
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 11 October 2022
Sec. Pediatric Endocrinology
Volume 10 - 2022 | https://doi.org/10.3389/fped.2022.1001290
Background: Signal transducer and activator of transcription 1 (STAT1) gain-of-function (GOF) mutations are characterized by chronic mucocutaneous candidiasis and autoimmune diseases. Type 1 diabetes mellitus is one of the well-characterized autoimmune conditions.
Case presentation: We reported a 5-year-old boy who presented with polydipsia and polyuria, with a medical history of chronic oral mucocutaneous candidiasis, recurrent respiratory infection, hepatosplenomegaly, and abnormal liver function. Genetic analysis identified a heterozygous GOF mutation (c.866A > G, p.Y289C) in STAT1.
Results: Various medicines were given to the boy during the follow-up, including insulin to keep blood glucose stable, intravenous immunoglobulin and antifungal agents for recurrent infections, and antituberculosis drugs (isoniazid, rifampicin) to combat tuberculosis infection. He did not show recurrent infection, but chronic oral mucocutaneous candidiasis still occurred twice per month. The blood glucose level was well controlled.
Conclusion: This article illustrates that early diagnosis and identification of STAT1 mutation are essential for assessing the severity of the disease and determining reasonable treatment options.
The signal transducer and activator of transcription 1 (STAT1) is one of the seven members of STATs. STATs transmit cell signals from extracellular to intracellular through Janus kinases (JAKs), activate target genes, and regulate cell growth and differentiation (1, 2). Mutations in the gene encoding the STAT protein family are associated with immune dysregulation syndrome of autoimmune deficiency (3). STAT1 can promote apoptosis and inhibit cell growth and differentiation. There are four types of primary immunodeficiency diseases caused by STAT1 mutation, including autosomal dominant gain-of-function (GOF) immunodeficiency, loss-of-function immunodeficiency, and autosomal recessive partial and complete immunodeficiency. Among these, the autosomal dominant inherited STAT1-GOF immunodeficiency is mainly associated with chronic mucosal candidiasis (CMC) and autoimmune diseases (3). As an autoimmune manifestation, diabetes mellitus is less common than hypothyroidism, and it is often diagnosed as type 1 diabetes mellitus (T1DM) at its onset. It is necessary to perform a genetic analysis to accurately diagnose monogenic autoimmune disease. In cases in which a clinical phenotype contains multiple system disorders, a genetic etiology may be indicated. Individuals suffering from autoimmunity due to a single gene defect may benefit from personalized treatment.
This report describes a boy with multiple system disorders who was identified with a STAT1-GOF mutation through whole exon sequencing (WES), and the prognosis was followed.
A 5 year and 4 month old boy was referred to us for polydipsia and polyuria. His blood glucose was 29.5 mmol/L, hemoglobin A1c (HbA1c) was 8.9%, and fasting C-peptide was 0.07 ng/ml. Serum anti-glutamate decarboxylase antibody and anti-islet cell antibody were both positive. He was diagnosed with T1DM at the onset. He had a history of recurrent oral mucocutaneous Candida fungi and respiratory infections after birth. At the age of 1 year, he suffered from herpetic pharyngitis with alanine transferase (ALT) 1,000–2,000 U/L, which was relieved by anti-infective and liver-protecting treatment. Increased ALT levels occurred several times after infection. A left under axillary abscess incision and drainage was performed when he was 3 years and 8 months old. He was the first child of non-consanguineous parents without any family history of hereditary diseases.
Physical examination showed the weight was 18 kg (25th percentile), and the height was 110 cm (25th percentile). The blood pressure was normal. He was conscious, his thyroid and cardiopulmonary systems were normal, and there was no evidence of an enlarged lymph node. The liver is 4 cm under the right rib, and the spleen is 5 cm below the left rib, with blunt edges, and there is no swelling in the lower limbs.
Laboratory investigation revealed normal blood routine analysis, thyroid function, immunoglobulin A (IgA), immunoglobulin M, immunoglobulin G, ammonia, lactic acid, blood amino acids, acylcarnitine, and urine organic acid analysis. The level of aspartate aminotransferase was 100 U/L, ALT was 16.2 U/L. Hepatophilic virus antibodies [anti-cytomegalovirus-immunoglobulin M (CMV-IgM), anti-Epstein–Barr virus (EBV)-IgM, hepatitis A/B/C/E virus antibodies] and autoimmune liver disease antibodies were negative. The thyroglobulin antibody, thyroid peroxidase antibody, and thyroid-stimulating hormone receptor antibody were negative. The percentage of CD4 lymphocytes was 21%, CD8 lymphocytes was 45%, and CD4/CD8 was 0.46, which indicated immunodeficiency, the detailed laboratory information was shown in Table 1.
WES identified a heterozygous de novo STAT1 mutation in the child, c.866A > G, p.Y289C, which was further confirmed by Sanger sequencing (Figure 1). It was previously shown to be a GOF mutation (4) and was defined as pathogenic according to criteria published by the American College of Medical Genetics and Genomics (ACMG) (5). The child was corrected diagnosed with monogenic autoimmune diabetes.
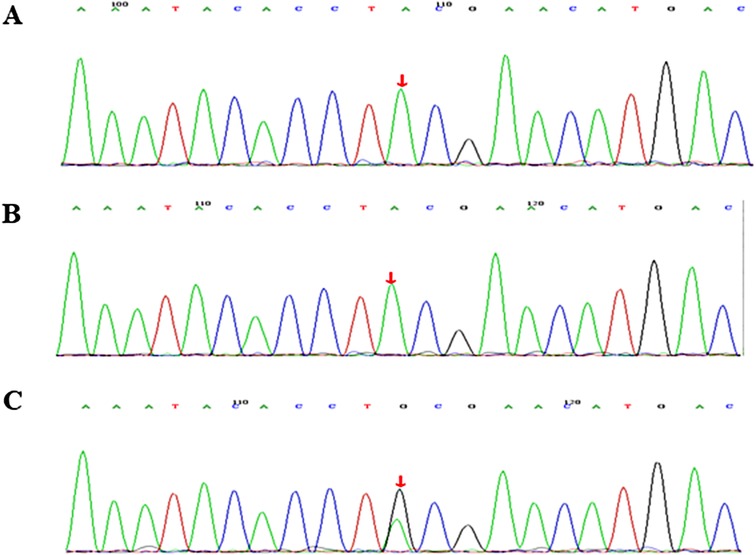
Figure 1. Molecular characterization of a family with STAT1 gain-of-function mutation. Sequence chromatogram showing a missense variant c.866A > G, p.Y289C. The variant was heterozygous in the proband (C). Sanger sequencing results of his father (A) and mother (B).
Based on the presence of CMC, immunodeficiency, autoimmune diabetes, and autoimmune hepatitis, as well as genetic results, the boy was diagnosed with syndromic CMC that was caused by a heterozygous GOF mutation of STAT1.
Upon admission, he was given rapid-acting insulin with meals and long-acting insulin at bedtime, with a total amount of 1.27 IU/kg/d, with blood glucose levels of 6–7 mmol/L before meals and 6–12 mmol/L after meals. During the follow-up, he was given oral antifungal therapy of fluconazole (75 mg/d) and intravenous injection of human immunoglobulin once a month for candidiasis and recurrent infections.
As of now, he was 8 years old and his insulin dosage was 1.1 IU/kg/d. Even under fluconazole therapy, he developed oral mucocutaneous Candida fungi twice every month, but he did not develop recurrent viral or bacterial infections. He was given antituberculosis drugs (isoniazide, rifampicin) for tuberculosis infection. The liver function was normal at the last follow-up, and the HbA1c was 6.5%. Ruxolitinib was recommended to the patient’s parents, but they refused because of concerns about side effects.
The mutations of STAT1 increase STAT1 phosphorylation by impairing nuclear dephosphorylation, which enhance signaling downstream of cytokines interferon α/β (IFN-α/β), IFN-γ, interleukin-27 (IL-27), STAT3-dependent IL-6 and IL-21 (6–9), and impair IL-17A, IL-17F immunity, resulting in lower T-cell counts producing IL-17A and/or IL-17F. CMC is most likely the consequence of impaired IL-17A and IL-17F immunity (10). Autoimmunity appears to be a consequence of stronger IFN-α/β signaling. The mutation reported in our study is located in the coiled-coil domain (CCD), which resulted in persistent STAT1 phosphorylation without appropriate dephosphorylation in all-natural killer cells in response to IFN-α (4).
More than one-third of patients with STAT1-GOF mutations presented with autoimmune manifestations. Autoimmunity predominantly affected endocrine organs, hypothyroidism was the most common endocrine disease, followed by diabetes mellitus and hyperthyroidism. Other autoimmune diseases contained hematological autoimmunity (hemolytic anemia or autoimmune thrombocytopenia, and autoantibody-positive pernicious anemia), systemic lupus erythematosus, scleroderma, autoimmune hepatitis, cutaneous diseases (vitiligo, alopecia, psoriasis), celiac disease, and inflammatory bowel disease (Crohn’s disease, ulcerative colitis, enteropathy with lymphocytic infiltration) (11). There have also been cases of patients with severe autoimmune symptoms resembling those found in patients with immune dysregulation-polyendocrinopathy-enteropathy-X-linked (IPEX) syndrome (12). Most patients with autoimmune manifestations were positive for autoantibodies.
The reason for diabetes mellitus here is different from that of T1DM. While both of them are autoimmune diseases with the associated islet autoantibodies, there is a crucial difference in their genetic susceptibility and hence their cause. The former is caused by a variant in a single gene in a monogenic autoimmune form of diabetes and is not associated with human leukocyte antigen (HLA). However, T1DM is a polygenic autoimmune disease with well-described associations with common variants in the HLA locus.
To date, over 20 cases of diabetes with a GOF mutation of STAT1 have been reported in the literature (11, 12–21): 11 females and 9 males. In 11 cases, the onset age was less than 1 year old. The clinical manifestations at the onset were thrush, skin esophageal/genital mucosa infections caused by Candida albicans, repeatedly bacterial infections (Staphylococcus aureus, Streptococcus pneumonia, Pseudomonas aeruginosa, Haemophilus influenza, etc.), virus (herpes simplex virus, varicella-zoster virus, CMV, EBV, infectious wart) infections caused pneumonia, sepsis, etc., or with invasive fungal infections, such as fungal pneumonia and esophagitis. Hepatosplenomegaly or autoimmune hepatitis occurred in seven cases, hypothyroidism, thrombocytopenia, and autoimmune hemolytic anemia occurred in six cases. Of the 20 patients, 4 died of severe infection, 2 died of intracranial hemorrhage, and 1 died of complications after transplantation. The oldest surviving person was 59 years old. Before 5 years old, T lymphocytes and immunoglobulin were normal. T lymphocyte and immunoglobulin gradually decreased with age, and significantly decreased during puberty.
The outcomes of patients with STAT1-GOF mutations were poor. It was estimated that 12.04% of patients with STAT1-GOF mutations died prematurely, which were caused by severe infections (i.e., coccidioidomycosis, cytomegalovirus, septicemia), cancers, and cerebral hemorrhage due to aneurysms (10). A retrospective study of 15 patients with severe and life-threatening infections who received hematopoietic stem cell transplantation found that 6 patients survived, 4 died of systemic severe infections, 4 died of multiorgan failure after transplantation, and 1 patient died of a ruptured aneurysm (14).
It has been reported that the granulocyte colony-stimulating factor, JAKs inhibitor, and histone deacetylase inhibitor can be used to treat chronic mucosal candidiasis and alleviate clinical symptoms. Ruxolitinib, a JAKs inhibitor, can decrease the level of phosphorylation of STAT1 (22), improving the function of damaged Th17 cells, increasing the expression of IL-17, reducing IFN-gamma, and alleviating autoimmune reactions. A diabetic patient who stopped insulin therapy under ruxolitinib medication was in good control of his blood sugar 1 year later (23). In spite of this, further research is needed to assess ruxolitinib’s therapeutic effects. In addition, long-term intravenous immunoglobulin and antifungal therapy have also been reported to be effective (22). The child in our study received intravenous immunoglobulin infusion and antifungal therapy, he did not show recurrent infection, but CMC still occurred twice per month. Meanwhile, the child did not develop other autoimmune diseases such as hypothyroidism or thrombocytopenia. Ruxolitinib was not tried on the child because of the parents’ unwillingness, insulin was given to keep glucose under good control.
In conclusion, STAT1 sequence analysis should be performed as soon as possible in patients suffering from recurrent respiratory infections and chronic mucosal candidiasis combined with autoimmune. Early diagnosis and identification of the type of STAT1 mutations are important for assessing the severity of the disease and determining the appropriate treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of Beijing Children’s Hospital, Capital Medical University, Beijing, China. Written informed consent to participate in this study was provided by the participant's legal guardian/next of kin.
All authors helped to perform the research. BC wrote the manuscript. ML contributed to the project management. YZ collected the clinical data and completed the genetic analysis. CG conceived and designed the project as well as revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors thank the family members of the patients involved in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. (2007) 282:20059–63. doi: 10.1074/jbc.R700016200
2. Goswami R, Kaplan MH. STAT transcription factors in T cell control of health and disease. Int Rev Cell Mol Biol. (2017) 331:123–80. doi: 10.1016/bs.ircmb.2016.09.012
3. van de Veerdonk FL, Plantinga TS, Hoischen A, Smeekens SP, Joosten LA, Gilissen C, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. (2011) 365:54–61. doi: 10.1056/NEJMoa1100102
4. Vargas-Hernández A, Mace EM, Zimmerman O, Zerbe CS, Freeman AF, Rosenzweig S, et al. Ruxolitinib partially reverses functional natural killer cell deficiency in patients with signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations. J Allergy Clin Immunol. (2018) 141:2142–55.e5. doi: 10.1016/j.jaci.2017.08.040
5. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
6. Boisson B, Quartier P, Casanova JL. Immunological loss-of-function due to genetic gain-of-function in humans: autosomal dominance of the third kind. Curr Opin Immunol. (2015) 32:90–105. doi: 10.1016/j.coi.2015.01.005
7. Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. (2013) 131:1611–23. doi: 10.1016/j.jaci.2012.11.054
8. Takezaki S, Yamada M, Kato M, Park M, Maruyama K, Yamazaki Y, et al. Chronic mucocutaneous candidiasis caused by a gain-of-function mutation in the STAT1 DNA-binding domain. J Immunol. (2012) 189(3):1521–6. doi: 10.4049/jimmunol.1200926
9. Okada S, Asano T, Moriya K, Boisson-Dupuis S, Kobayashi M, Casanova JL, et al. Human STAT1 gain-of-function heterozygous mutations: chronic mucocutaneous candidiasis and type I interferonopathy. J Clin Immunol. (2020) 40:1065–81. doi: 10.1007/s10875-020-00847-x
10. Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. (2012) 12:616–22. doi: 10.1097/ACI.0b013e328358cc0b
11. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. (2016) 127:3154–64. doi: 10.1182/blood-2015-11-679902
12. Lee JS, An Y, Yoon CJ, Kim JY, Kim KH, Freeman AF, et al. Germline gain-of-function mutation of STAT1 rescued by somatic mosaicism in immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like disorder. J Allergy Clin Immunol. (2020) 145:1017–21. doi: 10.1016/j.jaci.2019.11.028
13. Zimmerman O, Olbrich P, Freeman AF, Rosen LB, Uzel G, Zerbe CS, et al. STAT1 gain-of-function mutations cause high total STAT1 levels with normal dephosphorylation. Front Immunol. (2019) 10:1433. doi: 10.3389/fimmu.2019.01433
14. Leiding JW, Okada S, Hagin D, Abinun M, Shcherbina A, Balashov DN, et al. Hematopoietic stem cell transplantation in patients with gain-of-function signal transducer and activator of transcription 1 mutations. J Allergy Clin Immunol. (2018) 141:704–17.e5. doi: 10.1016/j.jaci.2017.03.049
15. Shahar E, Kriboy N, Pollack S. White cell enhancement in the treatment of severe candidosis. Lancet. (1995) 346:974–5. doi: 10.1016/s0140-6736(95)91599-0
16. Wildbaum G, Shahar E, Katz R, Karin N, Etzioni A, Pollack S. Continuous G-CSF therapy for isolated chronic mucocutaneous candidiasis: complete clinical remission with restoration of IL-17 secretion. J Allergy Clin Immunol. (2013) 132:761–4. doi: 10.1016/j.jaci.2013.04.018
17. Sharfe N, Nahum A, Newell A, Dadi H, Ngan B, Pereira SL, et al. Fatal combined immunodeficiency associated with heterozygous mutation in STAT1. J Allergy Clin Immunol. (2014) 133:807–17. doi: 10.1016/j.jaci.2013.09.032
18. Second J, Korganow AS, Jannier S, Puel A, Lipsker D. Rosacea and demodicidosis associated with gain-of-function mutation in STAT1. J Eur Acad Dermatol Venereol. (2017) 31:e542–4. doi: 10.1111/jdv.14413
19. Sáez-de-Ocariz M, Suárez-Gutiérrez M, Migaud M, Farrill-Romanillos PO, Casanova JL, Segura-Mendez NH, et al. Rosacea as a striking feature in family members with a STAT1 gain-of-function mutation. J Eur Acad Dermatol Venereol. (2020) 34:e265–7. doi: 10.1111/jdv.16241
20. Sampaio EP, Ding L, Rose SR, Cruz P, Hsu AP, Kashyap A, et al. Novel signal transducer and activator of transcription 1 mutation disrupts small ubiquitin-related modifier conjugation causing gain of function. J Allergy Clin Immunol. (2018) 141:1844–53.e2. doi: 10.1016/j.jaci.2017.07.027
21. Dotta L, Scomodon O, Padoan R, Timpano S, Plebani A, Soresina A, et al. Clinical and immunological data of nine patients with chronic mucocutaneous candidiasis disease. Data Brief. (2016) 7:311–5. doi: 10.1016/j.dib.2016.02.040
22. Weinacht KG, Charbonnier LM, Alroqi F, Plant A, Qiao Q, Wu H, et al. Ruxolitinib reverses dysregulated T helper cell responses and controls autoimmunity caused by a novel signal transducer and activator of transcription 1 (STAT1) gain-of-function mutation. J Allergy Clin Immunol. (2017) 139:1629–40.e2. doi: 10.1016/j.jaci.2016.11.022
Keywords: STAT1, chronic mucosal candidiasis, gain-of-function, diabetes mellitus, case report
Citation: Cao B, Liu M, Zhao Y and Gong C (2022) Chronic oral mucocutaneous candidiasis, recurrent respiratory infection, hepatosplenomegaly, and autoimmune diabetes mellitus: A case report of a gain-of-function mutation of STAT1 in a Chinese boy. Front. Pediatr. 10:1001290. doi: 10.3389/fped.2022.1001290
Received: 23 July 2022; Accepted: 16 September 2022;
Published: 11 October 2022.
Edited by:
Artur Mazur, University of Rzeszow, PolandReviewed by:
Claudio Pignata, University of Naples Federico II, Italy© 2022 Cao, Liu, Zhao and Chunxiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Xiu Gong Y2h1bnhpdWdvbmdAMTYzLmNvbQ==
Specialty Section: This article was submitted to Pediatric Endocrinology, a section of the journal Frontiers in Pediatrics
Abbreviations ACMG, American College of Medical Genetics and Genomics; ALT, alanine transferase; CCD, coiled-coil domain; CMC, chronic mucosal candidiasis; CMV, cytomegalovirus; EBV, Epstein–Barr virus; GOF, gain-of-function; HbA1c, hemoglobin A1c; HLA, human leukocyte antigen; IgM, immunoglobulin M; IFN-α/β, interferon α/β; IL, interleukin; IPEX, immune dysregulation-polyendocrinopathy-enteropathy-X-linked; JAKs, Janus kinases; STAT1, signal transducer and activator of transcription 1; T1DM, type 1 diabetes mellitus; WES, whole exon sequencing.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.