- 1Department of Otolaryngology-Head and Neck Surgery, Children's Hospital of Chongqing Medical University, Chongqing, China
- 2National Clinical Research Center for Child Health and Disorders, Chongqing, China
- 3Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing, China
- 4Chongqing Key Laboratory of Pediatrics, Chongqing, China
Objective: To investigate the clinical features and factors affecting the prognosis of children with profound sudden sensorineural hearing loss (SSNHL).
Methods: We retrospectively analyzed the clinical data of 147 children with profound SSNHL who received inpatient treatment at our department from January 2016 to January 2021. All children were administered with systemic steroid therapy and/or intratympanic steroid (ITS) treatment for 2 weeks. Statistical analyses were performed for the clinical features, treatment effectiveness, and factors affecting the prognosis using SPSS 23.0.
Results: The median age of the study population was 8 (6–10) years. The median treatment onset time was 8 (4–20) days. The most common concomitant symptom was tinnitus (45.58%). Laboratory findings showed that the percentages of children with abnormal leukocytes was 25.85%, abnormal platelet counts was 17.01%, abnormal cytomegalovirus IgG antibodies was 36.73% and abnormal Epstein–Barr (EB) virus IgG antibodies was 41.50%. The overall recovery rate of the treatment was 20.04%. The univariate analysis showed that age, treatment onset time, tinnitus, and ITS treatment were associated with the prognosis (p < 0.05). Regarding laboratory findings, the neutrophil count, lymphocyte count, and neutrophil-to-lymphocyte ratio differed significantly between the effective and invalid treatment effect groups (p < 0.05). The multivariable logistic regression analysis showed that treatment onset time [odds ratio (OR) = 0.936, 95% confidence interval (CI): 0.881–0.994] and ITS treatment (OR = 0.174, 95% CI: 0.044–0.0687) correlated with hearing recovery (p < 0.05).
Conclusion: In this study, the earlier the treatment start time of children with profound SSNHL, the better was the prognosis. Further, ITS could be an effective treatment option.
Introduction
Sudden sensorineural hearing loss (SSNHL) is defined as a rapidly developed hearing loss (occurs within a 72-h window) with an increased pure-tone threshold over 30 dB affecting at least three consecutive frequencies (1). Four types of audiogram configurations were defined based on the hearing loss pattern: ascending, descending, flat, and profound (2). The profound audiogram refers to the presence of a similar threshold across the frequency range and a hearing threshold over 80 dB HL (2, 3). Profound SSNHL accounting for approximately 34.1% of all cases of SSNHL, is associated with severe hearing loss and a poor prognosis, with 29.8% of the patients achieving a certain degree of recovery and only 3.6% of the patients achieving full recovery (4).
The incidence of SSNHL in children (age <18 years) is low, with only 20–30 cases per 100,000 children per year (5). Only 6.6%, 3.5%, and 1.2% of the patients with SSNHL are aged under 18 (6), 14 (7), and 9 years (8), respectively. In children, 55.3% of the cases of SSNHL are of profound hearing loss, and its effective treatment rate was found to be 31% (9). The profound SSNHL is the most severe form of hearing loss. Without timely treatment, it leads to permanent hearing loss, seriously affecting children's language and cognitive developments and increasing the burden on family and society.
The etiology and pathogenesis of SSNHL in children are unclear. Further, the treatment outcomes and factors affecting the prognosis are unknown, with no guidelines for the diagnosis or treatment. Most previous studies analyzed the risk factors and found a correlation between the hearing curve and the treatment effect (2, 10). However, factors affecting the prognosis of different types of SSNHL in children have not been investigated. Therefore, the aim of the present study was to retrospectively analyze the clinical characteristics and factors affecting the prognosis of profound SSNHL in children.
Materials and methods
Clinical data
We retrospectively enrolled 147 children diagnosed with profound SSNHL between January 2016 and January 2021 from the Children's Affiliated Hospital of Chongqing Medical University. We collected the clinical data, including the age, sex, affected side, treatment onset time, initial hearing level, accompanied tinnitus, vertigo, and aural fullness, hearing levels before and after treatment, complete blood count, serum biochemical findings, coagulation function, and viral serology results. The requirement to obtain informed consent was waived owing to the retrospective nature of the study. However, written informed consent was obtained from the guardians of the children for administering systemic steroid therapy (SST) and intratympanic steroid (ITS) treatment. The study protocol was approved by the Medical Ethics Committee of the Children's Affiliated Hospital of Chongqing Medical University.
All children met the diagnostic criteria for SSNHL in children laid down by the American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) (1). Inclusion criteria were: SSNHL diagnosed based on sudden, unexplained hearing loss within 72 h of more than 30 dB HL for at least three adjacent frequencies; age of 3–18 years; profound SSNHL determined based on full-frequency hearing loss with an average hearing level >80 dB HL; and availability of complete audiological examination and experimental results. Exclusion criteria were: hearing loss due to auditory nerve or middle or outer ear disease based on acoustic impedance, otoacoustic emissions, auditory brainstem response (ABR), otoscopy, ear computed tomography, or ear magnetic resonance imaging findings; suspected hearing loss caused by noise, medication, trauma, neurological disorders, or genetic factors; comorbidities; and data unavailability.
Inspection method
Laboratory examination
Routine blood, serum biochemical, coagulation function, and viral serology tests were performed. We collected the white blood cell count, platelet count, neutrophil count, lymphocyte count, neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), triglyceride level, cholesterol level, D2 polymer level, fibrinogen level, and cytomegalovirus and Epstein–Barr (EB) virus IgG and IgM antibodies.
Hearing tests
We performed hearing tests, including pure-tone audiometry, ABR, auditory steady state response (ASSR), and distortion product otoacoustic emission. Most children underwent pure-tone audiometry (according to ISO8253-1 standards), but those who could not cooperate in the pure-tone audiometry procedure underwent combined ABR and ASSR.
Therapeutic method
All children were treated for 2 weeks after admission. The primary treatment was SST with 1 mg/kg/day intravenous methylprednisolone for 5 days, with the maximum dose not exceeding 40 mg, followed by dose tapering. Twenty-seven children were administered with ITS once every 2 days for a total of 5 times using otoendoscopy. The ITS treatment was performed as follows: The child was placed in a seated position with the head tilted to the unaffected side; after local anesthesia with lidocaine, a tympanic membrane needle was introduced into the anterior portion of the tympanic membrane; subsequently, 0.4–0.8 ml of 4 mg/ml methylprednisolone was instilled in the middle ear cavity; the child was instructed to lie down and avoid swallowing for 15 min. Children on oral hormones were provided outpatient treatment and excluded from this study. After 2 weeks of treatment, hearing tests were performed again. Hearing levels were compared before and after the treatment to evaluate the treatment efficacy.
Treatment efficacy determination
Audiometry was performed at the initial visit and after 2 weeks of treatment. According to the Chinese Medical Association of Otolaryngology criteria for the sudden deafness treatment effect (3), complete recovery is defined as improvement in final hearing to a normal or pretreatment level; partial recovery is defined as a hearing improvement of more than 30 dB HL; slight recovery is defined as a hearing improvement of 15–30 dB HL; and no recovery is defined as a hearing improvement of less than 15 dB HL. The overall recovery rate was calculated with the following formula: (complete recovery + partial recovery + slight recovery)/total no. of cases × 100%.
Statistical analysis
Data were analyzed using SPSS 23.0 statistical software (IBM, Armonk, NY, USA). Normally distributed continuous data are expressed as mean ± standard deviation. Non-normally distributed data are expressed as median (1/4 quantile–3/4 quartile). Classification data are expressed as frequency (percentage). The t-test was performed for two-group comparisons of normally distributed data and the Mann–Whitney U test for non-normally distributed data. Associated factors were analyzed using the χ2 or rank test. A univariate analysis was performed for the relationship of hearing recovery with the sex, age, affected side, treatment onset time, initial hearing level, accompanied vertigo, tinnitus, and ear tightness, and blood parameter findings. A multivariate logistic regression analysis was performed for variables that were statistically significant in the univariate analysis to explore their impact on the prognosis. Statistical significance was set at p < 0.05.
Results
Clinical features of profound SSNHL in children
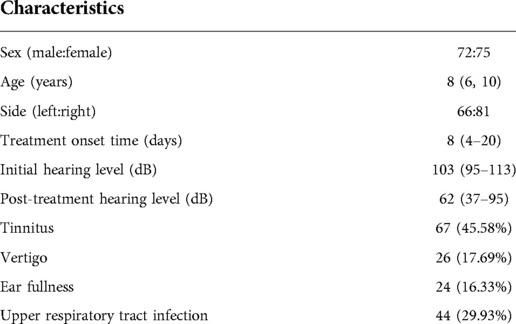
We enrolled 147 children, including 72 (48.98%) boys and 75 (51.02%) girls, aged 8 (6–10) years. The right and left ears were affected in 66 (44.90%) and 81 (55.10%) children, respectively. The treatment onset time was 8 (4–20) days. The initial hearing level was 103 (95–113) dB. Tinnitus, vertigo, ear fullness, and upper respiratory tract infection were found in 67 (45.58%), 26 (17.69%), 24 (16.33%), and 44 (29.93%) children, respectively (Table 1).
Laboratory examination results
Routine blood tests showed abnormal leukocyte, platelet, neutrophil, and lymphocyte counts in 38 (25.85%), 25 (17.01%), 21 (14.29%), and 17 (11.56%) children, respectively. Biochemical tests showed abnormal cholesterol and triglyceride levels in four (2.72%) and six (4.08%) children, respectively. Coagulation tests showed abnormal D2 polymer and fibrinogen levels in three (2.04%) children. Cytomegalovirus IgM and IgG antibodies were found in four (2.72%) and 54 (36.73%) children, respectively, while EB virus antibodies were found in five (3.40%) and 61 (41.50%) children, respectively (Table 2).
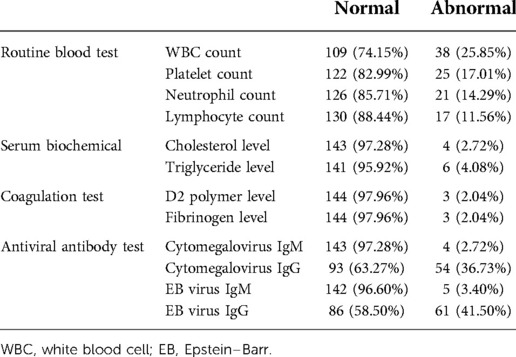
Table 2. Laboratory examination results of children with profound sudden sensorineural hearing loss.
Univariate analysis of the prognosis of children with profound SSNHL
Complete recovery, partial recovery, slight recovery, and no recovery were found in one (0.07%), nine (6.1%), 20 (13.6%), and 117 (79.6%) children, respectively, with a total response rate of 20.4%. SST alone was performed in 120 children and was effective in 17 (14.17%) children, while SST combined with ITS administration was performed in 27 children and was effective in 13 (48.15%) children. The univariate analysis showed that the age, treatment onset time, tinnitus, and ITS treatment were associated with the prognosis (p < 0.05), while the sex, affected side, initial hearing level, or accompanied vertigo, ear fullness, or upper respiratory infection was not associated with the treatment effect (p > 0.05; Table 3). The neutrophil count, lymphocyte count, and NLR significantly differed between the effective and invalid treatment effect groups (p < 0.05), while the leukocyte count, platelet count, PLR, triglyceride level, cholesterol level, D2 polymer level, fibrinogen level, or antiviral antibody positivity rate did not differ between the two groups (p > 0.05; Table 4).
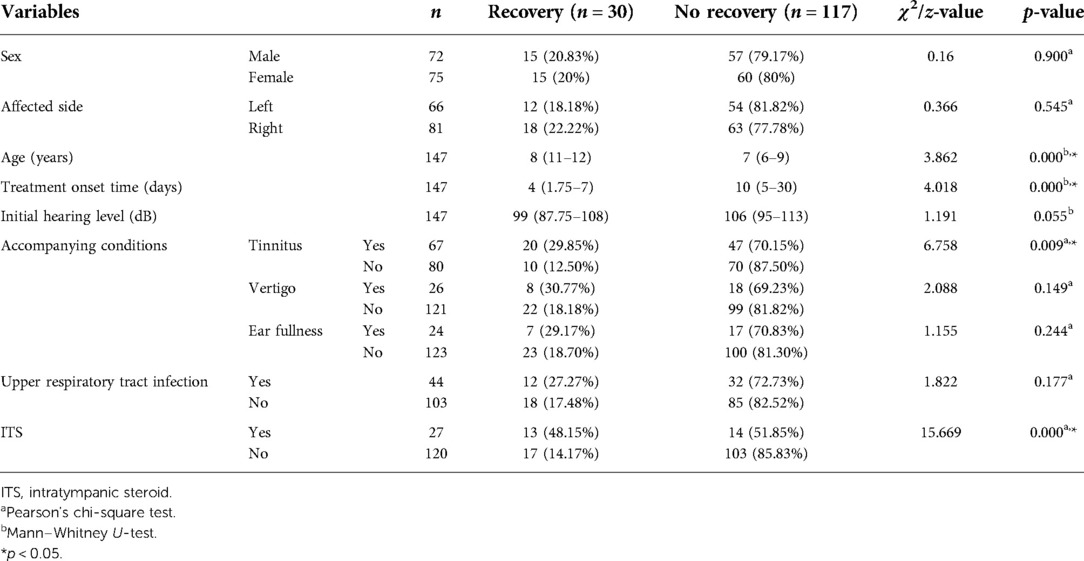
Table 3. Univariate analysis of the prognosis of children with profound sudden sensorineural hearing loss.

Table 4. Comparison of laboratory examination results between patients with and without hearing recovery.
Multivariate analysis of the treatment efficacy of children with profound SSNHL
The multivariate logistic regression analysis showed that the treatment onset time (odds ratio = 0.936, 95% confidence interval: 0.881–0.994) and ITS treatment (odds ratio = 0.174, 95% confidence interval: 0.044–0.0687) were associated with treatment efficacy (p < 0.05; Table 5).
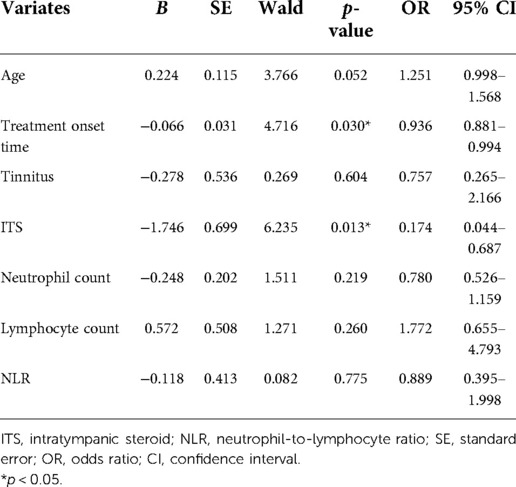
Table 5. Logistic multivariate regression analysis of the treatment efficacy in children with profound sudden sensorineural hearing loss.
Discussion
In this study, we analyzed the clinical characteristics of children with profound SSNHL and focused on investigating the risk factors to help evaluate the treatment effect. The age of onset of profound SSNHL was 8 years with no sex predilection, consistent with previous studies (11, 12). All children had unilateral SSNHL with no significant difference between the left and right sides (13). A frequent comorbidity with SSNHL was tinnitus, accounting for 45.58% of the cases, consistent with previous studies (14). Abnormal leukocyte count was found in 25.85% of the children. Cytomegalovirus IgM and IgG antibodies were found in 2.72% and 36.73% of the children, and EB virus IgM and IgG antibodies were found in 3.40% and 41.50% of the children, respectively, indicating that viral infection may be a pathogenic factor of profound SSNHL in children, similar to that reported by Pitaro et al. (14). In this study, 25 (17.01%) patients had elevated platelet count, and three (2.04%) patients had abnormal coagulation function, indicating that platelet count elevation and abnormal coagulation function may be pathogenic factors of profound SSNHL in children as they promote thrombosis and lead to cochlear microcirculation disorder. Viral infection and microcirculation disorder in children with SSNHL with the profound audiogram pattern can lead to thrombosis and vascular damage.
The effective treatment rate for profound SSNHL was 20.4% in the present study; it is associated with a poor prognosis and many influencing prognostic factors. In this study, age was associated with the treatment effectiveness of profound SSNHL in children. Patients were older (11–12 years) in the effective treatment group than in the invalid treatment effect group (6–9 years). This may be because younger children have poor perception and expression abilities, hindering the timely detection of unilateral deafness by parents, unlike older children. Kim et al. compared the prognosis of SSNHL between children (4–12 years) and adolescents (>12 years) and found that children had a significantly worse prognosis compared to adolescents (15), similar to our study findings. However, neither Qian et al. nor Li et al. found any correlation between age and SSNHL prognosis (2, 11).
In this study, children with tinnitus (29.85%) had a significantly better prognosis than those without tinnitus (12.50%; p < 0.05), indicating that tinnitus was a positive prognostic factor, consistent with previous studies (2, 6, 15). The presence of tinnitus suggests that inner ear hair cells are functional. Kruntorád et al. found that children with tinnitus have a 54.5% chance of hearing restitution, while those without tinnitus have a 35.3% chance (16). The recovery rate of combined tinnitus was higher than that reported in our study. A meta-analysis also revealed that patients with tinnitus had 2.2 times better hearing with partial or complete improvement than those without tinnitus (10). However, Li et al. analyzed prognostic factors of SSNHL in 101 children (113 ears) and found that tinnitus was not related to the prognosis (17). Ashtiani et al. found that tinnitus is a negative prognostic factor (18). The role of tinnitus in the prognosis of SSNHL with the profound audiogram pattern is controversial and should be further explored. In addition, vertigo and ear fullness were not associated with the prognosis (p > 0.05), consistent with previous studies (11, 16, 17).
A meta-analysis classified prognostic biomarkers of SSNHL in adults into six categories: inflammation, metabolism, coagulation, immunity, oxidation, and others. The analysis of the relationship of these factors with the prognosis of SSNHL revealed that low monocyte count, NLR, and fibrinogen level were correlated with the prognosis (19). The meta-analysis conducted by Cao et al. showed that the NLR, PLR, neutrophil count, and lymphocyte count were closely associated with the prognosis of SSNHL in adults (20). Studies also identified that NLR was a prognostic biomarker of SSNHL in adults (21, 22). Ha et al. showed that NLR had some prognostic value for SSNHL in children (23). In this study, blood routine, serum biochemical, coagulation, and viral antibody tests revealed that the neutrophil count and NLR were lower while the lymphocyte count was higher in the effective treatment group than in the invalid treatment effect group, consistent with Lee et al.'s study (24). Elevated neutrophil count and NLR and reduced lymphocyte count were associated with a poor prognosis, possibly because elevated NLR increases inflammation, and elevated neutrophil count can cause endothelial damage, leading to microcirculation damage, in addition to lymphocyte apoptosis occurrence during inflammation, reducing the lymphocyte count. The multivariate analysis showed that none of these parameters were independent risk factors for the prognosis of profound SSNHL in children, suggesting that these factors do not fully determine the onset or prognosis of profound SSNHL in children. Further basic and clinical studies should be performed in the future.
The AAO-HNS guidelines recommend early initiation of treatment, i.e., within 2 weeks of disease onset, for SSNHL (1). In this study, children with effective treatment had visited much earlier than those with invalid treatment effect, with an average treatment onset time of approximately 8 days. Liu et al. found that in adolescents with sudden deafness, early treatment (usually <8 days) had a better prognosis (25). Bulgurcu et al. also found that prognostic factors had no significant effect when the treatment for idiopathic sudden hearing loss was initiated within 7 days from the onset of hearing loss (26). Chung et al. performed a multivariate analysis of the prognostic factors of SSNHL and found that early treatment was associated with a good prognosis (6). Our study found that treatment onset time was an independent risk factor affecting the prognosis of SSNHL with the profound audiogram pattern, consistent with previous studies (2, 9, 27). This may be related to the microcirculation disorder of the inner ear. The later the treatment begins, the more likely it is to cause hypoxia and hair cell damage in the inner ear. Inner ear hair cell damage is reversible when treated early but may be permanent and irreversible otherwise. SSNHL in children is a medical emergency in the department of otolaryngology and should be diagnosed and treated as early as possible for good treatment efficacy.
The guidelines also recommended SST to treat SSNHL with ITS administration as a salvage treatment within 2–6 weeks (1). SST has become the most widely accepted treatment for both adults and children. However, studies investigating ITS administration in children have been scarce. ITS administration requires patient cooperation, and young children are less cooperative. It also carries risks of pain, bleeding, and infection, limiting its application in children (28). Previous studies revealed that ITS administration could benefit children (15). In this study, 27 children were administered with SST + ITS, with a significantly higher treatment response rate (48.15%) than those who were administered with SST alone (14.17%; p < 0.05), which suggests that ITS could be effective for profound SSNHL in children. SST is the first-line treatment of SSNHL in children, and salvage therapy with ITS treatment is helpful after treatment failure with SST (2, 29). However, data are lacking on ITS administration in children with profound SSNHL. Therefore, further large-scale multicenter prospective studies are required to investigate the efficacy and safety of ITS administration in children.
This study had some limitations. First, it was a retrospective study with a small sample size, which may lead to bias. Second, the treatment effect analysis of SSNHL were performed according to the Chinese standards, which differ from the international and Siegel standards. Third, ITS treatment in childen is controversial. This was not a randomized controlled trial. Future large-scale prospective studies should be performed with international standards for the diagnosis and treatment of SSNHL in children.
Conclusion
In this study, profound SSNHL in children had a poor prognosis. The prognosis was associated with the treatment onset time. Therefore, it should be treated as early as possible to improve treatment efficacy and restore hearing in children to avoid permanent hearing loss. ITS administration could be effective for profound SSNHL in children.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study protocol was approved by the Medical Ethics Committee of the Children’s Affiliated Hospital of Chongqing Medical University The requirement to obtain informed consent was waived owing to the retrospective nature of the study. However, written informed consent was obtained from the guardians of the children for administering systemic steroid therapy (SST) and intratympanic steroid (ITS) treatment.
Author contributions
LX collected data and wrote the paper. SS and JL statistical analysis and finished statistical analysis. YJ, YS and HY continued to check data and paper. LD proposed ideas and finished project administration. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review and MJEditor (www.mjeditor.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA, et al. Clinical practice guideline: sudden hearing loss (update) executive summary. Otolaryngol Head Neck Surg. (2019) 161(2):195–210. doi: 10.1177/0194599819859883
2. Qian Y, Zhong S, Hu G, Kang H, Wang L, Lei Y. Sudden sensorineural hearing loss in children: a report of 75 cases. Otol Neurotol. (2019) 39(8):1018–24. doi: 10.1097/MAO.0000000000001891
3. Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Guideline of diagnosis and treatment of sudden deafness (2015). Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 50(6):443–7. doi: 10.3760/cma.j.issn.1673-0860.2015.06.002
4. Wen YH, Chen PR, Wu HP. Prognostic factors of profound idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. (2014) 271(6):1423–9. doi: 10.1007/s00405-013-2593-y
5. Alexander T, Harris J. Incidence of sudden sensorineural hearing loss. Otol Neurotol. (2013) 34(9):1586–9. doi: 10.1097/MAO.0000000000000222
6. Chung JH, Cho SH, Jeong JH, Park CW, Lee SH. Multivariate analysis of prognostic factors foridiopathic sudden sensorineural hearing loss in children. Laryngoscope. (2015) 125(9):2209–15. doi: 10.1002/lary.25196
7. Jecmenica J, Bajec Opancina A. Sudden hearing loss in children. Clin Pediatr. (2014) 53(9):874–8. doi: 10.1177/0009922814533402
8. Na SY, Kim MG, Hong SM, Chung JH, Kang HM, Yeo SG. Comparison of sudden deafness in adults and children. Clin Exp Otorhinolaryngol. (2014) 7(3):165–9. doi: 10.3342/ceo.2014.7.3.165
9. Wang Y, Ren T, Jing JH, Gao N, Zhao H, Wang J. Clinical features and prognostic factors of pediatric idiopathic sudden hearing loss from moderate to profound degree. Am J Otolaryngol. (2021) 42(5):103027. doi: 10.1016/j.amjoto.2021.103027
10. Wood JW, Shaffer AD, Kitsko D, Ch DH. Sudden sensorineural hearing loss in children-management and outcomes: a meta-analysis. Laryngoscope. (2020) 131(2):425–34. doi: 10.1002/lary.28829
11. Li FJ, Wang DY, Wang HY, Wang L, Yang F, Lan L, et al. Clinical study on 136 children with sudden sensorineural hearing loss. Chin Med J. (2016) 129(8):946–52. doi: 10.4103/0366-6999.179791
12. Kizilay A, Koca ÇF. Pediatric sudden sensorineural hearing loss. J Craniofac Surg. (2017) 27(4):e364–6. doi: 10.1097/SCS.0000000000002630
13. Chen K, Jiang HY, Zong L, Wu X. Side-related differences in sudden sensorineural hearing loss in children. Int J Pediatr Otorhinolaryngol. (2018) 114:5–8. doi: 10.1016/j.ijporl.2018.08.022
14. Pitaro J, Bechor FA, Gavriel H, Marom T, Eviatar E. Sudden sensorineural hearing loss in children: etiology, management, and outcome. Int J Pediatr Otorhinolaryngol. (2016) 82:34–7. doi: 10.1016/j.ijporl.2015.12.022
15. Kim JY, Han JJ, Sunwoo WS, Koo JW, Oh SH, Park MH, et al. Sudden sensorineural hearing loss in children and adolescents: clinical characteristics and age-related prognosis. Auris Nasus Larynx. (2017) 45(3):447–55. doi: 10.1016/j.anl.2017.08.010
16. Kruntorád V, Urík M, Šikolová S. Sudden sensorineural hearing loss in children: a review of diagnosis, treatment, and prognosis. Otorinolaryngol Foniatr. (2022) 71(2):68–74. doi: 10.48095/ccorl202268
17. Li FJ, Xue XJ, Wang L, Yang FB, Wang HY, Guan J, et al. Prognostic factors of sudden sensorineural hearing loss in children. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2015) 29(22):1931–5. doi: 10.13201/j.issn.1001-1781.2015.22.001
18. Ashtiani MK, Firouzi F, Bastaninejad S, Dabiri S, Nasirmohtaram S, Saeedi N, et al. Efficacy of systemic and intratympanic corticosteroid combination therapy versus intratympanic or systemic therapy in patients with idiopathic sudden sensorineural hearing loss: a randomized controlled trial. Eur Arch Otorhinolaryngol. (2017) 275(1):89–97. doi: 10.1007/s00405-017-4808-0
19. Doo JG, Dg K, Kim Y, Yoo MC, Kim SS, Ryu J, et al. Biomarkers suggesting favorable prognostic outcomes in sudden sensorineural hearing loss. Int J Mol Sci. (2020) 21(19):1–16. doi: 10.3390/ijms21197248
20. Cao ZZ, Li ZY, Xiang HJ, Huang SY, Gao JJ, Zhan X, et al. Prognostic role of haematological indices in sudden sensorineural hearing loss: review and meta-analysis. Clin Chim Acta. (2018) 483:104–11. doi: 10.1016/j.cca.2018.04.025
21. Chen LQ, Zhang GH, Zhang ZH, Wang YF, Hu L, Wu JY. Neutrophil-to-lymphocyte ratio predicts diagnosis and prognosis of idiopathic sudden sensorineural hearing loss: a systematic review and meta-analysis. Medicine. (2018) 97(38):e12492. doi: 10.1097/MD.0000000000012492
22. Ni W, Song PJ, Jiang YD. Association between routine hematological parameters and sudden sensorineural hearing loss: a meta-analysis. J Otol. (2020) 16(1):47–54. doi: 10.1016/j.joto.2020.07.006
23. Ha R, Lim BW, Kim DH, Park JW, Cho CH, Lee JH. Predictive values of neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and other prognostic factors in pediatric idiopathic sudden sensorineural hearing loss. Int J Pediatr Otorhinolaryngol. (2019) 120:134–9. doi: 10.1016/j.ijporl.2019.02.023
24. Lee JS, Hong SK, Kim DH, Lee JH, Lee HJ, Park B, et al. The neutrophil-to-lymphocyte ratio in children with sudden sensorineural hearing loss: a retrospective study. Acta Otolaryngol. (2017) 137(1):35–8. doi: 10.1080/00016489.2016.1217561
25. Liu FX, Huang X. Analysis of the related factors affecting the prognosis of sudden deafness in adolescents. J Audiol Speech Dis. (2013) 21(4):369–71. doi: 10.3969/j.issn.1006-7299.2013.04.013
26. Bulğurcu S, Şahin B, Akgül G, Arslan İB, Çukurova İ. The effects of prognostic factors in idiopathic sudden hearing loss. Int Arch Otorhinolaryngol. (2018) 22(1):33–7. doi: 10.1055/s-0037-1603108
27. Uysal İÖ, Müderris T, Polat K, Yüce S, Gültürk S. Is the time from the onset to the treatment a prognostic indicator for hearing recovery in idiopathic sudden sensorineural hearing loss. Kulak Burun Bogaz Ihtis Derg. (2015) 25(2):70–6. doi: 10.5606/kbbihtisas.2015.53138
28. Dedhia K, Chi DH. Pediatric sudden sensorineural hearing loss: etiology, diagnosis and treatment in 20 children. Int J Pediatr Otorhinolaryngol. (2016) 88:208–12. doi: 10.1016/j.ijporl.2016.07.003
Keywords: sudden sensorineural hearing loss (SSNHL), profound, prognosis, children, intratympanic steroid
Citation: Xiao L, Su S, Liang J, Jiang Y, Shu Y, Yao H and Ding L (2022) Clinical features and prognostic factors of children with profound sudden sensorineural hearing loss. Front. Pediatr. 10:1023781. doi: 10.3389/fped.2022.1023781
Received: 20 August 2022; Accepted: 11 October 2022;
Published: 7 November 2022.
Edited by:
Norma De Oliveira Penido, Federal University of São Paulo, BrazilReviewed by:
Rafael da Costa Monsanto, University of Minnesota Twin Cities, United StatesSaba Battelino University Medical Centre Ljubljana, Slovenia
© 2022 Xiao, Su, Liang, Jiang, Shu, Yao and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Ding MTAyMDE0NTQ2NkBxcS5jb20=
Specialty Section: This article was submitted to Pediatric Otolaryngology, a section of the journal Frontiers in Pediatrics
 Ling Xiao
Ling Xiao Shuping Su1,2,3,4
Shuping Su1,2,3,4