- 1Division of Pediatric Hematology and Oncology, Royal Hospital, Muscat, Oman
- 2Division of Pediatric Hematology and Oncology Stead Family Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA, United States
Venous thromboembolism is a major hospital acquired complication in the pediatric population over the last two-decades, with a 130% increase in the past decade. Direct oral anticoagulants (DOACs) are a newer class of anticoagulant medication for the treatment and prophylaxis of VTEs that provide the primary advantages of an oral route of administration without a requirement to adjust dosing to achieve a therapeutic level. It is anticipated that these medications will quickly replace parenteral anticoagulants and clinicians should familiarize themselves with DOACs. In this article, we provide an overview of the pharmacological properties of DOACs, with a specific focus on rivaroxaban and dabigatran, which have been approved for use in pediatric patients. Each drug's characteristics are discussed along with data from their respective clinical trials.
Introduction
Venous thromboembolism (VTE) has emerged as a major hospital acquired complication in the pediatric population over the last two-decades. This is primarily related to advances in supportive interventions leading to disruption of Virchow's triad. Estimates show a 70% increase in the rate of hospitalized children diagnosed with VTE between 2001 and 2007 (from 18 per 100,000 hospitalized children in 1990s to 50 in 2000s) (1), and a further 130% increase from 2008 to 2019 (46 VTE cases per 10 000 hospitalizations to 106 in 2019) (2). A dedicated pediatric hemostasis-thrombosis inpatient consult service has noted, between 2011 and 2020, a 7-fold increase in daily census and 10-fold increase in consults for thromboprophylaxis, with the highest percentage of consult requests from orthopedic surgery and the cardiovascular intensive care unit (3). Therefore, anticoagulation therapy has become critical for management of these children, whether for the primary prevention or treatment of VTE.
Available therapeutic agents for the management of pediatric VTEs consist of a single oral medication (vitamin K antagonist) with the remainder being parenteral preparations: intravenous (IV) unfractionated heparin (UFH), subcutaneous (SQ) low-molecular weight heparin (LMWH), SQ fondaparinux, and IV direct thrombin inhibitors (DTIs) such as bivalirudin and argatroban (4); LMWH is the most commonly used medication for inpatient and outpatient VTE management. These medications require titration to therapeutic levels, multiple blood draws (some with strict time-based level collections) and subsequent medication dose adjustments to reach the desired levels. This not only worsens children's trypanophobia but can increase hospital length of stay and delay discharges.
Direct oral anticoagulants (DOACs) are a newer class of anticoagulant medication for the treatment and prophylaxis of VTEs that provide the primary advantages of an oral route of administration without a requirement to adjust dosing to achieve a therapeutic level. It is anticipated that these medications will quickly replace parenteral anticoagulants, specifically LMWH, and clinicians should familiarize themselves with DOACs. In this article, we provide an overview of pharmacological properties of DOACs with a specific focus on two DOACs, rivaroxaban and dabigatran, which have been approved for use in pediatric patients with VTE. Both DOACs have been approved by the Food and Drug Administration (FDA) in the United States and by the European Medicines Agency (EMA) in the European Union.
Development of oral anticoagulants and mechanism of action of DOACs
Anticoagulants were first used in the parenteral form in the 1940s with the discovery of UFH, with LMWH not in use until the 1980s, and the next advancement being in the 1990s with IV DTIs (bivalirudin and argatroban) (5). The first oral anticoagulant, warfarin, was initially licensed in 1954, and it gained popularity in patients with non-valvular atrial fibrillation for prevention of embolic strokes (6). Its use has been precarious due to the difficulty in maintaining therapeutic levels, with the need for frequent monitoring, along with complications of major and fatal bleeding events due to supratherapeutic dosing. It was another 70 years before the second oral anticoagulant (dabigatran) was approved in 2010. Since then, DOACs have revolutionized VTE treatment and thromboprophylaxis in many adult patient populations [currently contraindicated in patients with mechanical heart valves and during pregnancy] (7). Currently approved DOACs in adults are dabigatran etexilate (2010), rivaroxaban (2011), apixaban (2012), edoxaban (2014) and betrixaban (2017) (8).
DOACs exert their anticoagulant properties by affecting the common pathway in the coagulation cascade, with the two major oral medication classes being direct factor Xa inhibitors (DFXaI) and direct thrombin inhibitors (DTIs). An advantage of DOACs is their selective binding to their target factors (FXa for DFXaI and thrombin for DTIs) without the need for a cofactor. Conversely, medications such as indirect FXa inhibitors (e.g., heparins or fondaparinux), exert their anticoagulant effect by potentiating the cofactor antithrombin to bind to FXa (along with thrombin for UFH), thereby neutralizing their procoagulant effects by being removed from circulation (9).
Within the common pathway (Figure 1), FX is activated to FXa which in turn cleaves prothrombin to thrombin, whereby 1 molecule of FXa generates 1,000 molecules of thrombin. The prothrombinase complex (FXa-FVa complex) accelerates this activation 300,000 fold (10). This thrombin generation in turn re-potentiates the prothrombotic pathway by accelerating further activation of the cascade with continued thrombin generation. Therefore, direct inhibition of FXa or thrombin ultimately leads to significant reduction in thrombin generation and the resultant fibrin clot formation.
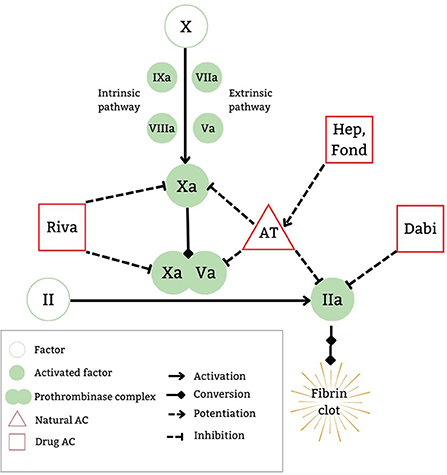
Figure 1. DOAC mechanism of action. AC, anticoagulatnt; AT, antithrombin; Dabi, dabigatran etexilate; Fond, fondaparinux; Hep, heparins; Riva, rivaroxaban.
Rivaroxaban and dabigatran etexilate are currently the first two DOACs approved for use in pediatric patients with VTE by the FDA and EMA. Pediatric dosing regimens are generally extrapolated from adult trial data; however, legislative changes have increased pediatric-specific data. The Best Pharmaceuticals for Children Act and Pediatric Research Equity Act have pushed to assess dosing and adverse events in pediatric patients via their own clinical trials (11), with rivaroxaban and dabigatran etexilate being amongst the first drugs to benefit from their own pediatric trials.
Below is a comparison of these two DOACs with their relevant trial data.
Rivaroxaban
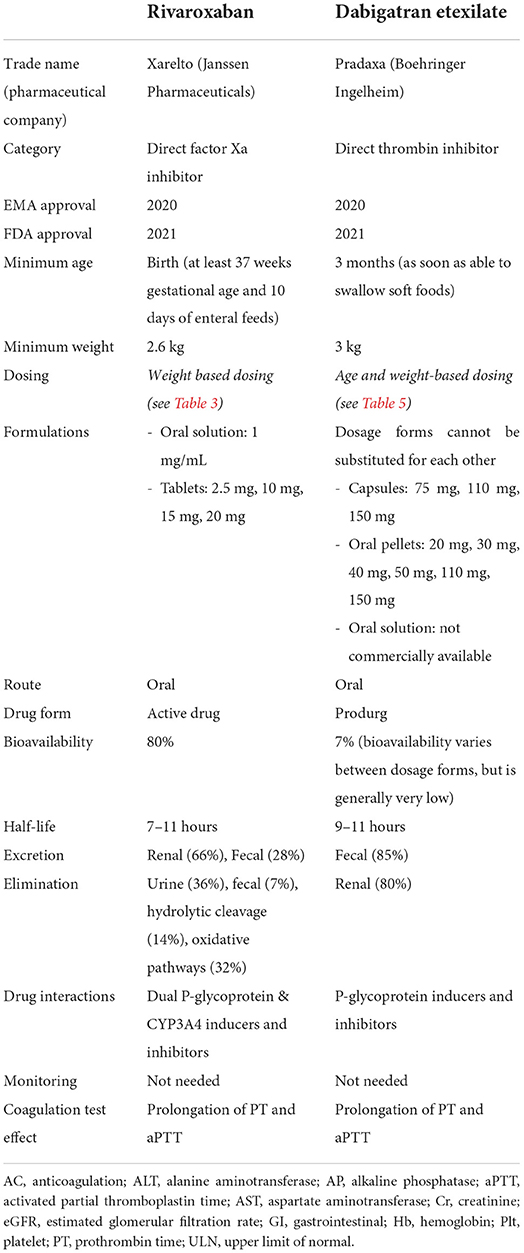
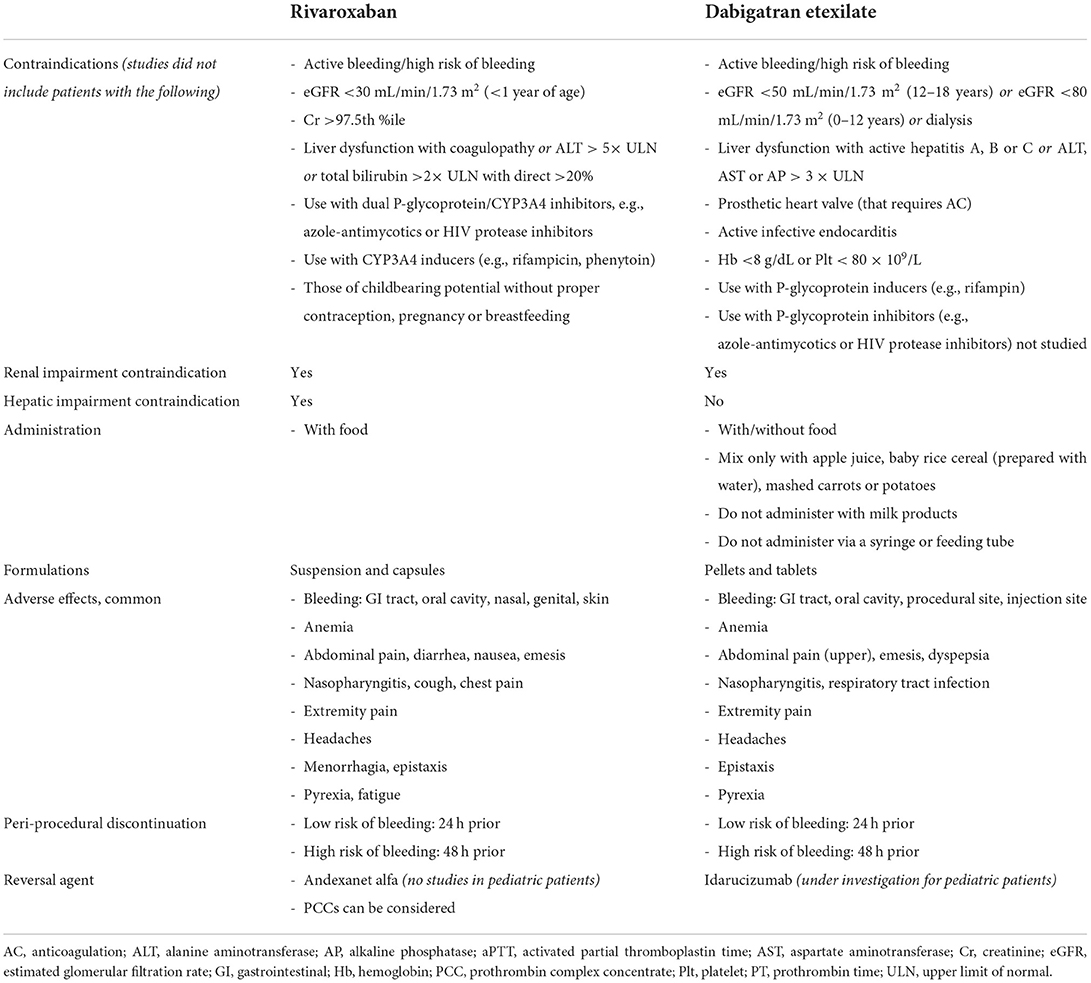
Rivaroxaban is a small molecule that reversibly binds directly to the S1 and S4 sites on FXa (12), with its advantage over indirect FXa inhibitors being its ability to bind both free FXa and FXa bound within the prothrombinase complex, thereby exerting a greater anticoagulant effect. It was initially EMA and FDA approved for adults in 2011, with pediatric approvals by EMA in 2020 (13) and the FDA in 2021 (14). The drug characteristics, pharmacokinetics, pharmacodynamics, safety and efficacy were studied to assess its safety and establish recommended dosage. Patients included were those up to 18 years of age with confirmed VTE—Table 1 (15–21). Neonates included were those at least 37 weeks of gestational age and who have enterally fed for at least 10 days, with a minimum weight of 2.6 kg. This group received a newly formulated oral suspension, in addition to the previously available tablets. Children with active malignancy and those on non-steroidal anti-inflammatory or antiplatelet agents were also eligible (21). The studies excluded patients with active/risk of bleeding, liver and/or renal dysfunction, those pregnant or breastfeeding, patients on strong inhibitors of both CYP3A4 and P-glycoproteins, and those using strong inducers of CYP3A4 (complete and detailed list of contraindications and patient exclusions is in Table 2) (16, 22).
Table 1 also lists rivaroxaban's pharmacodynamics and pharmacokinetics: it has 80% bioavailability (22), reaching its peak plasma concentration within 2–4 h, with a half-life of 7–11 h (12). Its excretion is predominantly renal (66%) with the remainder fecal (28%), with elimination being multifactorial: 36% renal, 7% fecal, 14% via hydrolytic cleavage and 32% via oxidative pathways (23). Rivaroxaban is to be taken with meals (due to studies that showed decreased bioavailability at higher doses) (22), and most of those surveyed on the suspension found it to be palatable (16). There is no requirement for direct or indirect monitoring of therapeutic levels, although there is prolongation of both prothrombin time (PT) and activated partial thromboplastin time (aPTT) (12): Anti-Xa levels can be measured in some high risk patients if there is concern about the pharmacodynamics of the drug (24). Drug dosing is noted in Table 3.
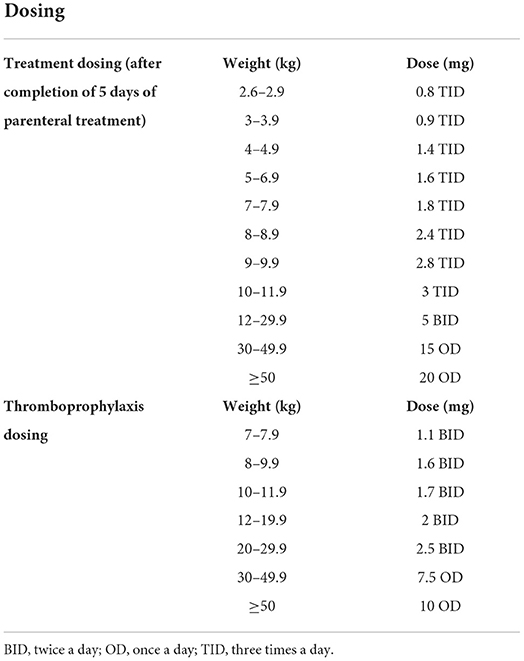
Table 3. Rivaroxaban dosing [modified from Young et al. (16) and Rivaroxaban package insert].
Comparing rivaroxaban to standard of care (SOC) in the EINSTEIN-Jr phase 3 trial, Male et al. (15) found that rivaroxaban had similar efficacy to that of SOC—Table 4. In this 2:1 randomized open label trial, 500 pediatric patients were enrolled between November 2014 and September 2018 in 107 institutions across 28 countries; the greatest proportion of patients were in the 12 to <18 years of age category (~55%). Patients studied included those with active malignancy, major organ disease, cardiac, gastrointestinal, renal and neurologic risk factors, known thrombophilia (inherited or acquired), and those on estrogens or progestins (15). Patients received 5–9 days of parenteral therapy with SOC (UFH, LMWH or fondaparinux) prior to being randomized, and they were then followed for the duration of their treatment course of 3 months (or 1 month for those <2 years of age with catheter related thrombosis). Six patients did not initiate study medication and 7 patients did not continue in the trial after initiation in the rivaroxaban group whereas 3 patients did not initiate study medication and 6 patients did not continue in the trial after initiation in the SOC group.
No patients in the rivaroxaban group had a major bleeding event while there were 2 (1%) in the SOC group (one intracranial and the other pulmonary), with 3% and <1% of patients having clinically relevant non-major bleeding (CRNMB) events in the intervention compared to SOC groups, respectively. Non-bleeding adverse events were mostly mild-moderate (summary of common adverse events in Table 2). Thrombus resolution occurred in 128 (38%) patients in the rivaroxaban group and 43 (26%) in the SOC group, which was clinically significant (p < 0.05), with a recurrence rate of 1 and 3%, respectively. There were no treatment related deaths.
Based on the pharmacokinetic data, peri-procedurally, rivaroxaban should be discontinued 24 and 48 h prior to procedures with low and high bleeding risks, respectively (26). For acute and emergent reversal of rivaroxaban in cases with life-threatening bleeding, andexanet alfa has been approved in adult patients in 2018. Andexanet alfa is an inactive recombinant factor Xa, and in the ANNEX-4 study (27), 80% of patients with acute major bleeding (intracranial or gastrointestinal) secondary to an oral DFXaI, achieved improved hemostatic function with administration of andexanet alfa. The reversal agent is expensive and not readily available in many institutions, with prothrombin complex concentrates (PCCs) being an alternative therapy (28). As there are no current pediatric specific data on its use, a recent survey of pediatric hematologists found a 44% preference for the use of andexanet alfa in pediatric patients requiring reversal for acute life threatening bleed due to FXaI, with 55% choosing PCCs (29). A newer investigational drug, ciraparantag, can reversibly bind and neutralize the effects of DFXaI, DTIs and heparins (30), with initial phase 2 clinical trials showing promise (31).
Dabigatran etexilate
Dabigatran etexilate, a DTI, is a prodrug which is converted to its active form dabigatran by carboxylesterases (32). It reversibly binds directly to the S1 site on thrombin (12), thereby inhibiting it's further role in clot formation and the positive feedback in propagating factors. It was the first DOAC to be approved by the FDA (in 2010) and EMA (2011), and was subsequently the first DOAC to be approved in pediatric patients by both EMA in 2020 (33) and the FDA in 2021 (34). The drug characteristics, pharmacokinetics and pharmacodynamics were well-studied in pediatric patients to assess its safety and establish dosage recommendations—Table 1 (25, 35–38). Its use was assessed in patients 3 months up to 18 years of age with confirmed VTE using an oral suspension, pellets or capsules (25). Patients excluded from the study (25) were those with active/risk of bleeding, liver and/or renal dysfunction, those with a prosthetic heart valve that requires AC, those with active infective endocarditis, patients who are anemic and/or thrombocytopenic, and avoided in those on P-glycoprotein inducers or on P-glycoprotein inhibitors (39) (complete and detailed list of contraindications is in Table 2).
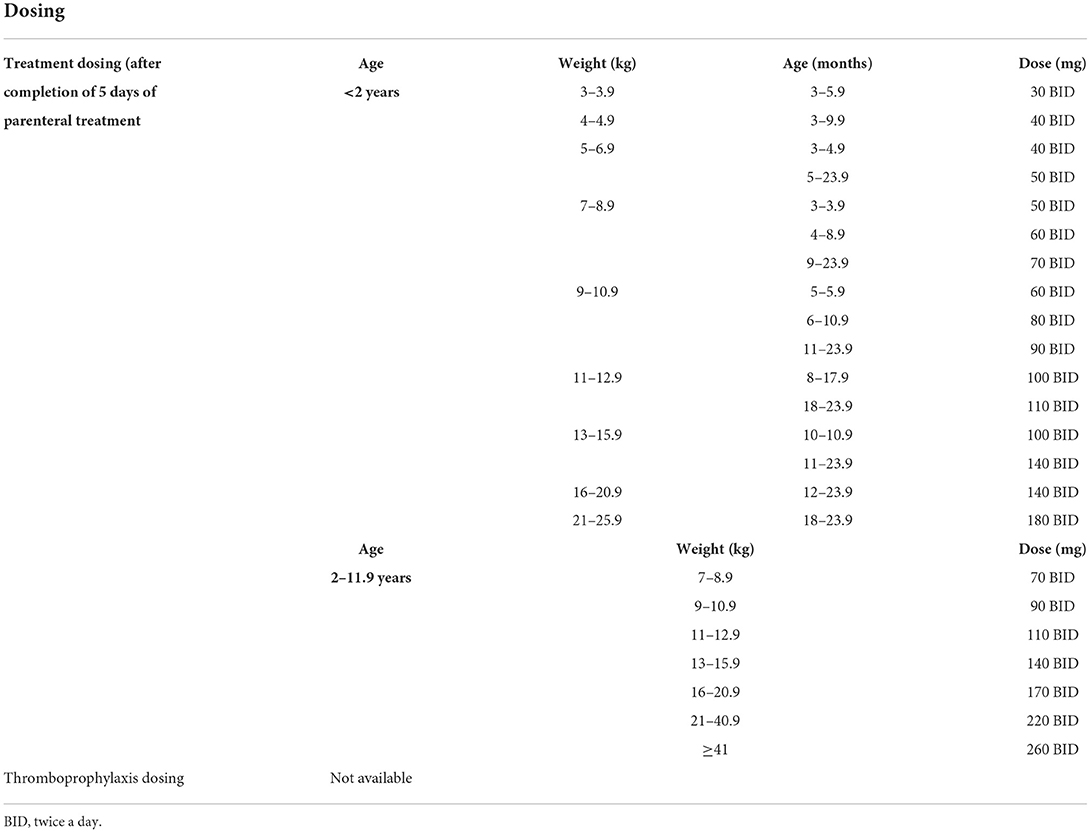
Dabigatran etexilate has low bioavailability of 7% per initial studies (40), although there are differences in bioavailability of the different dosage forms, therefore they cannot be substituted for each other (39). It has a peak effect of 1–2 h (delayed peak with meals high in fat content) with a half-life of 9–11 h (for pellets), and is excreted mainly unchanged in the feces (86%), with the active dabigatran primarily eliminated in the urine (80%) (40). Capsules and oral pellets are currently available for use (the oral solution is not commercially available). Oral pellets (39) can be administered in children as early as those who are able to swallow soft, solid foods by mixing with only the following: apple juice, baby rice cereal (prepared with water) and mashed carrots or potatoes. The pellets should not be administered with any milk products, should not be administered via a syringe or feeding tube, and should be administered within 30 minutes of mixing with juice or food. There is no requirement for direct or indirect monitoring of therapeutic levels due to predictable pharmacokinetics and pharmacodynamics (12), although there is prolongation of both PT, aPTT and thrombin time (TT) (41). Drug dosing is noted in Table 5.
The pediatric dabigatran trial (DIVERSITY) assessed dabigatran safety (37), with Halton et al. (25) showing the non-inferiority of dabigatran to SOC for the secondary prevention of VTE. The open label trial—Table 4—assessed 267 patients in 65 institutions across 26 countries from February 2014 to November 2019. Patients received 5–21 days of parenteral therapy with UFH, LMWH, fondaparinux or VKA (mean of ~15 days) prior to randomization and followed for 3 months (treatment duration). Patients aged 3 months to 18 years of age (the majority of patients were in the 12 to <18 years age group at 62%) were randomized 2:1 with 177 receiving dabigatran and 90 receiving SOC. Patients in the study included those with previous thromboembolism, thrombophilia (inherited or acquired) and those with congenital heart disease, malignancy, heart failure and diabetes. In the dabigatran group, 1 patient did not initiate the study medication and 8 patients did not continue in the trial after initiation, while in the SOC group, 5 patients did not continue in the trial after initiation.
Major bleeding (2%) and CRNMB (1%) events were the same in both groups; major bleeding events were procedural, urogenital, GI, and intracranial in the dabigatran group and retroperitoneal and GI in the SOC group. The common, non-bleeding adverse events are noted in Table 2. Thrombus resolution was achieved in 46% of those receiving dabigatran and 42% of those on SOC, with 4% and 8% having thrombus recurrence, respectively. There was 1 on-treatment death that was not related to treatment in the SOC group.
Based on pharmacokinetic data, peri-procedurally, dabigatran should be discontinued 24 and 48 h prior to procedures with low and high bleeding risks, respectively (42), in those with appropriate renal function. For acute and emergent reversal in cases of severe hemorrhage, idarucizumab, a monoclonal antibody that binds to dabigatran, has shown promise. In the RE-VERSE AD trial (43), emergent use of idarucizumab in patients with uncontrolled bleeding (or those who required urgent procedural intervention) was found to provide safe and durable reversal. Idarucizumabis is currently being investigated for its use as a reversal agent in children (44).
Conclusion
It is evident that DOACs administered to pediatric patients have provided similar safety profiles and efficacy in VTE management compared to SOC with traditional anticoagulants such as heparins and VKAs. The EINSTEIN-Jr and DIVERSITY trials have been instrumental in not only establishing the safety and efficacy of rivaroxaban and dabigatran, respectively, in pediatric patients, but in furthering the call for pediatric-specific interventional studies for newer therapeutics in pediatric hematology and beyond. Additional data on treatment and thromboprophylaxis in special patient populations at high risk for thrombosis is warranted. This is particularly for pediatric patients with heart disease (congenital and acquired) due to the multiple procedures they undergo and the use of prosthetic heart valves, which limits their potential use of DOACs. The trials also likely inadvertently excluded patients with active malignancy due to their repeat anemia and thrombocytopenia. Whitworth and Raffini provide further clinical aspects to be considered in these special patient populations (45).
Other DOACs (Apixaban, Betrixaban, Edoxaban) are currently under investigation in pediatric patients (46).
With the introduction of DOACs, the oral route of administration without the need for therapeutic monitoring is a triumph for patients and their healthcare teams alike. This data, along with newer studies such as the Kids-DOTT trial (showing the non-inferiority of 6 weeks vs. 3 months of AC in pediatric patients with provoked VTE) (47), is heralding an optimistic era in treating pediatric VTE.
Author contributions
MA-G and AS contributed to the conception and design of the manuscript. MA-G completed the initial literature review and wrote the first draft of the manuscript. Both authors contributed to the manuscript revision. Both authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AC, anticoagulation; CRNMB, clinically relevant non-major bleeding; DOAC, direct oral anticoagulant; DFXaI, direct factor Xa inhibitor; DTI, direct thrombin inhibitor; IV, intravenous; LMWH, low molecular weight heparin; SQ, subcutaneous; SOC, standard of care; UFH, unfractionated heparin; VTE, venous thromboembolism.
References
1. Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. (2009) 124:1001–8. doi: 10.1542/peds.2009-0768
2. O'Brien SH, Stanek JR, Witmer CM, Raffini L. The continued rise of venous thromboembolism across US children's hospitals. Pediatrics. (2022) 149:e2021054649. doi: 10.1542/peds.2021-054649
3. Carpenter SL, Bilynsky E, Wideman D, D'Agostino K, Amos LE, Sharma M, et al. Patterns and growth of a dedicated pediatric coagulation consult service in a Freestanding Children's Hospital. Blood. (2020) 136:33–4. doi: 10.1182/blood-2020-139686
4. Malec L, Young G. Treatment of venous thromboembolism in pediatric patients. Front Pediatr. (2017) 5:26. doi: 10.3389/fped.2017.00026
5. Franchini M, Liumbruno GM, Bonfanti C, Lippi G. The evolution of anticoagulant therapy. Blood Transfus. (2016) 14:175–84. doi: 10.2450/2015.0096-15
6. Pirmohamed M. Warfarin: almost 60 years old and still causing problems. British J Clin Pharmacol. (2006) 62:509–11. doi: 10.1111/j.1365-2125.2006.02806.x
7. Roberti R, Iannone LF, Palleria C, Curcio A, Rossi M, Sciacqua A, et al. Direct oral anticoagulants: from randomized clinical trials to real-world clinical practice. Front Pharmacol. (2021) 12:684638. doi: 10.3389/fphar.2021.684638
8. Chan N, Sobieraj-Teague M, Eikelboom JW. Direct oral anticoagulants: evidence and unresolved issues. Lancet. (2020) 396:1767–76. doi: 10.1016/S0140-6736(20)32439-9
9. Rezaie AR, Giri H. Anticoagulant and signaling functions of antithrombin. J Thromb Haemost. (2020) 18:3142–53. doi: 10.1111/jth.15052
10. Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. (2003) 23:17–25. doi: 10.1161/01.ATV.0000046238.23903.FC
11. Drug Research and Children. U.S. Food and Drug Administration (2016). Available online at: https://www.fda.gov/drugs/information-consumers-and-patients-drugs/drug-research-and-children (accessed June 7, 2022).
12. Samama MM. The mechanism of action of rivaroxaban–an oral, direct Factor Xa inhibitor–compared with other anticoagulants. Thromb Res. (2011) 127:497–504. doi: 10.1016/j.thromres.2010.09.008
14. FDA approves drug to treat help help prevent types of blood clots in certain pediatric populations. Food and Drug Admnistration (2021). Available online at: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-help-prevent-types-blood-clots-certain-pediatric-populations (accessed April 19, 2022).
15. Male C, Lensing AWA, Palumbo JS, Kumar R, Nurmeev I, Hege K, et al. Rivaroxaban compared with standard anticoagulants for the treatment of acute venous thromboembolism in children: a randomised, controlled, phase 3 trial. Lancet Haematol. (2020) 7:e18–27. doi: 10.1016/S2352-3026(19)30219-4
16. Young G, Lensing AWA, Monagle P, Male C, Thelen K, Willmann S, et al. Rivaroxaban for treatment of pediatric venous thromboembolism. An Einstein-Jr phase 3 dose-exposure-response evaluation. J Thromb Haemost. (2020) 18:1672–85. doi: 10.1111/jth.14813
17. Monagle P, Lensing AWA, Thelen K, Martinelli I, Male C, Santamaría A, et al. Bodyweight-adjusted rivaroxaban for children with venous thromboembolism (EINSTEIN-Jr): results from three multicentre, single-arm, phase 2 studies. Lancet Haematol. (2019) 6:e500–9. doi: 10.1016/S2352-3026(19)30161-9
18. Willmann S, Thelen K, Kubitza D, Lensing AWA, Frede M, Coboeken K, et al. Pharmacokinetics of rivaroxaban in children using physiologically based and population pharmacokinetic modelling: an EINSTEIN-Jr phase I study. Thromb J. (2018) 16:32. doi: 10.1186/s12959-018-0185-1
19. Thom K, Lensing AWA, Nurmeev I, Bajolle F, Bonnet D, Kenet G, et al. Safety and efficacy of anticoagulant therapy in pediatric catheter-related venous thrombosis (EINSTEIN-Jr CVC-VTE). Blood Adv. (2020) 4:4632–9. doi: 10.1182/bloodadvances.2020002637
20. Connor P, Sánchez van Kammen M, Lensing AWA, Chalmers E, Kállay K, Hege K, et al. Safety and efficacy of rivaroxaban in pediatric cerebral venous thrombosis (EINSTEIN-Jr CVT). Blood Adv. (2020) 4:6250–8. doi: 10.1182/bloodadvances.2020003244
21. Lensing AWA, Male C, Young G, Kubitza D, Kenet G, Patricia Massicotte M, et al. Rivaroxaban versus standard anticoagulation for acute venous thromboembolism in childhood. Design of the EINSTEIN-Jr phase III study. Thromb J. (2018) 16:34. doi: 10.1186/s12959-018-0188-y
22. Kreutz R. Pharmacokinetics and pharmacodynamics of rivaroxaban–an oral, direct factor Xa inhibitor. Curr Clin Pharmacol. (2014) 9:75–83. doi: 10.2174/1574884708666131111204658
23. Weinz C, Schwarz T, Kubitza D, Mueck W, Lang D. Metabolism and excretion of rivaroxaban, an oral, direct factor Xa inhibitor, in rats, dogs, and humans. Drug Metab Dispos. (2009) 37:1056–64. doi: 10.1124/dmd.108.025569
24. Ikeda K, Tachibana H. Clinical implication of monitoring rivaroxaban and apixaban by using anti-factor Xa assay in patients with non-valvular atrial fibrillation. Journal of Arrhythmia. (2016) 32:42–50. doi: 10.1016/j.joa.2015.08.001
25. Halton J, Brandão LR, Luciani M, Bomgaars L, Chalmers E, Mitchell LG, et al. Dabigatran etexilate for the treatment of acute venous thromboembolism in children (DIVERSITY): a randomised, controlled, open-label, phase 2b/3, non-inferiority trial. Lancet Haematol. (2021) 8:e22–33. doi: 10.2139/ssrn.3624247
26. Barnes GD, Mouland E. Peri-procedural management of oral anticoagulants in the DOAC era. Prog Cardiovasc Dis. (2018) 60:600–6. doi: 10.1016/j.pcad.2018.03.002
27. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, et al. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. (2019) 380:1326–35. doi: 10.1056/NEJMoa1814051
28. Dev P, Abousaab C, Zhou C, Sarode R. Efficacy of prothrombin complex concentrate in the management of oral factor Xa inhibitors associated major bleed assessed by ISTH and ANNEXA-4 criteria. J Thromb Thrombolysis. (2022) 53:249–56. doi: 10.1007/s11239-021-02536-x
29. Rodriguez V, Stanek J, Kerlin BA, Dunn AL. Andexanet alfa versus prothrombin complex concentrates/blood products as apixaban/rivaroxaban reversal agents: a survey among pediatric hematologists. Clin Appl Thromb Hemostasis. (2022) 28:107602962210788. doi: 10.1177/10760296221078842
30. Sullivan DW, Gad SC, Laulicht B, Bakhru S, Steiner S. Nonclinical safety assessment of PER977. Int J Toxicol. (2015) 34:308–17. doi: 10.1177/1091581815590667
31. Ansell J, Bakhru S, Laulicht BE, Tracey G, Villano S, Freedman D. Ciraparantag reverses the anticoagulant activity of apixaban and rivaroxaban in healthy elderly subjects. Eur Heart J. (2022) 43:985–92. doi: 10.1093/eurheartj/ehab637
32. Blommel ML, Blommel AL. Dabigatran etexilate: a novel oral direct thrombin inhibitor. Am J Health Syst Pharm. (2011) 68:1506–19. doi: 10.2146/ajhp100348
33. Agency. EM. Pradaxa. Procedure No. EMEA/H/C/000829/X/0122/G. (2020). Available online at: https://www.ema.europa.eu/en/documents/variation-report/pradaxa-h-c-829-x-0122-g-epar-assessment-report-extension_en.pdf (accessed September 4, 2022).
34. FDA Approves First Oral Blood Thinning Medication for Children: Food and Drug Administration. (2021). Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-blood-thinning-medication-children#:$\sim$:text=Today%2C%20the%20U.S.%20Food%20and,by%20injection%20for%20at%20least (accessed April 29, 2022).
35. Luciani M, Albisetti M, Biss B, Bomgaars L, Brueckmann M, Chalmers E, et al. Phase 3, single-arm, multicenter study of dabigatran etexilate for secondary prevention of venous thromboembolism in children: rationale and design. Res Pract Thromb Haemost. (2018) 2:580–90. doi: 10.1002/rth2.12093
36. Albisetti M, Biss B, Bomgaars L, Brandão LR, Brueckmann M, Chalmers E, et al. Design and rationale for the DIVERSITY study: an open-label, randomized study of dabigatran etexilate for pediatric venous thromboembolism. Res Pract Thromb Haemost. (2018) 2:347–56. doi: 10.1002/rth2.12086
37. Brandão LR, Albisetti M, Halton J, Bomgaars L, Chalmers E, Mitchell LG, et al. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. (2020) 135:491–504. doi: 10.1182/blood.2019000998
38. Röshammar D, Huang F, Albisetti M, Bomgaars L, Chalmers E, Luciani M, et al. Pharmacokinetic modeling and simulation support for age- and weight-adjusted dosing of dabigatran etexilate in children with venous thromboembolism. J Thromb Haemost. (2021) 19:1259–70. doi: 10.1111/jth.15277
39. PRADAXA® (dabigatran etexilate) oral pellets: Boehringer Ingelheim. (2021). Available online at: https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf (accessed May 21, 2022).
40. Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. (2008) 36:386–99. doi: 10.1124/dmd.107.019083
41. Dager WE, Gosselin RC, Kitchen S, Dwyre D. Dabigatran effects on the international normalized ratio, activated partial thromboplastin time, thrombin time, and fibrinogen: a multicenter, in vitro study. Ann Pharmacother. (2012) 46:1627–36. doi: 10.1345/aph.1R179
42. Schulman S, Carrier M, Lee AYY, Shivakumar S, Blostein M, Spencer FA, et al. Perioperative management of dabigatran. Circulation. (2015) 132:167–73. doi: 10.1161/CIRCULATIONAHA.115.015688
43. Pollack CV, Reilly PA, Van Ryn J, Eikelboom JW, Glund S, Bernstein RA, et al. Idarucizumab for dabigatran reversal — full cohort analysis. New England J Med. (2017) 377:431–41. doi: 10.1056/NEJMoa1707278
44. Albisetti M, Schlosser A, Brueckmann M, Gropper S, Glund S, Tartakovsky I, et al. Rationale and design of a phase III safety trial of idarucizumab in children receiving dabigatran etexilate for venous thromboembolism. Res Pract Thromb Haemost. (2018) 2:69–76. doi: 10.1002/rth2.12053
45. Whitworth H, Raffini L. Practical considerations for use of direct oral anticoagulants in children. Front Pediatr. (2022) 10:860369. doi: 10.3389/fped.2022.860369
46. Albisetti M. Use of direct oral anticoagulants in children and adolescents. Hämostaseologie. (2020) 40:064–73. doi: 10.1055/s-0039-3400491
47. Goldenberg NA, Kittelson JM, Abshire TC, Bonaca M, Casella JF, Dale RA, et al. Effect of anticoagulant therapy for 6 weeks vs 3 months on recurrence and bleeding events in patients younger than 21 years of age with provoked venous thromboembolism: the kids-DOTT randomized clinical trial. JAMA. (2022) 327:129–37. doi: 10.1001/jama.2022.3729
Keywords: direct oral anticoagulants (DOACs), venous thromboembolism (VTE), rivaroxaban, dabigatran, thrombosis
Citation: Al-Ghafry M and Sharathkumar A (2022) Direct oral anticoagulants in pediatric venous thromboembolism: Review of approved products rivaroxaban and dabigatran. Front. Pediatr. 10:1005098. doi: 10.3389/fped.2022.1005098
Received: 27 July 2022; Accepted: 12 September 2022;
Published: 13 October 2022.
Edited by:
Daniele Zama, Sant'Orsola-Malpighi Polyclinic, ItalyReviewed by:
Berardino Pollio, Ospedale Città della Salute e della Scienza, ItalySimone Bonetti, IRCCS Azienda Ospedaliero-Universitaria di Bologna Policlinico S. Orsola, Italy
Copyright © 2022 Al-Ghafry and Sharathkumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anjali Sharathkumar, anjali-sharathkumar@uiowa.edu
 Maha Al-Ghafry
Maha Al-Ghafry