T1DM cases, DKA, hospital attendance and hospitalizations, and any hypoglycemia and hyperglycemia at diagnosis and in those with established diabetes pre- and during the pandemic. Data on hypoglycemia and hyperglycemia were either reported or documented via CGM, glucometer, or logbook reviews.
DKA was defined as hyperglycaemia with blood glucose >11.00 mmol/L (200 mg/dL), venous blood gas-based pH <7.30, serum bicarbonate <15 mmol/L and the presence of ketones (ß-hydroxybutyrate ≥3 mmol/L in blood, moderate-large ketonuria) (39). Hospital attendance and hospitalizations refers to diabetes-related healthcare facility including emergency department visits or overnight hospital admissions. Hypoglycemia was defined as a plasma glucose level ≤3.9 mmol/L (70 mg/dL) and hyperglycemia was defined as a plasma glucose level ≥13.3 mmol/L (240 mg/dL) (39).
The Hamilton Integrated Research Ethics Board approved the study and granted a waiver of consent due to the anonymous and aggregate nature of the data used in the analyses. The study was performed in accordance with the guidelines of the Tri-Council Policy Statement 2 and the basic principles of Good Clinical Practice.
Continuous variables are presented as means (SD) with range where indicated, and dichotomous variables are reported as numbers (%). Tests for collinearity were conducted to ensure predictors used in the model were not highly correlated. A variance inflation factor <5 was used to meet this assumption of non-collinearity (40).
We utilized the paired t-test for comparisons of continuous variables and McNemar's test for binary variables to report differences in repeated measures pre-pandemic when compared to the pandemic phase. To assess differences in the proportions of patients diagnosed pre-pandemic to those diagnosed during the pandemic and their clinical presentations, we utilized the Wald χ2 test. To assess the effect of time as a confounding variable on our measures of interest, we also utilized these tests to assess the differences in the pre-pandemic data from 2018 when compared to data from 2019.
The generalized estimating equation (GEE) model was applied to compare HbA1c levels before and during the pandemic and was adjusted for age, sex, treatment type, sensor use, weight and diabetes-related morbidities (41, 42). GEE models were also used to assess changes in hypoglycemia, hyperglycemia, DKA, and hospital attendance and hospitalizations (41). These models were adjusted for age, sex, treatment type, and sensor use (41, 43). For this analysis, we utilized the first-order auto-regressive working correlation matrix that recognizes correlations are highest between adjacent times and systematically decrease with increasing distance between time points (41, 43).
Continuous variables were reported using mean difference (MD) with a 95% confidence interval (CI) and binary variables were presented as an odds ratio (OR) with a 95% CI.
To assess the robustness of the findings, we employed a sensitivity analysis to assess the impact of missing data (44, 45). We used the multiple imputations (MI) approach to address missing data, reported as a number and percent, and re-ran the GEE model for outcomes. The data analyses were performed using SPSS 28.0 (46). Significance was set at alpha = 0.05.
We did not adjust alpha for multiple testing because of the exploratory nature of the analyses (47).
Due to missing data for BMI z-score during the pandemic, mostly due to missing hospital-measured heights to allow accurate calculation of BMI z-score, weight was instead adjusted for in the model and it was not included as an outcome variable.
Participants' characteristics are reported in Table 1. We included 346 patients who met the inclusion criteria (Figure 1). The age at study inclusion was 10.30 ± 3.50 years (range 2.00–16.20 years). Participants had diabetes for 4.50 ± 3.30 years (range 1.00–15.70 years) at inclusion. There were 159 (46.10%) female articipants, and puberty data were not available.
There was a significant increase in the number of newly diagnosed patients during the first year of the pandemic, when compared to the two years in the pre-pandemic phase (χ2 = 16.52, p < 0.001).
The number of patients using insulin pump therapy increased over time compared to MDI treatment and was stable during the pandemic, as pump start activities shifted to community-based starts with lockdowns (pump use pre-pandemic 2018: 46.00%, 2019: 59.00%; pandemic: 59.80%; p = 0.317). In children using MDI treatment, 82.50% had HbA1c measures taken during the pandemic, while 88.90% of children on pumps having pandemic HbA1c data (χ2 = 2.68, p = 0.101). In an exploratory analysis adjusting for age, sex, and diabetes duration, trends for HbA1c did not differ between children using MDI or pump therapy when comparing pre-pandemic and pandemic periods (OR = 0.99; 95% CI 0.94, −1.04, p = 0.579). The use of CGM increased over time and climbed further during the pandemic (pre-pandemic 2018: 44.00%, 2019: 50.80%; pandemic: 60.70%; p < 0.001).
There was a trend for a rise in BMI z-score during the pandemic when compared to the pre-pandemic phase (n = 110, MD = −0.13; 95% CI, −0.22, −0.04, p = 0.004) driven by the increase in the weight percentile during the pandemic (n = 122, MD = −2.23; 95% CI, −4.16, −0.29, p = 0.024). In an exploratory sex-based analysis for BMI z-score, females had a more significant rise in BMI z-score than males during the pandemic (Males n = 62, MD = −0.11, p = 0.068; Females n = 48, MD = −0.16, p = 0.023).
There were 216 (63.00%) patients with only 1 HbA1c value during the pandemic. There was no significant change in HbA1c when comparing the pre-pandemic to the pandemic phases (n = 343, MD = −0.10; 95% CI, −0.21, 0.01, p = 0.076) (Table 2).
However, more newly diagnosed T1DM patients presented in DKA (χ2 = 12.94, p < 0.001) and more patients required hospital attendance and hospitalization at diagnosis in the unadjusted analysis (χ2 = 50.94, p < 0.001). In contrast, patients with established diabetes had less reported DKA (pre-pandemic 2018: 5.50%%, 2019: 5.80%; pandemic: 2.90%; p < 0.001) and hospital attendance and hospitalizations (pre-pandemic 2018: 9.00%, 2019: 9.80%; pandemic: 7.50%; p < 0.001) during the pandemic. Hospital attendance and hospitalizations were not significantly different during the pandemic in the GEE analysis (Table 3).
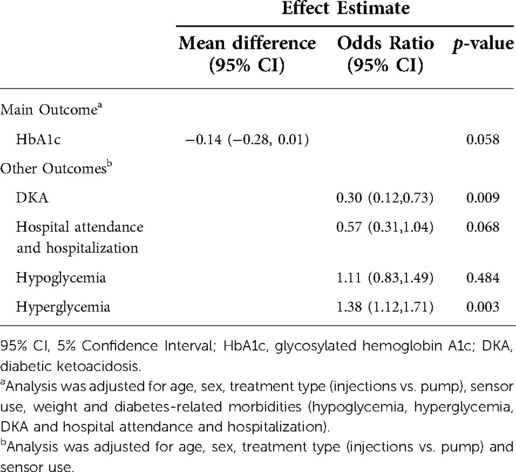
Table 3. Changes in diabetes control and diabetes-related morbidities during the COVID-19 pandemic (n = 344).
The occurrence of any hypo- or hyperglycemia, in patients with T1DM was sustained from pre-pandemic levels during the pandemic. A significant number of children had hypoglycemia and this trend continued into the pandemic (pre-pandemic 2018: n = 323, 96.40%, 2019: n = 332, 97.40%; pandemic: n = 330, 96.80%; p = 0.819). The hyperglycemic events were noted in the majority of patients in all years of study with a slight rise during the pandemic (pre-pandemic 2018: n = 278, 83.70%, 2019: n = 277, 82.00%; pandemic 2020: n = 301, 88.80%; p = 0.011) (Table 2).
To determine if the changes during the pandemic were different when compared to the pre-pandemic phase (2018–2019), we compared the data from the pre-pandemic phase. A significant increase in pump therapy use (2018: 46.00%, 2019: 59.00%; p < 0.001), CGM use (2018: 44.00%, 2019: 50.80%; p < 0.001), weight percentile (n = 333, MD = 1.53; 95% CI, 2.46, 0.59; p = 0.001) and BMI z-score (n = 333, MD = 0.06; 95% CI, 0.10, 0.02; p = 0.002) was found when comparing the data between 2018 and 2019.
The results of the GEE analysis model of the impact of the first year of the COVID-19 pandemic on glycemic control and diabetes-related morbidities are reported in Table 3 (n = 344).
During the pandemic, participants with established diabetes maintained their glycemic control (MD −0.14; 95% CI, −0.28,0.01; p = 0.058) with significantly less DKA (OR 0.30, 95% CI, 0.12,0.73; p = 0.009) and an increase in any hyperglycemic events (OR 1.38, 95% CI, 1.12,1.71; p = 0.003) when compared to the pre-pandemic period. There was no change in hospital attendance and hospitalizations (OR 0.57, 95% CI, 0.31,1.04; p = 0.068) or hypoglycemic events (OR 1.11, 95% CI, 0.83,1.49; p = 0.484).
To address the effect of missing data during the pandemic with virtual visits, we conducted multiple imputations and repeated the GEE model analyses which confirmed the results, and that the missingness of data did not impact the analysis conclusions (Table 4).
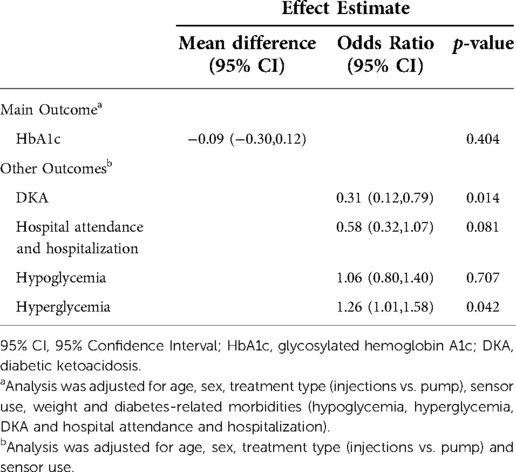
Table 4. Sensitivity analysis using multiple imputations and generalized estimating equation model to assess glycemic outcomes (n = 346).
The COVID-19 pandemic fundamentally shifted models of care delivery for children living with T1DM. The short-term outcomes of patients living with T1DM during the pandemic continue to unravel, and it will be many years before the full impact of the pandemic on diabetes outcomes is fully understood.
This retrospective study compared data from the two years pre-pandemic to the first year of the pandemic in children living with T1DM. When assessing glycemic control, the HbA1c remained stable during the first year of the pandemic. This conclusion, while contrary to our hypothesis, is consistent with some of the emerging evidence for stability of pandemic time glycemic control trends (20, 48–53), and some studies even reported an improvement in glycemic control (22, 54). The stability or potential improvement in glycemic control during the pandemic may be related to enhanced awareness of children and caregivers during lockdowns with more attention paid to glycemic trends, increased patient-caregiver collaboration with managing glycemia, and the development of predictable management routines (22, 54). Importantly, the data on stability of glycemic trends in T1DM were reported from upper-middle or high-income countries with infrastructure to conduct virtual visits and sustained access to glucose monitoring supplies and treatments (20, 22, 48, 49, 51, 52, 54). A study from India based on reported self-monitoring of glucose data reported a deterioration in glycemic control during lockdowns due to the reduced availability of insulin and glucose test strips in the early stages of the pandemic (55). For virtual and hybrid models of diabetes care to be universally effective and sustainable, the disparity in access to diabetes healthcare professionals, supplies, and digital resources need to be eliminated.
The number of patients presenting with new onset T1DM rose during the pandemic, and more patients presented in DKA when compared to the pre-pandemic phase. In contrast, fewer patients with established T1DM diagnosis had DKA during the pandemic phase. While the reported number of cases for these outcomes were relatively small, the data are consistent with current evidence reporting an increase in DKA and its severity at diagnosis (21, 26–29, 56–58). These findings are likely related to delays in seeking care due to concerns about the increased risk in contracting the COVID-19 virus in healthcare facilities, and caregivers abiding by health systems messaging to divert care away from hospitals to prioritize resource utilization for COVID-19 activities (26). Public health education campaigns may be helpful in this phase of the pandemic to remind caregivers of the symptoms of diabetes and DKA to allow early diabetes diagnosis and mitigate the risk and severity of DKA.
Some studies reported an increase in DKA rates in children with established diabetes during the first lockdown within the first five months of the pandemic, with a return to or below pre-pandemic levels within the first few months (48, 56).
Consistent with our results, there is evidence to suggest that the pandemic was not associated with increased hospital attendance and hospitalizations in those with established diabetes (20). The rapid adoption of telemedicine and diabetes team accessibility, the pre-existing high levels of access to technologies including CGM and pump therapy, and increased attention to glycemic trends likely helped limit hospital attendance and hospitalizations during the pandemic (20–22, 24, 59, 60).
The pandemic was associated with higher rates of hyperglycemia when compared to the pre-pandemic phase despite stable glycemic control. It is possible that reported hyperglycemia is related to enhanced attention to glucose profiles while in lockdown, the use of CGM, and alterations in dietary patterns and physical activity levels, with children being less active and using technology for longer periods than in pre-pandemic times (22, 54, 61–63). Another explanation is that while the reporting of hyperglycemia was based on the occurrence of these events, the duration of time when the patient had a normal glucose likely contributed to stable glycemic control.
The burden of hypoglycemia remained high throughout the study period. Hypoglycemia is a frequent accompaniment of T1DM (64–67). For patients living with T1DM and their families, glycemic variability and avoiding hypo- and hyperglycemia are a consistent and substantial burden in diabetes management (64–69).
Recent reports suggest that glucose sensor data of time in range (TIR), hypoglycemia, and hyperglycemia were stable or improved early in the pandemic; the trends reverted when patients returned to school and other activities with the relaxation of social distancing measures (23, 49, 51–54). There is a need to assess approaches to maintain glycemic trends by limiting glycemic excursions in T1DM.
Our data suggest that the weight percentile and BMI z-score increased during lockdowns and this trend continued from the pre-pandemic phase. The possible explanations for the pandemic results include the impact of age and growth, puberty, diabetes duration, anabolic effects of insulin over time on body mass, and children having lower physical activity, sedentary behaviours, and changes in dietary habits during lockdown when compared to the pre-pandemic phase (48–51, 70–74). Further assessment of the factors contributing to this upward body mass trend in children with diabetes is warranted.
We noted a significant increase in the use of glucose monitoring technology including CGM when comparing the pre-pandemic years of 2018 and 2019. As CGM use was also noted to increase during the pandemic year, it is likely that this trend was not because of the pandemic alone. Rather, patients were already transiting to use technology and likely would have continued to adopt these tools even without the pandemic.
This study compared glycemic trends and diabetes-related outcomes before and after the start of the pandemic. The longitudinal data available for a relatively large sample size and using the GEE approach allowed data comparison over time.
There were some limitations to the study. This is a single center retrospective study from a high-income country, which may limit generalizability. In addition, the confounder of time may lead to some measures changing over time regardless of the pandemic. The HbA1c data were also limited with some patients only having one or two HbA1c values during the pandemic, when compared to four annual tests in the pre-pandemic phase due to disruption of point-of-care clinic and laboratory-based services. This limitation required a pragmatic decision to use existing data. The disruption of children's access to laboratories and point-of-care testing facilities was significant during the early part of the pandemic, and the data available reflected this limitation.
In patients with T1DM, glycemic control was comparable during the first year of the COVID-19 pandemic to the pre-pandemic phase. The number of new patients with T1DM and those presenting in DKA at diagnosis increased during the pandemic, while those with established diabetes saw a decline in DKA. The burden of hypo- and hyperglycemia was substantial throughout the study period.
There is a need for healthcare systems to equip children and families living with T1DM with the technological and digital infrastructure necessary to support diabetes care and maintain glycemic outcomes and lower the risk of co-morbidities and complications. These approaches to care require a commitment to equity in access of all children to maintain and improve diabetes control and outcomes globally.
The raw data supporting the conclusions of this article will be made available by the authors, upon reasonable justification.
The studies involving human participants were reviewed and approved by Hamilton Integrated Research Ethics Board. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
RR and MCS generated the research question. All authors conceptualized the study design. Data collection was done by RR with the two acknowledged individuals, Saini and Cargnelli. Statistical analyses were performed by RR, LT, JEE, and MCS. All authors contributed to result interpretation. RR and MCS drafted the manuscript and all authors edited and approved the final version. MCS is the guarantor. All authors contributed to the article and approved the submitted version.
We would like to thank Amandeep Saini and Claudia Cargnelli for their contributions in extracting the study data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020. Geneva, Switzerland (2020).
3. COVID-19 Intervention Timeline in Canada: Canadian Institute for Health Information (2021). Available from: Https://www.cihi.ca/en/covid-19-intervention-timeline-in-canada
4. World Health Organization. Infection prevention and control in the context of coronavirus disease (COVID-19): A living guideline, 25 April 2022: updated chapter: mask use, part 1: health care settings. World Health Organization (2022).
5. Ryan RE, Parkhill A, Schonfeld L, Walsh L, Lowe D, Merner B, et al. What are relevant, feasible and effective approaches to promote acceptance, uptake and adherence to physical distancing measures for COVID-19 prevention and control? World Health Organization. Regional Office for Europe (2021).
6. Daniel J. Education and the COVID-19 pandemic. Prospects. (2020) 49(1):91–6. doi: 10.1007/s11125-020-09464-3
7. Lemay DJ, Bazelais P, Doleck T. Transition to online learning during the COVID-19 pandemic. Comput Hum Behav Rep. (2021) 4:100130. doi: 10.1016/j.chbr.2021.100130
8. Brodeur A, Gray D, Islam A, Bhuiyan S. A literature review of the economics of COVID-19. J Econ Surv. (2021) 35(4):1007–4. doi: 10.1111/joes.12423
9. Fung A, Irvine M, Ayub A, Ziabakhsh S, Amed S, Hursh BE. Evaluation of telephone and virtual visits for routine pediatric diabetes care during the COVID-19 pandemic. J. Clin. Transl. Endocrinol. (2020) 22:100238. doi: 10.1016/j.jcte.2020.100238
10. Prahalad P, Leverenz B, Freeman A, Grover M, Shah S, Conrad B, et al. Closing disparities in pediatric diabetes telehealth care: lessons from telehealth necessity during the COVID-19 pandemic. Clin Diabetes. (2022) 40(2):153–7. doi: 10.2337/cd20-0123
11. Tornese G, Schiaffini R, Mozzillo E, Franceschi R, Frongia AP, Scaramuzza A. The effect of the COVID-19 pandemic on telemedicine in pediatric diabetes centers in Italy: results from a longitudinal survey. Diabetes Res. Clin. Pract. (2021) 179:109030. doi: 10.1016/j.diabres.2021.109030
12. Pierce JS, Gurnurkar S, Vyas N, Carakushansky M, Owens L, Patton SR. Feasibility of implementing a pediatric diabetes clinic via telehealth. Diabetes Spectr. (2021) 34(2):190–7. doi: 10.2337/ds20-0060
13. American Diabetes Association Professional Practice Committee. Children and adolescents: standards of medical care in diabetes—2022. Diabetes Care. (2021) 45(Suppl. 1):S208–31. doi: 10.2337/dc22-S014
14. Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. (2018) 42(Suppl 1):S1–S325.29650079
15. Hattersley AT, Greeley SA, Polak M, Rubio-Cabezas O, Njølstad PR, Mlynarski W, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Stages of type 1 diabetes in children and adolescents. (2018).
16. Wittmeier KD, Protudjer JL, Wicklow BA. Reflections on virtual care for chronic conditions during the COVID-19 pandemic. Can J Diabetes. (2021) 45(1):1–2. doi: 10.1016/j.jcjd.2020.11.013
17. Lee JM, Albanese-O'Neill A, Demeterco-Berggren C, Corathers SD, Vendrame F, Weinstock RS, et al. Adoption of telemedicine for type 1 diabetes care during the COVID-19 pandemic. Diabetes Technol Ther. (2021) 23(9):642–51. doi: 10.1089/dia.2021.0080
18. Braune K, Boss K, Schmidt-Herzel J, Gajewska KA, Thieffry A, Schulze L, et al. Shaping workflows in digital and remote diabetes care during the COVID-19 pandemic via service design: prospective, longitudinal, open-label feasibility trial. JMIR Mhealth Uhealth. (2021) 9(4):e24374. doi: 10.2196/24374
19. Buggs-Saxton C. Care of Pediatric Patients with Diabetes During the Coronavirus Disease 2019 (COVID-19) Pandemic. Pediatr Clin North Am. (2021) 68(5):1093–101. doi: 10.1016/j.pcl.2021.05.014
20. Choudhary A, Adhikari S, White PC. Impact of the COVID-19 pandemic on management of children and adolescents with type 1 diabetes. BMC Pediatr. (2022) 22(1):1–7. doi: 10.1186/s12887-022-03189-2
21. Kaushal T, Ambler-Osborn L, Turcotte C, Quinn H, Laffel L. Rapid adoption of telemedicine along with emergent use of continuous glucose monitors in the ambulatory care of young persons with new-onset type 1 diabetes in the time of COVID-19: a case series. Telemed J E Health. (2022) 28(1):107–14. doi: 10.1089/tmj.2020.0554
22. Lazzeroni P, Motta M, Monaco S, Laudisio S, Furoncoli D, Maffini V, et al. Improvement in glycaemic control in paediatric and young adult type 1 diabetes patients during COVID-19 pandemic: role of telemedicine and lifestyle changes. Acta Biomed. (2021) 92(5).34738562
23. Rachmiel M, Lebenthal Y, Mazor-Aronovitch K, Brener A, Levek N, Levran N, et al. Glycaemic control in the paediatric and young adult population with type 1 diabetes following a single telehealth visit-what have we learned from the COVID-19 lockdown? Acta Diabetol. (2021) 58(6):697–705. doi: 10.1007/s00592-021-01673-2
24. Wood CL, Clements SA, McFann K, Slover R, Thomas JF, Wadwa RP. Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol Ther. (2016) 18(1):7–14. doi: 10.1089/dia.2015.0123
25. Levin K, Madsen JR, Petersen I, Wanscher CE, Hangaard J. Telemedicine diabetes consultations are cost-effective, and effects on essential diabetes treatment parameters are similar to conventional treatment: 7-year results from the Svendborg Telemedicine Diabetes Project. SAGE Publications (2013).
26. Kamrath C, Mönkemöller K, Biester T, Rohrer TR, Warncke K, Hammersen J, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. Jama. (2020) 324(8):801–4. doi: 10.1001/jama.2020.13445
27. Wolf RM, Noor N, Izquierdo R, Jett D, Rewers A, Majidi S, et al. Increase in newly diagnosed type 1 diabetes in youth during the COVID-19 pandemic in the United States: a multi-center analysis. Pediatr Diabetes. (2022) 23(4):433–8. doi: 10.1111/pedi.13328
28. Unsworth R, Wallace S, Oliver NS, Yeung S, Kshirsagar A, Naidu H, et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the UK. Diabetes Care. (2020) 43(11):e170–e1. doi: 10.2337/dc20-1551
29. Rahmati M, Keshvari M, Mirnasuri S, Yon DK, Lee SW, Il Shin J, et al. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: a systematic review and meta-analysis. J Med Virol. (2022) 94(11):5112–27. doi: 10.1002/jmv.27996
30. Schiaffini R, Deodati A, Rapini N, Pampanini V, Cianfarani S. Increased incidence of childhood type 1 diabetes during the COVID-19 pandemic. Figures from an Italian tertiary care center. J Diabetes. (2022) 14(8):562–3. doi: 10.1111/1753-0407.13298
31. Dilek S, Gürbüz F, Turan İ, Celiloğlu C, Yüksel B. Changes in the presentation of newly diagnosed type 1 diabetes in children during the COVID-19 pandemic in a tertiary center in Southern Turkey. J Pediatr Endocrinol Metab. (2021) 34(10):1303–9. doi: 10.1515/jpem-2021-0287
32. Hernández Herrero M, Terradas Mercader P, Latorre Martinez E, Feliu Rovira A, Rodríguez Zaragoza N, Parada Ricart E. New diagnoses of type 1 diabetes mellitus in children during the COVID-19 pandemic. Regional multicenter study in Spain]. Endocrinol Diabetes Nutr. (2022) 69(9):709–14. doi: 10.1016/j.endinu.2021.12.003
33. McKeigue PM, McGurnaghan S, Blackbourn L, Bath LE, McAllister DA, Caparrotta TM, et al. Relation of incident type 1 diabetes to recent COVID-19 infection: cohort study using e-health record linkage in Scotland. Diabetes Care. (2022) (2022):dc220385. doi: 10.2337/dc22-0385. [Epub ahead of print]35880797
34. Birkebaek NH, Kamrath C, Grimsmann JM, Aakesson K, Cherubini V, Dovc K, et al. Impact of the COVID-19 pandemic on long-term trends in the prevalence of diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes: an international multicentre study based on data from 13 national diabetes registries. Lancet Diabetes Endocrinol. (2022).36202118
35. Sellers EAC, Pacaud D. Diabetic ketoacidosis at presentation of type 1 diabetes in children in Canada during the COVID-19 pandemic. Paediatr Child Health. (2021) 26(4):208–9. doi: 10.1093/pch/pxab017
36. Dżygało K, Nowaczyk J, Szwilling A, Kowalska A. Increased frequency of severe diabetic ketoacidosis at type 1 diabetes onset among children during COVID-19 pandemic lockdown: an observational cohort study. Pediatr Endocrinol Diabetes Metab. (2020) 26(4):167–75. doi: 10.5114/pedm.2020.101003
37. Must A, Anderson S. Body mass index in children and adolescents: considerations for population-based applications. Int J Obes. (2006) 30(4):590–4. doi: 10.1038/sj.ijo.0803300
38. Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD clinical practice consensus guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. (2018) 19:155–77. doi: 10.1111/pedi.12701
39. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care. (2020) 43(Suppl. 1):S14–31.31862745
40. O’brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. (2007) 41(5):673–90. doi: 10.1007/s11135-006-9018-6
41. Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. (1986):121–30. doi: 10.2307/2531248
43. Ballinger GA. Using generalized estimating equations for longitudinal data analysis. Organ Res Methods. (2004) 7(2):127–50. doi: 10.1177/1094428104263672
44. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. (2006) 15(5):291–303. doi: 10.1002/pds.1200
45. Thabane L, Mbuagbaw L, Zhang S, Samaan Z, Marcucci M, Ye C, et al. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. (2013) 13(1):1–12. doi: 10.1186/1471-2288-13-92
47. Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. (2017).
48. Hammersen J, Reschke F, Tittel SR, Pappa A, Dost A, Köstner K, et al. Metabolic control during the SARS-CoV-2 lockdown in a large German cohort of pediatric patients with type 1 diabetes: results from the DPV initiative. Pediatr Diabetes. (2022) 23(3):351–61. doi: 10.1111/pedi.13319
49. Minuto N, Bassi M, Montobbio C, Vinci F, Mercuri C, Perri FN, et al. The effect of lockdown and physical activity on glycemic control in Italian children and young patients with type 1 diabetes. Front Endocrinol (Lausanne). (2021) 12. doi: 10.3389/fendo.2021.690222
50. Shah N, Karguppikar M, Bhor S, Ladkat D, Khadilkar V, Khadilkar A. Impact of lockdown for COVID-19 pandemic in Indian children and youth with type 1 diabetes from different socio-economic classes. J Pediatr Endocrinol Metab. (2021) 34(2):217–23. doi: 10.1515/jpem-2020-0460
51. Wu X, Luo S, Zheng X, Ding Y, Wang S, Ling P, et al. Glycemic control in children and teenagers with type 1 diabetes around lockdown for COVID-19: a continuous glucose monitoring-based observational study. J Diabetes Investig. (2021) 12(9):1708–17. doi: 10.1111/jdi.13519
52. Tinti D, Savastio S, Grosso C, De Donno V, Trada M, Nugnes M, et al. Impact of lockdown during COVID-19 emergency on glucose metrics of children and adolescents with type 1 diabetes in piedmont, Italy. Acta Diabetol. (2021) 58(7):959–61. doi: 10.1007/s00592-021-01702-0
53. Silverii GA, Delli Poggi C, Dicembrini I, Monami M, Mannucci E. Glucose control in diabetes during home confinement for the first pandemic wave of COVID-19: a meta-analysis of observational studies. Acta Diabetol. (2021) 58(12):1603–11. doi: 10.1007/s00592-021-01754-2
54. Lombardo F, Salzano G, Bombaci B, Basile P, Lucania G, Alibrandi A, et al. Has COVID-19 lockdown improved glycaemic control in pediatric patients with type 1 diabetes? An analysis of continuous glucose monitoring metrics. Diabetes Res Clin Pract. (2021) 178:108988. doi: 10.1016/j.diabres.2021.108988
55. Verma A, Rajput R, Verma S, Balania VK, Jangra B. Impact of lockdown in COVID 19 on glycemic control in patients with type 1 diabetes Mellitus. Diabetes Metab Syndr. (2020) 14(5):1213–6. doi: 10.1016/j.dsx.2020.07.016
56. Danne T, Lanzinger S, de Bock M, Rhodes ET, Alonso GT, Barat P, et al. A worldwide perspective on COVID-19 and diabetes management in 22,820 children from the SWEET project: diabetic ketoacidosis rates increase and glycemic control is maintained. Diabetes Technol Ther. (2021) 23(9):632–41. doi: 10.1089/dia.2021.0110
57. Marks BE, Khilnani A, Meyers A, Flokas ME, Gai J, Monaghan M, et al. Increase in the diagnosis and severity of presentation of pediatric type 1 and type 2 diabetes during the COVID-19 pandemic. Horm Res Paediatr. (2021) 94(7–8):275–84. doi: 10.1159/000519797
58. Elgenidy A, Awad AK, Saad K, Atef M, El-Leithy HH, Obiedallah AA, et al. Incidence of diabetic ketoacidosis during COVID-19 pandemic: a meta-analysis of 124,597 children with diabetes. Pediatr Res. (2022) 1–12. doi: 10.1038/s41390-022-02241-2
59. De Guzman KR, Snoswell CL, Taylor ML, Senanayake B, Haydon HM, Batch JA, et al. A systematic review of pediatric telediabetes service models. Diabetes Technol Ther. (2020) 22(8):623–38. doi: 10.1089/dia.2019.0489
60. Tejera-Perez C, Moreno-Pérez Ó, Rios J, Reyes-García R. People living with type 1 diabetes point of view in COVID-19 times (COVIDT1 study): disease impact, health system pitfalls and lessons for the future. Diabetes Res Clin Pract. (2021) 171:108547. doi: 10.1016/j.diabres.2020.108547
61. Stockwell S, Trott M, Tully M, Shin J, Barnett Y, Butler L, et al. Changes in physical activity and sedentary behaviours from before to during the COVID-19 pandemic lockdown: a systematic review. BMJ Open Sport Exerc Med. (2021) 7(1):e000960. doi: 10.1136/bmjsem-2020-000960
62. Wicaksana AL, Hertanti NS, Ferdiana A, Pramono RB. Diabetes management and specific considerations for patients with diabetes during coronavirus diseases pandemic: a scoping review. Diabetes Metab Syndr. (2020) 14(5):1109–20. doi: 10.1016/j.dsx.2020.06.070
63. Okuyama J, Seto S, Fukuda Y, Funakoshi S, Amae S, Onobe J, et al. Mental health and physical activity among children and adolescents during the COVID-19 pandemic. Tohoku J Exp Med. (2021) 253(3):203–15. doi: 10.1620/tjem.253.203
64. Coolen M, Broadley M, Hendrieckx C, Chatwin H, Clowes M, Heller S, et al. The impact of hypoglycemia on quality of life and related outcomes in children and adolescents with type 1 diabetes: a systematic review. PLoS One. (2021) 16(12):e0260896. doi: 10.1371/journal.pone.0260896
65. Urakami T. Severe hypoglycemia: is it still a threat for children and adolescents with type 1 diabetes? Front Endocrinol (Lausanne). (2020) 609.
66. Bulsara MK, Holman CDAJ, Davis EA, Jones TW. The impact of a decade of changing treatment on rates of severe hypoglycemia in a population-based cohort of children with type 1 diabetes. Diabetes Care. (2004) 27(10):2293–8. doi: 10.2337/diacare.27.10.2293
67. Karges B, Rosenbauer J, Kapellen T, Wagner VM, Schober E, Karges W, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. (2014) 11(10):e1001742. doi: 10.1371/journal.pmed.1001742
68. Rewers A, Chase HP, Mackenzie T, Walravens P, Roback M, Rewers M, et al. Predictors of acute complications in children with type 1 diabetes. Jama. (2002) 287(19):2511–8. doi: 10.1001/jama.287.19.2511
69. Brazeau AS, Messier V, Talbo MK, Gagnon C, Taleb N, Fortier I, et al. Self-reported severe and nonsevere hypoglycemia in type 1 diabetes: population surveillance through the BETTER patient engagement registry: development and baseline characteristics. Can J Diabetes. (2022):S1499-2671(22)00134-4. doi: 10.1016/j.jcjd.2022.05.010
70. Moore SA, Faulkner G, Rhodes RE, Brussoni M, Chulak-Bozzer T, Ferguson LJ, et al. Impact of the COVID-19 virus outbreak on movement and play behaviours of Canadian children and youth: a national survey. Int J Behav Nutr Phys Activ. (2020) 17(1):1–11. doi: 10.1186/s12966-020-00987-8
71. Chang T-H, Chen Y-C, Chen W-Y, Chen C-Y, Hsu W-Y, Chou Y, et al. Weight gain associated with COVID-19 lockdown in children and adolescents: a systematic review and meta-analysis. Nutrients. (2021) 13(10):3668. doi: 10.3390/nu13103668
72. Nsamenang SA, Gutierrez CA, Manayathu Jones J, Jenkins G, Tibelius SA, DiGravio AM, et al. Impact of SARS-CoV-2 pandemic on the mental and physical health of children enrolled in a paediatric weight management clinic. Paediatr Child Health. (2022) 27(Suppl. 1):S72–S7. doi: 10.1093/pch/pxac014
73. Carroll N, Sadowski A, Laila A, Hruska V, Nixon M, Ma DW, et al. The impact of COVID-19 on health behavior, stress, financial and food security among middle to high income Canadian families with young children. Nutrients. (2020) 12(8):2352. doi: 10.3390/nu12082352
Keywords: COVID-19, type 1 diabetes mellitus, child, diabetic ketoacidosis, hypoglycemia, glycemic control, HbA1c
Citation: Rajan R, Athale U, Ewusie JE, McAssey K, Thabane L and Samaan MC (2022) An exploratory analysis of the impact of the COVID-19 pandemic on pediatric type 1 diabetes mellitus patient outcomes: A single-center study. Front. Pediatr. 10:1038345. doi: 10.3389/fped.2022.1038345
Received: 6 September 2022; Accepted: 2 November 2022;
Published: 18 November 2022.
Edited by:
Stefano Zucchini, Sant’Orsola-Malpighi Polyclinic, ItalyReviewed by:
Riccardo Schiaffini, Bambino Gesù Children’s Hospital (IRCCS), Italy© 2022 Rajan, Athale, Ewusie, McAssey, Thabane and Samaan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Constantine Samaan c2FtYWFuY0BtY21hc3Rlci5jYQ==
Specialty Section: This article was submitted to Pediatric Endocrinology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.