
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Pediatr. , 24 January 2022
Sec. Pediatric Hematology and Hematological Malignancies
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.814033
This article is part of the Research Topic Risk Factors and New Treatment Options in Pediatric Venous Thromboembolism View all 7 articles
Thromboembolism (TE), including venous thromboembolism (VTE), arterial TE, arterial ischemic stroke (AIS), and myocardial infarction (MI), is considered a relatively rare complication in the pediatric population. Yet, the incidence is rising, especially in hospitalized children. The vast majority of pediatric TE occurs in the setting of at least one identifiable risk factor. Most recently, acute COVID-19 and multisystem inflammatory syndrome in children (MIS-C) have demonstrated an increased risk for TE development. The mainstay for the management pediatric TE has been anticoagulation. Thrombolytic therapy is employed more frequently in adult patients with ample data supporting its use. The data for thrombolysis in pediatric patients is more limited, but the utilization of this therapy is becoming more commonplace in tertiary care pediatric hospitals. Understanding the data on thrombolysis use in pediatric TE and the involved risks is critical before initiating one of these therapies. In this paper, we present the case of an adolescent male with acute fulminant myocarditis and cardiogenic shock likely secondary to MIS-C requiring extracorporeal life support (ECLS) who developed an extensive thrombus burden that was successfully resolved utilizing four simultaneous catheter-directed thrombolysis (CDT) infusions in addition to a review of the literature on the use of thrombolytic therapy in children.
A 12-year-old 87.9 kg male presented with five days of fever and two days of midsternal chest pain and respiratory distress. Initial lab evaluations showed a mild leukocytosis with neutrophil predominance, elevated d-dimer (3,954 ng/mL), elevated inflammatory markers (fibrinogen 1,288 mg/dL; lactic acid 3.8 mmol/L; C-reactive protein 26 mg/L; ferritin 379 ng/mL), a troponin of 12.5 ng/mL, and a BNP of 1,740 pg/mL. Influenza and SARS-CoV-2 PCR testing were negative. It was noted that his father worked in a poultry plant with multiple known SARS-CoV-2 positive co-workers. Electrocardiogram showed diffuse ST elevation and a chest radiograph showed bilateral airspace opacities and prominent heart size. He was intubated shortly after arrival due to hemodynamic instability. An echocardiogram revealed severely depressed biventricular systolic function, moderate mitral valve regurgitation, and a small right atrial (RA) thrombus. Due to refractory cardiogenic shock from suspected myocarditis, he was cannulated onto veno-arterial (VA)-ECLS via the right femoral artery with termination in the descending aorta and right femoral vein with termination at the inferior vena cava (IVC) and RA junction.
Unfractionated heparin (UFH) infusion was used for anticoagulation (100 units/kg IV at the time of cannulation, followed by an infusion at 25 units/kg/h), but despite reasonable iSTAT kaolin activated clotting times (ACTs) of 180–200 s, therapeutic heparin assays (0.35–0.7 units/mL) could not be achieved. Significant fibrin deposits in the ECLS circuit developed in the first hour after cannulation and the patient was transitioned to bivalirudin [0.25 mg/kg/h starting dose with escalation up to 1.15 mg/kg/h based on activated partial thromboplastin time (aPTT) goal of 2–3 times his baseline (60–85 s)] due to concern for heparin resistance. One significant limitation to bivalirudin that we discussed was its inability to provide clot dissolution in areas of stagnant flow, for example a poorly contracting ventricle with little inflow/outflow, but the inability to achieve therapeutic heparin assays was deemed a more significant risk.
Four hours post-cannulation, repeat echocardiogram revealed a massive thrombus burden [right ventricular apex thrombus, large right pulmonary artery (RPA) thrombus from the branch bifurcation throughout the mid and distal portions, large left pulmonary artery (LPA) thrombus in the proximal portion, mural thrombus in the left ventricular apex (3.1 cm), and large thrombus in the ascending aorta (3.4 x 1.4 cm) with extension into the transverse arch].
After confirming that the patient had no intracranial abnormalities by non-contrast head computed tomography (CT), systemic thrombolysis with recombinant tissue plasminogen activator (rtPA) (100 mg over 2 h) was initiated via his right internal jugular central venous line. Due to concerns that the extensive nature of the thrombus burden that may not be resolved with a single systemic thrombolysis infusion and his ongoing significant thrombotic risk, this was followed by a continuous rtPA infusion at our institutional maximum dose of 1 mg/h. A bivalirudin infusion was run currently during the rtPA infusions with an aPTT goal of ~1.5–2 times his baseline (50–60 s). Repeat echocardiographic imaging after 12 h revealed little change in thrombotic burden with slight extension of the aortic thrombus farther into the transverse arch.
CDT was initiated at this time as it was felt that systemic rtPA was ineffective secondary to a recirculation effect due to the proximity of the site of infusion to the ECLS venous drainage cannula in the right atrium. The patient was taken to the cardiac catheterization laboratory and individual catheters were placed at four target sites via the left femoral vessels: one was placed retrograde through a left femoral arterial sheath to the aortic root, one was placed into the apex of the left ventricle via trans-septal approach, and the others were placed in the distal RPA and LPA (Figure 1). A dose of 0.25 mg/h of rtPA with 15 mL/h of fresh frozen plasma (FFP) was infused through each catheter. With the ineffectiveness of systemic thrombolysis likely due to an ECLS recirculation effect, we had significant concern for the lack of blood flow to the thrombus sites and felt that without concurrent exogenous plasminogen supplementation thrombolysis would have been ineffective. The three catheters utilizing a transvenous approach were placed through a single DrySeal sheath (Gore Medical, Flagstaff, AZ) to minimize the need for individual vascular access sites for each catheter. Bivalirudin infusion was continued with an aPTT goal of ~1.5–2 times his baseline (50–60 s). Follow up echocardiogram 24 h after initiation of CDT revealed complete thrombus resolution. CDT was continued for another 12 h and CT angiography confirmed the resolution of all TE. No bleeding complications were noted during his time on ECLS.
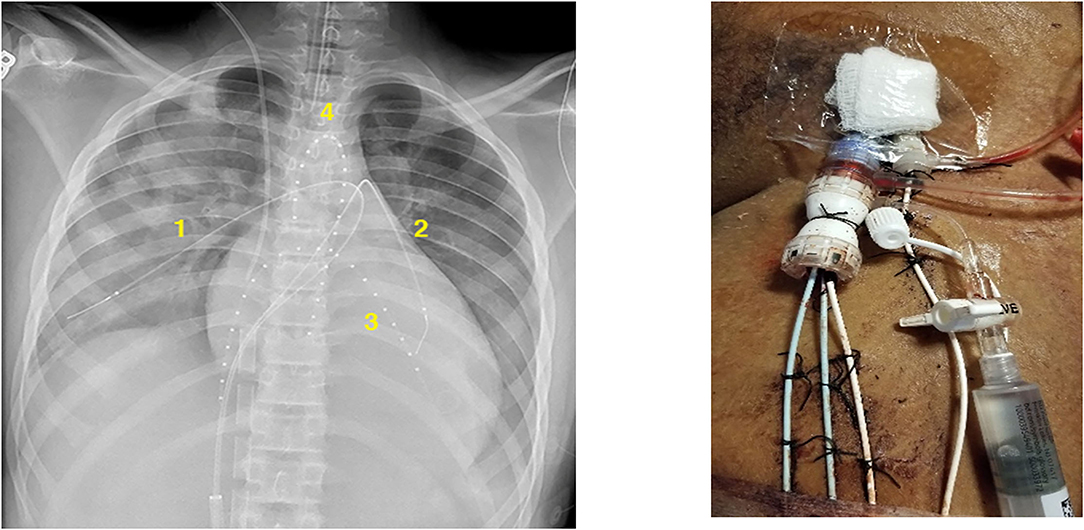
Figure 1. Thrombolysis catheter placement in the right pulmonary artery (1), left pulmonary artery (2), left ventricular cavity (3), and aortic root (4).
The patient was decannulated from VA-ECLS on hospital day (HD) 9. By HD 11, his echocardiogram revealed normal bilateral ventricular systolic function. He remained on therapeutic anticoagulation with bivalirudin until HD 15 when he was transitioned to enoxaparin. An extensive thrombophilia work-up revealed no inherited cause for his hypercoagulability. On HD 15, speech deficiencies were noted and a magnetic resonance imaging (MRI) of his brain revealed no acute abnormalities but did find a chronic punctate lesion in the right cerebellum. After spending 9 days in inpatient rehabilitation, he was discharged home with his family. He was noted only to have mild articulation difficulties that did not impact intelligibility and mild gross motor difficulties (unsteady gait). He was anticoagulated for a total of 12 months with no residual effect noted from his acute illness at the time of anticoagulation cessation.
Although anticoagulation is utilized in the management of acute pediatric TE, anticoagulation alone may not be sufficient to prevent long-term morbidity associated with acute TE events (1). Thrombolysis refers to the use of the exogenous serine proteases tissue plasminogen activator (tPA) and urokinase-type plasminogen activator for more rapid dissolution of thrombus burden (2). Clinical studies for all thrombolytic agents are lacking in children, but studies have shown that tPA may be more efficient at stimulating thrombolysis as it binds preferentially to plasminogen that is fibrin bound (3). Due to its short half-life and the seemingly improved efficiency compared to other agents, rtPA has become the most commonly utilized thrombolytic agent in children (1, 4).
Thrombolytic therapy has been employed for the management of pediatric TE for decades and the use is increasing (5, 6). A major contributing factor to the rise in pediatric thrombolysis is the increase in pediatric interventional radiologists and cardiologists with an expertise in this therapy (1). Thrombolytic therapy can be administered either systemically or via an endovascular route, including CDT or pharmaco-mechanical thrombolysis. Currently there are no studies comparing the routes of administration in children, which limits the ability to address the relative risk and benefit comparison between systemic and endovascular thrombolysis, but there are case series that suggest CDT may be safer and be more efficacious than systemic thrombolysis (1, 7, 8).
The recommended rtPA dosing for as well as the concomitant use of unfractionated heparin with systemic thrombolysis widely varies (2). Administered treatment regimens include a low dose (0.01–0.06 mg/hg/h) infusion for 6–72 h or a high dose (0.1–0.6 mg/kg/h) for 2–6 h. Laboratory monitoring to assess thrombolytic response and bleeding risk is recommended every 6–12 h and includes a complete blood count, prothrombin time, partial thromboplastin time, fibrinogen, and D-dimer (1, 9). Almost 80% of pediatric patients receiving systemic thrombolysis achieve complete or partial TE resolution, but up to 15% have major bleeding complications (fatal bleeding, hemoglobin drop of at least 2 g/dL in a 24 h time period, any bleed requiring surgical intervention, and specific locations: retroperitoneal, pulmonary, and intracranial) (6, 10). An increased risk for major bleeding in children receiving systemic thrombolysis has been associated with lower fibrinogen activity right after completion of thrombolysis and longer rtPA infusions (11).
Endovascular thrombolysis dosing is typically 0.01–0.03 mg/kg with a maximum dose of 1–2 mg/h. Laboratory monitoring is similar to that of systemic thrombolysis (1). Partial or complete resolution is seen in up to 93% of pediatric patients receiving endovascular thrombolysis (6). While the directed therapy may lower the risk of major bleeding (reported in up to 3% of children who undergo endovascular thrombolysis), it does require more healthcare utilization, including: longer intensive care stays, utilization of interventionalists, and general anesthesia (1).
Specific indications for thrombolysis in pediatric TE are lacking due to the lack of clinical trials (2). In fact, recent American Society of Hematology guidelines for the management of pediatric venous thromboembolism suggest using anticoagulation alone in acute pediatric VTE and sub-massive pulmonary embolism (PE) over the use of thrombolysis followed by anticoagulation due to the concern that the potential benefit would outweigh the inherent risks (major bleeding—particularly in neonates) in most clinical scenarios (4, 7). In general, thrombolytic therapy is reserved for life-, limb-, and organ-threatening events, including PE with hemodynamic compromise, in centers with access to pediatric interventional radiology or interventional cardiology expertise (4, 7). Yet, with improved laboratory monitoring capabilities, radiographic imaging, and interventional radiology and surgical interventions, the use of thrombolysis in pediatrics has risen in the last 10–20 years and recent guidelines acknowledged that certain patients could benefit from this therapy (1, 7). Thrombolysis is also more likely to be considered in centers with access to interventionalists. Generally, single or potentially two concomitant rtPA CDT infusions may be utilized and there is a report of three distinct simultaneous infusions, but our case, to our knowledge, is the first reported use of four simultaneous rtPA infusions for CDT (12, 13).
Despite the lack of specific evidence in pediatric populations, there are many reported case series and cohort reports on the use of thrombolysis in pediatric populations that show similar results in efficacy, major bleeding, and TE recurrence rates (Table 1) (14–39). Even though there are many reports on the use of thrombolysis in pediatric patients, the decision to utilize any mechanism of this therapy should be decided on a case-by-case basis (40).
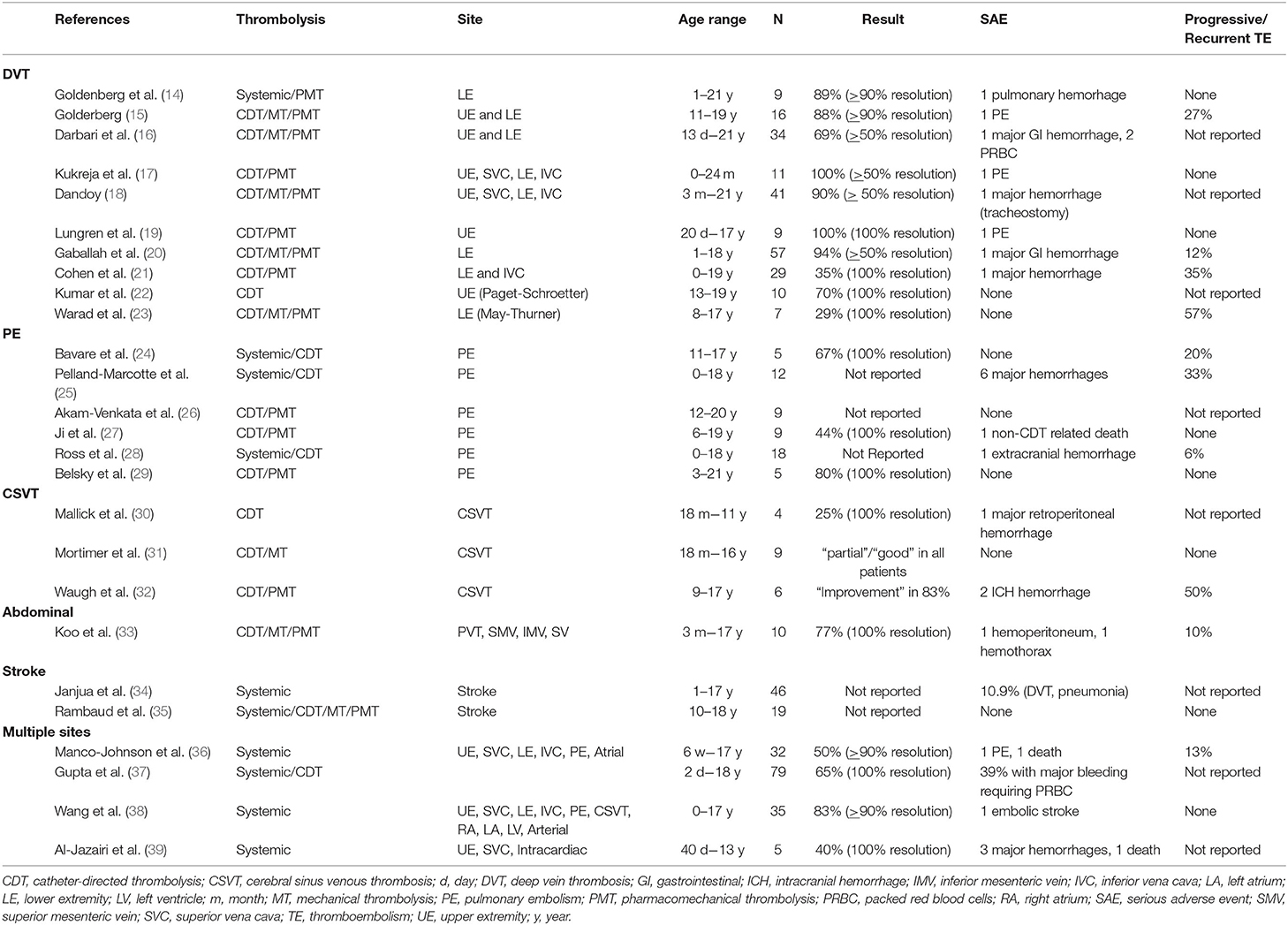
Table 1. Summary of case series and case cohorts on thrombolysis in children based on thromboembolism type.
CHEST recently published consensus recommendations for high-risk and intermediate-risk PE (40). For high-risk PE in adults, there is evidence showing systemic thrombolysis reduces recurrent PE and mortality risk and treatment algorithms for children do recommend systemic thrombolysis in this setting, with consideration of CDT if the facility has staff experienced with this therapy (7). ECLS should also be available in patients with confirmed or suspected high-risk PE in which thrombolysis is being considered (40). For intermediate-risk PE, current adult guidelines recommend against the use of systemic thrombolysis due to the increased risk of major bleeding (41). Yet, there are pediatric case series with more favorable outcomes utilizing CDT in intermediate-risk PE (24, 26, 27, 29). Thus, if thrombolysis is pursued in intermediate-risk PE, CDT may be preferable due to the lower complication rate (40).
More recently, symptomatic IVC and iliofemoral DVT, including those related to May-Thurner anatomy, have been considered indications for thrombolysis. There is evidence to support this decision as it appears to improve function and pain in the short term and it could reduce the risk for the development of post-thrombotic syndrome in the long term (PTS) (14, 15, 18). Similar to the adult data, the combination of CDT and iliac vein stenting followed by anticoagulation is the most common treatment for VTE In the setting of May-Thurner, the evidence suggests that complications using this strategy are low (42). It seems reasonable to consider thrombolysis in pediatric patients with May-Thurner based on the short-term benefits, seemingly low complication rate, and potential long-term functional gains.
In the setting of Paget-Schroetter in adults, there is significant evidence showing the success of thrombolytic therapy and CDT has become the primary method for establishing primary reperfusion (43). The data is much more limited in pediatrics as there is a case series that suggests CDT followed by rib enumeration and anticoagulation is safe and efficacious, but there was no a significant difference in the reported PTS or health related quality-of-life scores between the group that did get thrombolysis and the one that did not (22). There was also no difference in the rate of recurrent DVT. Thus, the need for thrombolysis is still unclear, but could still be considered in certain patients considered to the highest risk for recurrence.
The evidence for the use of thrombolysis in pediatric stroke is also very limited and is generally extrapolated from adult data. There is a recent case series that shows that thrombolysis can be utilized in adolescent stroke for acute revascularization relatively safely and efficaciously (35). Yet, the main multi-institutional trial evaluating the safety and efficacy of thrombolysis in children (TIPS trial) was not able to enroll any patients despite screening 93 patients and confirming 43 with an arterial ischemic stroke (44). Stroke recognition with rapid diagnosis and stroke management strategies were quite varied as the TIPS trial was being organized, but this study did lead to significant increase in acute stroke teams for centers participating in the trial (45). The development of acute pediatric stroke alert teams may fill in existing knowledge gaps, but, until then, current data only suggests that decision for acute revascularization with thrombolytic therapy in pediatric patients should involve multidisciplinary collaboration and be made on a case-by-case basis (35).
ECLS is a technique that maintains gas exchange, tissue oxygenation, and cardiac output in patients with temporary, reversible cardiac and respiratory failure (46). Since the 1990s, ECLS has been increasingly utilized in pediatric intensive care units (47). As anticoagulation is required for pediatric ECLS, major difficulty in the management is balancing the risk for both hemorrhage and thrombosis. TE in children can lead to the need for ECLS and TE can occur while on ECLS, and the management of these situations can be quite challenging. There are clinical situations, including the one presented in our case, where the benefits of thrombolysis outweigh the risk and the use of CDT in pediatric ECLS has been safe and effective (48–51).
There are also reports of neonates and infants receiving thrombolysis while on ECLS: a 4-day-old with acute respiratory failure on ECLS that developed a RA thrombus, a 4-day-old with an acute MI and cardiogenic shock, a 12-day-old with respiratory failure in the setting of a congenital diaphragmatic hernia that developed an aortic thrombus to the level of the kidneys in the setting of a umbilical arterial catheter on ECLS, and a 7-month-old with a bidirectional Glenn shunt thrombosis prior to ECLS (13, 52–54). Generally, CDT is preferred in neonates compared to systemic TPA due to the lower risk for bleeding, which is only amplified on ECLS (55). There is also a special consideration in neonates, namely developmental hemostasis and decreased plasminogen levels compared to adults. Some of the reported neonatal cases required a concomitant FFP infusion to ensure adequate thrombolysis was achieved. This principle was employed in our case as there was concern that the stagnant flow within the central vasculature would have limited plasminogen availability for successful thrombolysis. Despite the reported successful utilization of thrombolysis on ECLS in children, the therapy should only be considered in certain pediatric patients after consultation with the family and providers trained in thrombolysis with a full understanding of the risks and benefits (55).
Acute COVID-19 infections have been associated with hypercoagulability and thromboembolic complications (56). There is a report of an adolescent successfully utilizing dual CDT for segmental and subsegmental PE in the setting of acute COVID-19 as well as a 21-year-old with recurrent PE undergoing successful CDT for bilateral PE (12, 57). Yet, in adolescents and children, MIS-C has been shown to have a higher TE incidence than acute COVID-19 infections (58). And while CDT has been successfully utilized in the setting of acute COVID-19, it appears that our case may represent the first reported successful use of thrombolysis in the setting of MIS-C (49).
Thrombolysis has been utilized anecdotally for the management of acute COVID-19 infections with TE complications, but the evidence is limited (49, 59). The logistical challenges that thrombolysis present in a patient with acute COVID-19, including the complexity of transporting patients with COVID-19 due to the potential nosocomial spread of the virus, may limit the ability to utilize this therapy (60). Despite this limited evidence and the logistical challenges, the National PERT Consortium has stated that the indications for thrombolysis for acute COVID-19 associated pulmonary embolism remain unchanged (61). Extrapolating from this recommendation, it seems that thrombolytic therapy can still be considered in the setting of an acute COVID-19 infection understanding there may be instances where the risk is too significant to proceed.
Heparin resistance, similar to our patient, in acute COVID-19 has been described, mostly in adults admitted to intensive care units (56, 62). Typically, patients receiving CDT and some receiving systemic thrombolysis will also receive prophylactic dosing of unfractionated heparin. Yet, there are instances where heparin may not be effective, and an alternative anticoagulant must be considered. There have been two reported cases in children of the successful use of bivalirudin with CDT. The first is a 2-year old with a history of a mechanical heart valve that presented in shock and developed significant thrombotic burden while on high doses of UFH (63). The other is a teenager with known antithrombin deficiency with inadequate anticoagulation on low molecular weight heparin (64). Our case supplements the fact that bivalirudin can be utilized safely and effectively in heparin resistant children during thrombolysis.
As the incidence of pediatric TE has increased, the utilization of thrombolysis has as well. While there is some evidence of the acute and chronic benefits of thrombolysis in pediatric patients, most of the data is extrapolated from adult studies. Thrombolysis can be utilized safely in children, but it requires multidisciplinary collaboration with experienced providers from numerous specialties (hematology, interventional radiology, interventional cardiology and/or critical care) as well as close laboratory monitoring. Without specific evidence from randomized trials in children on the risks and benefits of thrombolysis, providers must be vigilant in the patients selected for the therapy and ensure appropriate monitoring is undertaken to ensure the most optimal outcomes.
GW and HV contributed to the conception and design of the manuscript. GW wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tarango C, Manco-Johnson MJ. Pediatric thrombolysis: a practical approach. Front Pediatr. (2017) 5:260. doi: 10.3389/fped.2017.00260
2. Raffini L. Thrombolysis for intravascular thrombosis in neonates and children. Curr Opin Pediatr. (2009) 21:9–14. doi: 10.1097/MOP.0b013e32831ef537
3. Hoylaerts M, Rijken DC, Lijnen HR, Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. (1982) 257:2912–9. doi: 10.1016/S0021-9258(19)81051-7
4. Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, et al. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. (2012) 141(Suppl. 2):e737S−801S. doi: 10.1378/chest.11-2308
5. Ino T, Benson LN, Freedom RM, Barker GA, Aipursky A, Rowe RD. Thrombolytic therapy for femoral artery thrombosis after pediatric cardiac catheterization. Am Heart J. (1988) 115:633–9. doi: 10.1016/0002-8703(88)90815-0
6. Albisetti M. Thrombolytic therapy in children. Thromb Res. (2006) 118:95–105. doi: 10.1016/j.thromres.2004.12.018
7. Monagle P, Cuello CA, Augustine C, Bonduel M, Brandão LR, Capman T, et al. American Society of Hematology 2018 Guidelines for management of venous thromboembolism: treatment of pediatric venous thromboembolism. Blood Adv. (2018) 2:3292–316. doi: 10.1182/bloodadvances.2018024786
8. Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. (2011) 123:1788–830. doi: 10.1161/CIR.0b013e318214914f
9. Witmer C, Raffini L. Treatment of venous thromboembolism in pediatric patients. Blood. (2020) 135:335–43. doi: 10.1182/blood.2019001847
10. Mitchell LG, Goldenberg NA, Male C, Kenet G, Monagle P, Nowak-Göttl U, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. (2011) 9:1856–8. doi: 10.1111/j.1538-7836.2011.04433.x
11. Newall F, Browne M, Savoia H, Campbell J, Barnes C, Monagle P. Assessing the outcome of systemic tissue plasminogen activator for the management of venous and arterial thrombosis in pediatrics. J Pediatr Hematol Oncol. (2007) 29:269–73. doi: 10.1097/MPH.0b013e318047b78b
12. Kotula JJ, Balakumar N, Khan D, Patel B. Bilateral pulmonary emboli in a teenager with positive SARS-CoV-2 antibody. Pediatr Pulmonol. (2021) 56:271–3. doi: 10.1002/ppul.25132
13. Ryerson L, Seaman C, Holinski P, Bauman M, Massicotte P. Successful lysis of thrombus with tissue plasminogen activator in a bidirectional glenn on venoarterial extracorporeal life support. Insights Pediatr Cardiol. (2016) 1:1.
14. Goldenberg NA, Durham JD, Knapp-Clevenger R, Manco-Johnson MJ. A thrombolytic regimen for high-risk deep venous thrombosis may substantially reduce the risk of postthrombotic syndrome in children. Blood. (2007) 110:45–53. doi: 10.1182/blood-2006-12-061234
15. Goldenberg NA, Branchford B, Wang M, Ray C, Durham JD, Manco-Johnson MJ. Percutaneous mechanical and pharmacomechanical thrombolysis for occlusive deep vein thrombosis of the proximal limb in adolescent subjects: findings from an institution-based prospective inception cohort study of pediatric venous thromboembolism. J Vasc Interv Radiol. (2011) 22:121–32. doi: 10.1016/j.jvir.2010.10.013
16. Darbari DS, Desai D, Arnaldez F, Desai K, Kallen J, Strouse J, et al. Safety and efficacy of catheter directed thrombolysis in children with deep venous thrombosis. Br J Haematol. (2012) 159:376–8. doi: 10.1111/bjh.12025
17. Kukreja KU, Lungren MP, Patel MN, Johnson ND, Racadio JM, Dandoy C, et al. Endovascular venous thrombolysis in children younger than 24 months. J Vasc Interv Radiol. (2014) 25:1158–64. doi: 10.1016/j.jvir.2014.04.003
18. Dandoy CE, Kukreja KU, Gruppo RA, Patel MN, Tarango C. Outcomes in children with deep vein thrombosis managed with percutaneous endovascular thrombolysis. Pediatr Radiol. (2015) 45:719–26. doi: 10.1007/s00247-014-3209-4
19. Lungren MP, Ward TJ, Patel MN, Racadio JM, Kukreja K. Endovascular thrombolysis to salvage central venous access in children with catheter-associated upper extremity deep vein thrombosis: technique and initial results. J Thromb Thrombolysis. (2015) 40:274–9. doi: 10.1007/s11239-015-1209-3
20. Gaballah M, Shi J, Kukreja K, Raffini L, Tarango C, Keller M, et al. Endovascular thrombolysis in the management of iliofemoral thrombosis in children: a multi-institutional experience. J Vasc Interv Radiol. (2016) 27:524–30. doi: 10.1016/j.jvir.2015.12.753
21. Cohen CT, Sartain SE, Sangi-Haghpeykar H, Kukreja KU, Desai SB. Clinical characteristics and outcomes of combined thrombolysis and anticoagulation for pediatric and young adult lower extremity and inferior vena cava thrombosis. Pediatr Hematol Oncol. (2021) 38:528–42. doi: 10.1080/08880018.2021.1889729
22. Kumar R, Harsh K, Saini S, O'Brien SH, Stanek J, Warren P, et al. Treatment-Related Outcomes in Paget-Schroetter Syndrome-A Cross-Sectional Investigation. J Pediatr. (2019) 207:226–32.e1. doi: 10.1016/j.jpeds.2018.11.018
23. Warad DM, Rao AN, Bjarnason H, Rodriguez V. Clinical outcomes of May-Thurner syndrome in pediatric patients: a single institutional experience. TH Open. (2020) 4:e189–e96. doi: 10.1055/s-0040-1714694
24. Bavare AC, Naik SX, Lin PH, Poi MJ, Yee DL, Bronicki RA, et al. Catheter-directed thrombolysis for severe pulmonary embolism in pediatric patients. Ann Vasc Surg. (2014) 28:1794.e1–7. doi: 10.1016/j.avsg.2014.03.016
25. Pelland-Marcotte MC, Tucker C, Klaassen A, Avila ML, Amid A, Amiri N, et al. Outcomes and risk factors of massive and submassive pulmonary embolism in children: a retrospective cohort study. Lancet Haematol. (2019) 6:e144–e53. doi: 10.1016/S2352-3026(18)30224-2
26. Akam-Venkata J, Forbes TJ, Schreiber T, Kaki A, Elder M, Turner DR, et al. Catheter-directed therapy for acute pulmonary embolism in children. Cardiol Young. (2019) 29:263–9. doi: 10.1017/S1047951118002135
27. Ji D, Gill AE, Durrence WW, Shah JH, Paden ML, Patel KN, et al. Catheter-directed pharmacologic thrombolysis for acute submassive and massive pulmonary emboli in children and adolescents-an exploratory report. Pediatr Crit Care Med. (2020) 21:e15–22. doi: 10.1097/PCC.0000000000002172
28. Ross CE, Shih JA, Kleinman ME, Donnino MW. Pediatric massive and submassive pulmonary embolism: a single-center experience. Hosp Pediatr. (2020) 10:272–6. doi: 10.1542/hpeds.2019-0290
29. Belsky J, Warren P, Stanek J, Kumar R. Catheter-directed thrombolysis for submassive pulmonary embolism in children: a case series. Pediatr Blood Cancer. (2020) 67:e28144. doi: 10.1002/pbc.28144
30. Mallick AA, Sharples PM, Calvert SE, Jones RW, Leary M, Lux AL, et al. Cerebral venous sinus thrombosis: a case series including thrombolysis. Arch Dis Child. (2009) 94:790–4. doi: 10.1136/adc.2008.154708
31. Mortimer AM, Bradley MD, O'Leary S, Renowden SA. Endovascular treatment of children with cerebral venous sinus thrombosis: a case series. Pediatr Neurol. (2013) 49:305–12. doi: 10.1016/j.pediatrneurol.2013.07.008
32. Waugh J, Plumb P, Rollins N, Dowling MM. Prolonged direct catheter thrombolysis of cerebral venous sinus thrombosis in children: a case series. J Child Neurol. (2012) 27:337–45. doi: 10.1177/0883073811421827
33. Koo KSH, Lamar DL, Shaw DWW, Monroe EJ, Shivaram GM. Catheter-directed thrombolysis for portal vein thrombosis in children: a case series. J Vasc Interv Radiol. (2018) 29:1578–83. doi: 10.1016/j.jvir.2018.07.018
34. Janjua N, Nasar A, Lynch JK, Qureshi AI. Thrombolysis for ischemic stroke in children: data from the nationwide inpatient sample. Stroke. (2007) 38:1850–4. doi: 10.1161/STROKEAHA.106.473983
35. Rambaud T, Legris N, Bejot Y, Bellesme C, Lapergue B, Jouvent E, et al. Acute ischemic stroke in adolescents. Neurology. (2020) 94:e158–e69. doi: 10.1212/WNL.0000000000008783
36. Manco-Johnson MJ, Nuss R, Hays T, Krupski W, Drose J, Manco-Johnson ML. Combined thrombolytic and anticoagulant therapy for venous thrombosis in children. J Pediatr. (2000) 136:446–53. doi: 10.1016/S0022-3476(00)90006-4
37. Gupta AA, Leaker M, Andrew M, Massicotte P, Liu L, Benson LN, et al. Safety and outcomes of thrombolysis with tissue plasminogen activator for treatment of intravascular thrombosis in children. J Pediatr. (2001) 139:682–8. doi: 10.1067/mpd.2001.118428
38. Wang M, Hays T, Balasa V, Bagatell R, Gruppo R, Grabowski EF, et al. Low-dose tissue plasminogen activator thrombolysis in children. J Pediatr Hematol Oncol. (2003) 25:379–86. doi: 10.1097/00043426-200305000-00006
39. Al-Jazairi AS, Al-Gain RA, Bulbul ZR, Cherfan AJ. Clinical experience with alteplase in the management of intracardiac and major cardiac vessels thrombosis in pediatrics: a case series. Ann Saudi Med. (2010) 30:227–32. doi: 10.4103/0256-4947.62840
40. Ross C, Kumar R, Pelland-Marcotte MC, Mehta S, Kleinman ME, Thiagarajan RR, et al. “Acute management of high- and intermediate-risk pulmonary embolism in children: a review”. Chest. (2021). doi: 10.1016/j.chest.2021.09.019 [Online ahead of print].
41. Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. (2020) 4:4693–738. doi: 10.1182/bloodadvances.2020001830
42. Hansrani V, Moughal S, Elmetwally A, Al-Khaffaf H. A review into the management of May-Thurner syndrome in adolescents. J Vasc Surg Venous Lymphat Disord. (2020) 8:1104–10. doi: 10.1016/j.jvsv.2020.05.006
43. Karaolanis G, Antonopoulos CN, Koutsias SG, Giosdekos A, Metaxas EK, Tzimas P, et al. A systematic review and meta-analysis for the management of Paget-Schroetter syndrome. J Vasc Surg Venous Lymphat Disord. (2021) 9:801–10.e5. doi: 10.1016/j.jvsv.2021.01.011
44. Rivkin MJ, deVeber G, Ichord RN, Kirton A, Chan AK, Hovinga CA, et al. Thrombolysis in pediatric stroke study. Stroke. (2015) 46:880–5. doi: 10.1161/STROKEAHA.114.008210
45. Bernard TJ, Rivkin MJ, Scholz K, deVeber G, Kirton A, Gill JC, et al. Emergence of the primary pediatric stroke center: impact of the thrombolysis in pediatric stroke trial. Stroke. (2014) 45:2018–23. doi: 10.1161/STROKEAHA.114.004919
46. Gaffney AM, Wildhirt SM, Griffin MJ, Annich GM, Radomski MW. Extracorporeal life support. BMJ. (2010) 341:c5317. doi: 10.1136/bmj.c5317
47. Sandhu HS, Fortenberry JD, MacLaren G. Editorial: improving extracorporeal life support outcomes in children. Front Pediatr. (2019) 7:140. doi: 10.3389/fped.2019.00140
48. Frazier WJ, Hall MW, Coley BD, Catheter-directed thrombolysis during Extra-Corporeal Membrane Oxygenation (ECMO) in a child with massive Pulmonary Embolus (PE): 638. Crit Care Med. (2004) 32:A179. doi: 10.1097/00003246-200412001-00634
49. Visveswaran GK, Morparia K, Narang S, Sturt C, Divita M, Voigt B, et al. Severe acute respiratory syndrome coronavirus 2 infection and thrombosis: phlegmasia cerulea dolens presenting with venous gangrene in a child. J Pediatr. (2020) 226:281–4.e1. doi: 10.1016/j.jpeds.2020.07.032
50. Machado DS, Tule M, Philip J, Wynn T, Lazarowicz M, Machuca T, et al. Bivalirudin and alteplase for pulmonary embolism requiring veno-arterial extracorporeal membrane oxygenation in an adolescent. J Extra Corpor Technol. (2020) 52:327–31. doi: 10.1182/ject-2000013
51. Badheka A, Bangalore Prakash P, Allareddy V. Successful use of extracorporeal membrane oxygenation in a child with obstructive shock due to massive bilateral pulmonary embolism. Perfusion. (2018) 33:323–5. doi: 10.1177/0267659117736380
52. Garcia A, Gander JW, Gross ER, Reichstein A, Sheth SS, Stolar CJ, et al. The use of recombinant tissue-type plasminogen activator in a newborn with an intracardiac thrombus developed during extracorporeal membrane oxygenation. J Pediatr Surg. (2011) 46:2021–4. doi: 10.1016/j.jpedsurg.2011.06.039
53. Deutsch MA, Cleuziou J, Noebauer C, Eicken A, Vogt M, Hoerer J, et al. Successful management of neonatal myocardial infarction with ECMO and intracoronary r-tPA lysis. Congenit Heart Dis. (2014) 9:E169–74. doi: 10.1111/chd.12117
54. Gunnarsson B, Heard CM, Martin DJ, Brecher ML, Steinhorn RH. Successful lysis of an obstructive aortic and renal artery thrombus in a neonate on extracorporeal membrane oxygenation. J Perinatol. (2000) 20:555–7. doi: 10.1038/sj.jp.7200466
55. Van Ommen CH, Neunert CE, Chitlur MB. Neonatal ECMO. Front Med (Lausanne). (2018) 5:289. doi: 10.3389/fmed.2018.00289
56. White D, MacDonald S, Bull T, Hayman M, de Monteverde-Robb R, Sapsford D, et al. Heparin resistance in COVID-19 patients in the intensive care unit. J Thromb Thrombolysis. (2020) 50:287–91. doi: 10.1007/s11239-020-02145-0
57. Hirschbaum JH, Bradley CP, Kingsford P, Mehra A, Kwan W. Recurrent massive pulmonary embolism following catheter directed thrombolysis in a 21-year-old with COVID-19: a case report. Eur Heart J Case Rep. (2021) 5:ytab140. doi: 10.1093/ehjcr/ytab140
58. Whitworth H, Sartain SE, Kumar R, Armstrong K, Ballester L, Betensky M, et al. Rate of thrombosis in children and adolescents hospitalized with COVID-19 or MIS-C. Blood. (2021) 138:190–8. doi: 10.1182/blood.2020010218
59. Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. (2020) 18:1752–5. doi: 10.1111/jth.14828
60. Kipshidze N, Dangas G, White CJ, Siddiqui F, Lattimer CR, Carter CA, et al. Viral coagulopathy in patients with COVID-19: treatment and care. Clin Appl Thromb Hemost. (2020) 26:1076029620936776. doi: 10.1177/1076029620936776
61. Rosovsky RP, Grodzin C, Channick R, Davis GA, Giri JS, Horowitz J, et al. Diagnosis and treatment of pulmonary embolism during the coronavirus disease 2019 pandemic: a position paper from the National PERT consortium. Chest. (2020) 158:2590–601. doi: 10.1016/j.chest.2020.08.2064
62. Beun R, Kusadasi N, Sikma M, Westerink J, Huisman A. Thromboembolic events and apparent heparin resistance in patients infected with SARS-CoV-2. Int J Lab Hematol. (2020) 42:19–20. doi: 10.1111/ijlh.13230
63. Regling K, Callaghan MU, Rajpurkar M. Bivalirudin during thrombolysis with catheter-directed tPA in a heparin-refractory patient: a case report. Pediatr Blood Cancer. (2020) 67:e28094. doi: 10.1002/pbc.28094
Keywords: thrombosis, thrombolysis, pediatrics, extracorporeal life support (ECLS), COVID-19, May-Thurner, Paget-Schroetter
Citation: Woods GM, Kim DW, Paden ML and Viamonte HK (2022) Thrombolysis in Children: A Case Report and Review of the Literature. Front. Pediatr. 9:814033. doi: 10.3389/fped.2021.814033
Received: 12 November 2021; Accepted: 23 December 2021;
Published: 24 January 2022.
Edited by:
Cornelia Heleen Van Ommen, Sophia Children's Hospital, NetherlandsReviewed by:
John S. Kim, University of Colorado, United StatesCopyright © 2022 Woods, Kim, Paden and Viamonte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary M. Woods, Z2FyeS53b29kc0BjaG9hLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.