
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 14 November 2022
Sec. Pediatric Cardiology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1012738
This article is part of the Research Topic Advances in the Care of the Pediatric Pulmonary Hypertension Patient: From the Neonate to the Adolescent-Young Adult Patient View all 15 articles
 Ivonne Wieland1*
Ivonne Wieland1* Franziska Diekmann2
Franziska Diekmann2 Julia Carlens3
Julia Carlens3 Laura Hinze1
Laura Hinze1 Katharina Lambeck1
Katharina Lambeck1 Thomas Jack2
Thomas Jack2 Georg Hansmann2*
Georg Hansmann2*
Background and objectives: Emerging evidence suggests that increased degradation of von Willebrand factor and decrease in high molecular weight multimers occurs in patients with pulmonary hypertension (PH). However, the link between acquired von Willebrand Syndrome (AVWS) type 2 and PH remains poorly understood.
Material and methods: We retrospectively evaluated the charts of 20 children with PH who underwent bilateral lung transplantation (LuTx) between 2013 and 2022. Von Willebrand variables were determined in 14 of these patients; 11 patients had complete diagnostics including multimer analysis.
Results: We confirmed AVWS in 82% of the children studied (9 of 11 patients by multimer analysis). The two remaining patients had suspected AVWS type 2 because of a VWF:Ac/VWF:Ag ratio of <0.7. Platelet dysfunction or suspicion of VWD type 1 were found in two separate patients. All but one of the 14 children with severe PH had a coagulation disorder. Most patients (9 proven, 2 suspected) had AVWS type 2. Notably, 3 of 5 patients (60%) with normal VWF:Ac/VWF:Ag ratio >0.7 had abnormal VWF multimers, indicating AVWS type 2. Hemostatic complications were observed in 4 of 12 (33%) patients with VWS and 3 of 6 (50%) patients without diagnostics and therapy.
Conclusion: For children with moderate to severe PH, we recommend systematic analysis of von Willebrand variables, including multimer analysis, PFA-100 and platelet function testing. Awareness of the diagnosis “AVWS” and adequate therapy may help to prevent these patients from bleeding complications in case of surgical interventions or trauma.
Von Willebrand factor (VWF) is a protein, which is required for adhesion, playing an important role in hemostasis. Various hereditary types (type 1–3) of von Willebrand disease (VWD) are described, in which mainly a reduction of VWF and/or VWF-multimers is involved (1). According to published data on the prevalence of VWD, hereditary VWD is found in up to 1% of the population, but the proportion of patients with VWD with clinical relevance is even smaller (one in 10,000 people) (1). VWD type 1 with reduced VWF, is the most common type (70%–80%), followed by type 2 that is characterised by reduction or loss of large VWF multimers (20%–25%), and type 3 with a complete loss of VWF (<1%–5%) (1, 2). Acquired von Willebrand Syndrome (AVWS) was reported to have a prevalence of 0.04% to 0.13% in the population (3). Generally, AVWS is associated with an underlying disorder. According to the International Society on Thrombosis and Haemostasis (ISTH) registry (4) and a recent report (5), the most common conditions associated with AVWS are: lymphoproliferative/haematological malignancy (48%), cardiovascular (21%), myeloproliferative (15%), other neoplastic (5%) and autoimmune disorders (2%–5%) or various other causes (plasma-mediated hyperfibrinolysis, glycogen storage disease, uremia, hypothyroidism) (<10%) (4, 5).
In the paediatric population, AVWS tends to be underdiagnosed and often unknown. However, data on AVWS in childhood are rare and mostly case reports or small case series. Most data are reported for AVWS in pediatric patients with congenital heart diseases (6–14).
Acquired AVWS as type 2A is especially common in adult patients with aortic valve stenosis (vAS) (15). 67%–92% of patients with severe aortic stenosis (vAS) are reported to develop AVWS, and 21% of those patients suffer from bleedings (16). Pathophysiologically, it was assumed that the development of AVWS in vAS is caused by the acceleration of blood flow at the aortic valve, resulting in shear stress for large plasma proteins like VWF, leading to consecutive proteolytic cleavage of VWF and a decrease or loss of the high molecular weight multimers (HMWMs). In addition to aortic stenosis, AVWS was reported for instance in hypertrophic obstructive cardiomyopathy (HOCM), tetralogy of Fallot, pulmonary hypertension and mitral regurgitation (17).
According to the World Symposium on Pulmonary Hypertension (WSPH, 2018), pulmonary hypertension (PH) is a condition that is divided into 5 subgroups (18–20).
The pathobiology of pulmonary arterial hypertension (PAH) is a complex and multifactorial process, in which peripheral artery loss and obstructive vascular remodeling cause a rise in pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), resulting in progressive right heart failure and death (21). Inflammation, delayed shear adaptation and endothelial cell dysfunction seem to play crucial roles in this process (22–25). Wall shear stress-dependent changes in pulmonary arterial lumen diameter were found to be a persistent remodeling response (26).
Especially in young patients, the etiology of PH (groups 1–5) is very heterogeneous across the different age groups, but most frequently associated with congenital heart disease (CHD; group 1 PH = pulmonary arterial hypertension), followed by developmental lung disease (group 3 PH; mainly bronchopulmonary dysplasia) and so-called idiopathic PAH forms (18, 19, 21). The estimated incidence for idiopathic PAH (IPAH)/heritable PAH (HPAH) and (non-transient) CHD-associated PAH is 0.7 and 2.2/million, and the estimated prevalence is 4.4 and 15.6/million children, respectively. (19).
From the late 1980s, emerging evidence suggested that PAH patients have increased degradation of VWF and a decrease in high molecular weight multimers, and/or platelet dysfunction, which are typical findings of AVWS type 2 (27). Abnormal flow or shear stress through the pulmonary vessels has been suggested to cause PAH in 1995 (28). A study on 30 PAH patients showed that those patients with abnormalities in VWF had a reduced 1-year survival rate compared with those with normal VWF (29).
Apart from isolated cases, such as the case report on an adolescent woman with PAH and menorrhagia suffering from AVWS type 2 (30), very little data on AVWS in pediatric PH has been published so far. In another cohort of 16 patients with CHD, 5 had PAH. All of them suffered from AVWS type 2 and presented with bleeding symptoms such as epistaxis, menorrhagia or gum bleeding (31). Surprisingly, in a small study on 8 children with PAH, all of them had AVWS type 1 with a normal multimer analysis differing from other reports describing an association with abnormalities in VWF or AVWS type 2 (32).
In this retrospective study, we analyzed the von Willebrand variables in all children with severe PH and right ventricular (RV) failure undergoing lung transplantation (LuTx) at Hannover Medical School between December 2013 and February 2022. We hypothesized that most end-stage PH patients have evident AVWS type 2 that is relevant to patient management perioperatively (e.g., VWF supplementation).
We retrospectively evaluated the charts of 20 children with PH undergoing lung transplantation at Hannover Medical School between December 2013 and February 2022 for von Willebrand variables (von Willebrand factor activity (VWF:Ac); von Willebrand factor antigen (VWF:Ag), multimers) and, when performed, for platelet function analysis (Figure 1).
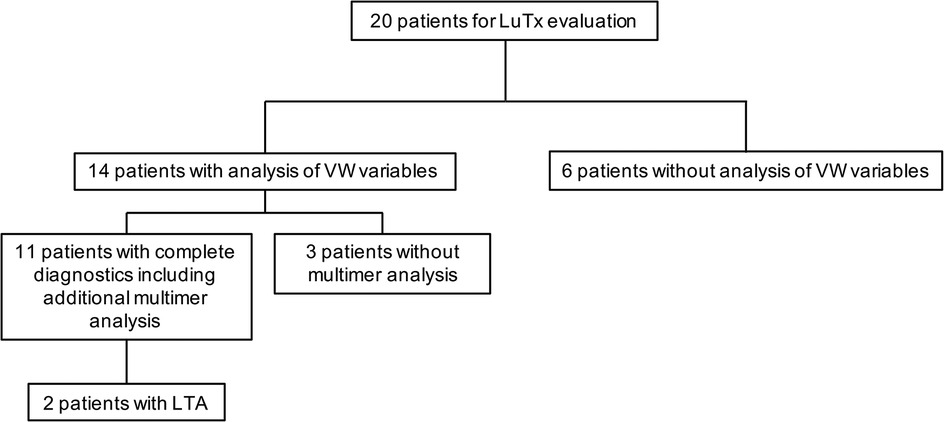
Figure 1. Diagnostic workup. The indicated VW variables include von Willebrand factor activity (VWF:Ac) and von Willebrand factor antigen (VWF:Ag). LTA, light transmission aggregometry; LuTx, lung transplantation; VW, von Willebrand.
Measurement of von Willebrand parameters were performed once during evaluation for lung transplantation in the central laboratory of MHH. VWF:Ag and VWF:Ac were determined in the Siemens instruments tubimetricly. PFA-100 was measured on COLL/EPI and COLL/ADP membranes on a Siemens instrument. For platelet function analysis, light transmission aggregometry (LTA, so called “Born-Aggregation”) was performed in the APACP 4S Plus/DiaSys instrument.
Von Willebrand Multimer analysis was done externally by the laboratory AMEDES, Hamburg, following standardized protocols.
Patients with a VWF:Ac/VWF:Ag ratio <0.7 are suspected of having VWS type 2 according to the ASH guidelines (33). AVWS type 2 was confirmed with a loss of largest and reduction of large multimers in multimer analysis independent of the VWF:Ac/VWF:Ag ratio.
Von Willebrand variables (VWF:Ag and/or VWF:Ac) ≤ 50% were classified as suspected VWD type 1. We cannot differ between congenital or acquired.
Von Willebrand variables, at least in our laboratory, were determined in only 14 of the 20 patients. Twelve of these 14 patients were female. The mean age at transplantation was 10.9 years (range 1.9–21.3 years) (Table 1, Supplementary Table S1).
Nineteen patients were classified as group 1 PH (idiopathic (n = 8) or heritable PAH (n = 5), PAH-CHD (PAH associated with congenital heart disease, n = 3) and PVOD/PCH [pulmonary veno-occlusive disease PVOD)/pulmonary capillary hemangiomatosis (PCH), n = 3] and one as group 3 PH (PH associated with developmental lung disease) (Table 1, Supplementary Table S1).
All patients underwent ECMO peri transplantation. The management of PAH patients undergoing LuTx at our center using veno-arterial ECMO (VA-ECMO) support has previously been published (34, 35). Only patients with known or suspected VWS received VWF supplementation during VA-ECMO support pre and post LuTx, no patient without known VWS. We further identified complications related to hemorrhage and thromboembolic events in all 20 pediatric patients pre and post lung transplantation (LuTx).
Von Willebrand variables (VWF:Activity (VWF:Ac) and VWF:Antigen (VWF:Ag)) in our locale laboratory were determined in 14 of these patients, but the diagnostic workup was complete in only 11 patients and confirmed by multimer analysis (Figure 1).
In these 14 patients, we could find 9 (64%) with confirmed AVWS type 2, 2 (14%) with suspected AVWS type 2, 1 (7%) platelet dysfunction and 1 (7%) with suspected VWD type 1 (without excluded type 2 because of missing multimer analysis) (Figure 2, Table 2).
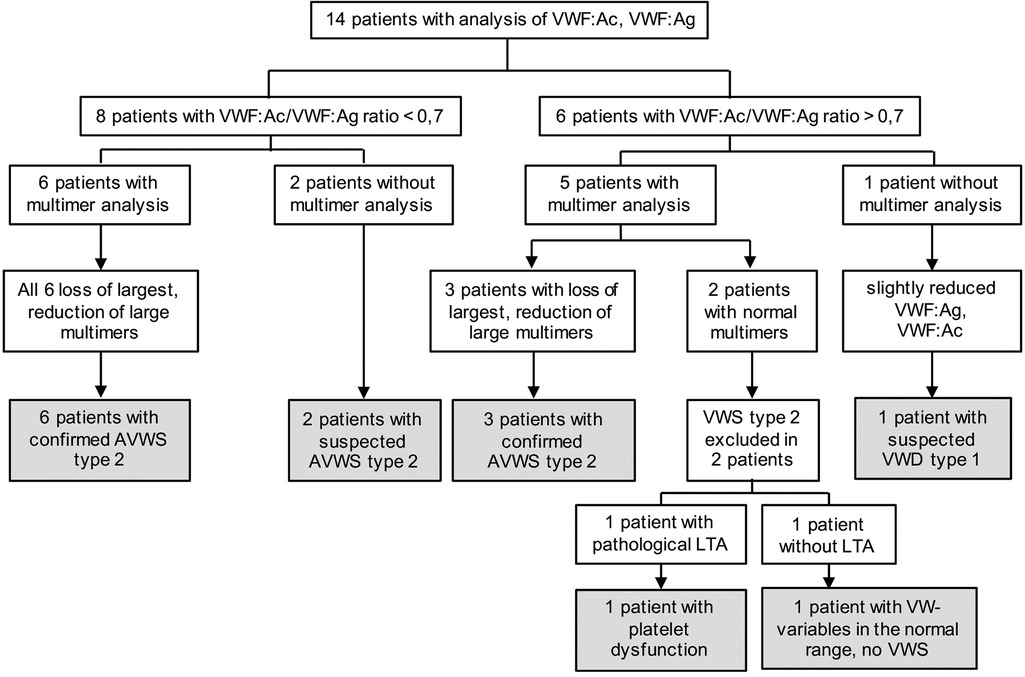
Figure 2. Results of diagnostic workup. AVWS, acquired von Willebrand syndrome; LTA, light transmission aggregometry, VWF: Ac, von Willebrand factor activity; VWF: Ag, von Willebrand factor antigen; VWS, von Willebrand syndrome.
Eight of the 14 patients (57%) were suspected for AVWS type 2 due to a ratio of <0.7. In six of these patients, multimer analysis was performed and all of them (100%) demonstrated a decrease or loss of HMWM, which is typical of AVWS type 2. The remaining two patients were classified as suspected AVWS type 2 because of missing multimer analysis.
Six of the 14 patients (43%) had a normal VWF:Ac/VWF:Ag ratio (>0.7). In five of them, multimer analysis was performed. Despite a normal VWF:Ac/VWF:Ag ratio of >0.7, 3 of these 5 patients (60%) had a decrease or loss of HMWM, indicating these patients suffer from AVWS type 2. In 2 of these 3 patients, PFA-100 was performed and was highly increased (>300s).
Of the 2 patients with excluded AVWS type 2 by multimer analysis, one patient (patient 11) had pathological results in platelet function analysis. Despite normal platelet numbers, PFA-100 was highly increased (>300s) and in LTA, aggregations of each stimulus (ADP 10 µM, adrenalin 5 µM, collagen 5 µg/ml and ristocetin 1.0 mg/ml) were decreased, indicating platelet dysfunction in this patient. Due to the slightly reduced von Willebrand variables, patient 8 is suspected for at least VWD type 1, without fully excluded type 2 because of missing multimer analysis.
Four patients were bridged to transplantation on veno-arterial ECMO. All patients were transplanted on cardiopulmonary bypass or ECMO and all but one (patient number 6) remained on planned ECMO support after transplantation. Patients on ECMO and/or cardiopulmonary bypass received anticoagulation. Patients with known or suspected VWS were treated preventively with VWF containing concentrate during VA-ECMO to prevent bleeding complications.
We observed bleeding complications (hematothorax) in 4 patients (patients 5, 16, 18, 19) in the first days after transplantation. Two (2 of 12; 17%) of these occurred in patients with confirmed/suspected AVWS and two (2 of 6; 33%) in patients without extended hemostatic diagnostic work-up. One additional patient (number 1) suffered from hematothorax because of cannula dislocation during emergency ECMO cannulation before transplantation. Because the bleeding was caused by cannula dislocation, we do not include this patient in the group with bleeding complications.
We observed thromboembolic complications in 6 patients (patients 2, 5, 12, 13, 16, 18): four developed emboli after lung transplantation with ischemia in limb arteries during ECMO therapy or shortly after ECMO explantation, one suffered an infarction of the A. cerebri media on ECMO and one suffered a spinal cord ischemia before lung transplantation while on bridge-to-transplantation ECMO support. Three (3 of 12; 25%) of these occurred in patients with confirmed/suspected AVWS and three (3 of 6; 50%) in patients without extended hemostatic diagnostic work-up.
Both bleeding and thromboembolic events occurred in 3 patients [1 with AVWS (patient 16) and 2 without diagnostic workup (patients 5, 18)]. Details are summarized in Table 3.
The detection of potential bleeding disorders is important in patients with advanced cardiovascular and pulmonary disease, including group 1 PH (PAH) and group 3 PH, especially if those patients undergo major surgery such as lung transplantation or creation of endogenous Potts shunt (35–37).
In all but one of the 14 children with severe PH from our cohort with diagnostics, we detected a coagulation disorder. AVWS type 2 (confirmed or suspected: 11/13; 85%) was the most common coagulation disorder. We found confirmed AVWS in 9 of 14 (64%) patients [9 of 11 children with complete workup (82%)]. Two more patients were suspected for AVWS type 2 because of VWF:Ac/VWF:Ag ratio <0.7. Two others suffered from platelet dysfunction (n = 1) or were suspected of having at least VWS type 1 (n = 1; without fully excluded type 2 because of missing multimer analysis). In one patient without coagulation abnormality, platelet function analysis was missing.
Multimer analysis is proving AVWS type 2 and should be performed in all patients if AVWS type 2 is suspected. However, multimer analysis is a time-consuming method only available in some specialized laboratories. For urgent clinical questions other tests are required that initially indicate the presence of AVWS type 2 - the VWF:Ac/VWF:Ag ratio <0.7 (33). In all our patients with a VWF:Ac/VWF:Ag ratio <0.7, multimer analysis confimed AVWS type 2. However, 60% of the patients with a normal VWF:Ac/VWF:Ag ratio >0.7 also had a decrease or loss of HMWM, indicating these patients suffer from AVWS type 2 too. This finding is in line with other reports (13, 38). For instance, Icheva et al. reported for their cohort of patients with CHD a very high specificity (100%) of the ratio VWF:RCo/VWF:Ag (RCo; Ristocetin-Cofactor) to detect an AVWS, but a very low sensitivity (38%). (13). In contrast, Tiede et al. found a sensitivity of 86% (38). Because of the relatively low sensitivity of the VWF:Ac/VWF:Ag ratio, some patients with AVWS type 2 can be missed using only this method. Possibly, this diagnostic uncertainty could be reduced by additional analysis of PFA-100, for which a sensitivity of >90% has been described for VWD type 2 (39). Concerning the sensitivity and specificity of the different tests, further investigations are necessary.
The pathophysiology of AVWS in PH seems to be comparable to that in patients with aortic stenosis and may also result from increased shear stress for large plasma proteins like VWF. The latter leads to consecutive proteolytic cleavage of VWF and a decrease or loss of HMWMs (24).
We found a higher proportion of patients with AVWS type 2 in our pediatric cohort with end-stage PH than previously reported (32). In the small study of Pelland-Marcotte, 8 of 14 patients with PAH have shown bleeding symptoms and/or laboratory abnormalities but with normal VW-multimers, surprisingly excluding type 2 AVWS. One of these patients was in NYHA functional class (FC) III and underwent lung transplantation. The 7 other patients were in NYHA FC I (n = 4), NYHA FC I-II (n = 2) and NYHA II-III (n = 1). In contrast, we only analyzed patients with severe PAH immediately prior to LuTx. Therefore, the results of our study may indicate that the risk of AVWS increases with the severity of PAH. This observation is in line with the much earlier report of Lopes et al., demonstrating that patients with PAH and abnormalities in circulating VWF have a reduced 1-year-survival (29).
The clinical importance of an increased bleeding risk in patients with AVWS is the subject of a controversial discussion (15, 40–42). On the one hand, published data show that AVWS is not or only mildly associated with a relevant bleeding risk in adults with aortic stenosis (16). No increased bleeding tendency was reported, neither in daily life nor during surgery of aortic valve stenosis in adults (40–42). On the other hand, a meta-analysis of patients with AVWS, including data from two different registries, demonstrated an increased rate of patients (77%) with bleeding diathesis in the subgroup of patients with underlying cardiovascular disease (4). However, data on the prevalence and clinical bleeding tendency in PH patients with AVWS, especially in children with PH, are missing. Recently, a report on a patient with end-stage PAH and a major bleeding following reverse Potts shunt procedure was published (43). Two other small clinical studies showed that 4 of 5 patients with PAH (31) and 7 of 8 patients with PH (32) suffered from bleeding symptoms (31, 32), suggesting a high percentage of bleeding in VWS associated with PAH. The most common bleeding problems included epistaxis, menorrhagia, and perioperative bleedings like hereditary VWD (30–32, 43).
Re-Thoracotomy for major bleeding (hematothorax) is a major and common complication in the first hours and days after lung transplantation in young patients with severe PAH. In 117 lung transplanted pediatric patients from our center (including children with pulmonary hypertension and other diagnoses), 13 (11.1%) required re-thoracotomy for hematothorax after transplantation (35). Based on our findings and previous experience (35, 36), we substitute VWF containing concentrate in all PAH patients with confirmed or suspected VWS type 1 or 2, prior to, during and after invasive procedures or surgery, and also during VA-ECMO while being heparinized.
In the group of patients with VWS, 4 of 12 patients (33%) suffered from hemostatic complications (2 embolisms, 1 hematothorax, 1 both). In the group without extended diagnostics, three of six patients (50%) had hemostatic complications (1 thromboembolism and 2 both, thromboembolisms and hematothoraces). These complications were usually in temporal relation to the ECMO support. The etiology of these complications is likely multifactorial. Patients on VA-ECMO support require anticoagulation with heparin but were all substituted with coagulative agents at the same time. Both bleedings and thrombotic events are well known complications in ECMO treatment. Despite substitution with VWF containing concentrate to improve hemostasis, we did not observe an increase of thromboembolic events in the group with VWS (25% vs. 50%). Due to the small number of patients, a statistic analysis between AVWS and clinical outcome was not possible. Further investigations are necessary to determine whether hemostatic diagnostics can improve clinical outcome.
The limitation of our study is the relatively small number of patients. However, these patients showed a low heterogeneity and a comparable disease severity at the time of analysis shortly before LuTx.
Due to the high risk of bleeding complications in patients with severe PH, we recommend analysis of von Willebrand variables (VWF:Ag, VWF:Ac), multimer analysis, PFA-100 and platelet function testing [light transmission aggregometry (LTA, “Born-Aggregation”)] in all patients with severe PH. At least, multimer analysis is evidential for AVWS type 2 and should be performed in all of these patients. Early suspicion of evident AVWS type 2, before receiving the result of the mandatory multimer analysis, should be raised in case of a VWF:Ac/VWF:Ag ratio <0.7 and a prolongation of PFA-100. Of note, because of relatively low sensitivity of this ratio, some patients with AVWS could be missed. The recommendation to use the VWF:Ac/VWF:Ag ratio is for practical reasons, because multimer analysis is a time-consuming method and not available in most hospitals.
Early diagnosis of AVWS type 2 in critically ill PH patients undergoing ECMO cannulation and/or major surgery could give the health care providers the opportunity to treat or prevent potential hemorrhagic events and may improve the patients' safety and outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The authors meet the following criteria: 1. Substantial contributions to conception and design, 2. Acquisition of samples, 3. Analysis of data, 4. Interpretation of data, 5. Drafting the article or revising it critically for important intellectual content, 6. Final approval of the version to be published. IW 1, 3–6; FD 3, 5, 6; JC 1–6; LH 3, 5, 6; KL 3, 5, 6; TJ 3–6; GH 1–6. All authors contributed to the article and approved the submitted version.
G.H. receives funding from the German Research Foundation (DFG KFO311, grant HA4348/6-2, and DFG grant HA4348/2-2), the Federal Ministry of Education and Research (BMBF; 01KC2001B, 03VP08053) and the European Pediatric Pulmonary Vascular Disease Network (www.pvdnetwork.org).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.1012738/full#supplementary-material.
1. Leebeek FW, Eikenboom JC. Von Willebrand's disease. N Engl J Med. (2016) 375:2067–80. doi: 10.1056/NEJMra1601561
2. Breaky VR, Carcao M. Von Willebrand disease in children. In: Blanchette VS, Brandao LR, Breakey VR, Revel-Vilk S, editors. Sickkids handbook of pediatric thrombosis and hemostasis. 2nd ed. Basel: Karger (2017). p. 97–112.
3. Lison S, Dietrich W, Spannagl M. Unexpected bleeding in the operating room: the role of acquired von Willebrand disease. Anesth Analg. (2012) 114:73–81. doi: 10.1213/ANE.0b013e318236b16a
4. Federici AB, Rand JH, Bucciarelli P, Budde U, van Genderen PJJ, Mohri H, et al. Subcommittee on von Willebrand factor. Acquired von Willebrand syndrome: data from an international registry. Thromb Haemost. (2000) 84:345–9; Erratum in: Thromb Haemost (2000) 84(4):739. doi: 10.1055/s-0037-1614018
5. Leebeek FWG. New developments in diagnosis and management of acquired hemophilia and acquired von Willebrand syndrome. Hemasphere. (2021) 5:e586. doi: 10.1097/HS9.0000000000000586
6. Avila ML, Lee K-J, Bouskill V, Rand ML, James P, Carcao M. Acquired von Willebrand syndrome in paediatric patients with congenital heart disease: challenges in the diagnosis and management of this rare condition. Haemophilia. (2015) 21:e89–92. doi: 10.1111/hae.12567
7. Virk H, Jain R, Kumar N, Uppal V, Bose SK, Bansal D, et al. Varying test results: a clue to pediatric acquired von Willebrand syndrome. Pediatr Blood Cancer. (2019) 66:e27651. doi: 10.1002/pbc.27651
8. Callaghan MU, Wong TE, Federici AB. Treatment of acquired von Willebrand syndrome in childhood. Blood. (2013) 122(12):2019–22. doi: 10.1182/blood-2012-10-435719
9. Sandrock-Lang K, Glonnegger H, Zieger B. Acquired von Willebrand syndrome in children. Hämostaseologie. (2022) 42:117–22. doi: 10.1055/a-1790-6156
10. Arslan MT, Ozyurek R, Kavakli K, Levent E, Ulger Z, Gurses D, et al. Frequency of acquired von Willebrand's disease in children with congenital heart disease. Acta Cardiol. (2007) 62:403–8. doi: 10.2143/AC.62.4.2022285
11. Rauch R, Budde U, Koch A, Girisch M, Hofbeck M. Acquired von Willebrand syndrome in children with patent ductus arteriosus. Heart. (2002) 88:87–8. doi: 10.1136/heart.88.1.87
12. Wiegand G, Hofbeck M, Zenker M, Budde U, Rauch R. Bleeding diathesis in noonansyndrome: is acquired von Willebrand syndrome the clue? Thromb Res. (2012) 130(5):e251–4. doi: 10.1016/j.thromres.2012.08.314
13. Icheva V, Nowak-Machen M, Budde U, Jaschonek K, Neunhoeffer F, Kump M, et al. Acquired von Willebrand syndrome in congenital heart disease surgery: results from an observational case-series. J Thromb Haemost. (2018) 16:2150–8. doi: 10.1111/jth.14208
14. Loeffelbein F, Funk D, Nakamura L, Zieger B, Grohmann J, Siepe M, et al. Shear-stress induced acquired von Willebrand syndrome in children with congenital heart disease. Interact Cardiovasc Thorac Surg. (2014) 19:926–32. doi: 10.1093/icvts/ivu305
15. Vincentelli A, Susen S, Le Tourneau T, Six I, Fabre O, Juthier F, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. (2003) 349:343–9. doi: 10.1056/NEJMoa022831
16. Langer AL, Connell NT. Acquired von Willebrand syndrome. Hematol Oncol Clin North Am. (2021) 35:1103–16. doi: 10.1016/j.hoc.2021.07.005
17. Horiuchi H, Doman T, Kokame K, Saiki Y, Matsumoto M. Acquired von Willebrand syndrome associated with cardiovascular diseases. J Atheroscler Thromb. (2019) 26:303–14. doi: 10.5551/jat.RV170
18. Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, et al. Updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European pediatric pulmonary vascular disease network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. (2019) 38:879–901. doi: 10.1016/j.healun.2019.06.022
19. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. (2022) 43:3618–3731. doi: 10.1093/eurheartj/ehac237
20. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53:1801913. doi: 10.1183/13993003.01913-2018
21. Hansmann G. Pulmonary hypertension in infants, children, and young adults. J Am Coll Cardiol. (2017) 69:2551–69. doi: 10.1016/j.jacc.2017.03.575
22. Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. (2019) 53:1801887. doi: 10.1183/13993003.01887-2018
23. Kurakula K, Smolders VFED, Tura-Ceide O, Jukema JW, Quax PHA, Goumans MJ. Endothelial dysfunction in pulmonary hypertension: cause or consequence? Biomedicines. (2021) 9:57. doi: 10.3390/biomedicines9010057
24. Szulcek R, Happé CM, Rol N, Fontijn RD, Dickhoff C, Hartemink KJ, et al. Delayed microvascular shear adaptation in pulmonary arterial hypertension. Role of platelet endothelial cell adhesion molecule-1 cleavage. Am J Respir Crit Care Med. (2016) 193:1410–20. doi: 10.1164/rccm.201506-1231OC
25. Bonnet S, Provencher S. Shear stress maladaptation in pulmonary arterial hypertension. An ageless concept. Am J Respir Crit Care Med. (2016) 193:1331–2. doi: 10.1164/rccm.201601-0113ED
26. Pewowaruk R, Lamers L, Roldán-Alzate A. Longitudinal evolution of pulmonary artery wall shear stress in a swine model of pulmonary artery stenosis and stent interventions. Ann Biomed Eng. (2021) 49:1477–92. doi: 10.1007/s10439-020-02696-6
27. Rabinovitch M, Andrew M, Thom H, Trusler GA, Williams WG, Rowe RD, et al. Abnormal endothelial factor VIII associated with pulmonary hypertension and congenital heart defects. Circulation. (1987) 76:1043–52. doi: 10.1161/01.cir.76.5.1043
28. Lopes AA, Maeda NY. Abnormal degradation of von Willebrand factor main subunit in pulmonary hypertension. Eur Respir J. (1995) 8:530–6. doi: 10.1183/09031936.95.08040530
29. Lopes AA, Maeda NY, Bydlowski SP. Abnormalities in circulating von Willebrand factor and survival in pulmonary hypertension. Am J Med. (1998) 105:21–6. doi: 10.1016/s0002-9343(98)00138-7
30. Sokkary NA, Dietrich JE, Venkateswaran L. Idiopathic pulmonary hypertension causing acquired von Willebrand disease and menorrhagia. J Pediatr Adolesc Gynecol. (2011) 24:e107–9. doi: 10.1016/j.jpag.2011.04.004
31. Hashmi SK, Velasquez MP, Yee DL, Hui SK, Mahoney D Jr, Srivaths LV. Pediatric acquired von Willebrand syndrome in cardiopulmonary disorders: do laboratory abnormalities predict bleeding risk? J Pediatr Hematol Oncol. (2017) 39:121–5. doi: 10.1097/MPH.0000000000000738
32. Pelland-Marcotte MC, Humpl T, James PD, Rand ML, Bouskill V, Reyes JT, et al. Idiopathic pulmonary arterial hypertension – a unrecognized cause of high-shear high-flow haemostatic defects (otherwise referred to as acquired von Willebrand syndrome) in children. Br J Haematol. (2018) 183:267–75. doi: 10.1111/bjh.15530
33. James PD, Connell NT, Ameer B, Di Paola J, Eikenboom J, Giraud N, et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. (2021) 5:280–300. doi: 10.1182/bloodadvances.2020003265
34. Tudorache I, Sommer W, Kühn C, Wiesner O, Hadem J, Fühner T, et al. Lung transplantation for severe pulmonary hypertension–awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation. (2015) 99:451–8. doi: 10.1097/TP.0000000000000348
35. Iablonskii P, Carlens J, Mueller C, Aburahma K, Niehaus A, Boethig D, et al. Indications and outcome after lung transplantation in children under 12 years of age: a 16-year single center experience. J Heart Lung Transplant. (2022) 41:226–36. doi: 10.1016/j.healun.2021.10.012
36. Hansmann G, Diekmann F, Chouvarine P, Ius F, Carlens J, Schwerk N, et al. Full recovery of right ventricular systolic function in children undergoing bilateral lung transplantation for severe PAH. J Heart Lung Transplant. (2022) 41:187–98. doi: 10.1016/j.healun.2021.10.014
37. Grady RM, Canter MW, Wan F, Shmalts AA, Coleman RD, Beghetti M, et al. International registry potts shunt. Pulmonary-to-systemic arterial shunt to treat children with severe pulmonary hypertension. J Am Coll Cardiol. (2021) 78(5):468–77. doi: 10.1016/j.jacc.2021.05.039
38. Tiede A, Priesack J, Werwitzke S, Bohlmanni K, Oortwijn B, Lenting P, et al. Diagnostic workup of patients with acquired von Willebrand syndrom: a retrospective single-centre cohort study. J Throomb Haemost. (2008) 6:569–76. doi: 10.1111/j.1538-7836.2008.02909.x
39. Favalore EJ. The utility of PFA-100 in identification of von Willebrand disease: a concise review. Semin Thromb Hemost. (2006) 32:537–45. doi: 10.1055/s-2006-947869
40. Casonato A, Sponga S, Pontara E, Cattini MG, Basso C, Thiene G, et al. von Willebrand factor abnormalities in aortic valve stenosis: pathophysiology and impact on bleeding. Thromb Haemost. (2011) 106:58–66. doi: 10.1160/TH10-10-0634
41. Mikkelsen MM, Fenger-Eriksen C, Johnsen SP, Christensen TD, Sørensen B. No excess surgical blood loss in patients with acquired type 2A von Willebrand disease. Scand Cardiovasc J. (2011) 45:120–6. doi: 10.3109/14017431.2010.545143
42. Solomon C, Budde U, Schneppenheim S, Czaja E, Hagl C, Schoechl H, et al. Acquired type 2A von Willebrand syndrome caused by aortic valve disease corrects during valve surgery. Br J Anaesth. (2011) 106:494–500. doi: 10.1093/bja/aeq413
Keywords: acquired von willebrand syndrome (AVWS), pulmonary hypertension, bleeding risk, high molecular weight multimers, children
Citation: Wieland I, Diekmann F, Carlens J, Hinze L, Lambeck K, Jack T and Hansmann G (2022) Acquired von Willebrand syndrome (AVWS) type 2, characterized by decreased high molecular weight multimers, is common in children with severe pulmonary hypertension (PH). Front. Pediatr. 10:1012738. doi: 10.3389/fped.2022.1012738
Received: 5 August 2022; Accepted: 14 October 2022;
Published: 14 November 2022.
Edited by:
Stephanie Handler, Medical College of Wisconsin, United StatesReviewed by:
Connie Miller, Centers for Disease Control and Prevention (CDC), United States© 2022 Wieland, Diekmann, Carlens, Hinze, Lambeck, Jack and Hansmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ivonne Wieland d2llbGFuZC5pdm9ubmVAbWgtaGFubm92ZXIuZGU= Georg Hansmann Z2VvcmcuaGFuc21hbm5AZ21haWwuY29t
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.