
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 21 December 2021
Sec. Pediatric Hematology and Hematological Malignancies
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.788360
This article is part of the Research TopicAllogeneic Hematopoetic Stem Cell Transplantation for Children with Acute Lymphoblastic Leukemia in the Era of ImmunotherapyView all 24 articles
 Anita Lawitschka1,2
Anita Lawitschka1,2 Leila Ronceray1
Leila Ronceray1 Dorothea Bauer1
Dorothea Bauer1 Michael Rittenschober1
Michael Rittenschober1 Natalia Zubarovskaya1
Natalia Zubarovskaya1 Rene Geyeregger2
Rene Geyeregger2 Winfried F. Pickl3
Winfried F. Pickl3 Zoya Kuzmina4*
Zoya Kuzmina4*Objectives: Chronic graft-versus-host disease (cGvHD) following haematopoietic stem cell transplantation (HSCT) shares many similarities with de novo autoimmune disorders, being associated with the presence of autoantibodies. However, data on the implication of autoantibodies in paediatric HSCT recipients are scarce. In this single-centre study of paediatric patients with acute lymphoblastic leukaemia (ALL) surviving longer than 3 months, our objectives were to evaluate autoantibody expression and investigate the correlation with cGvHD and immune reconstitution using serially monitored parameters.
Methods: We investigated circulating autoantibodies together with cellular and humoral parameters [including major T- and B-cell subsets, natural killer (NK) cells, and immunoglobulin levels] in 440 samples from 74 patients (median age 10.9 years, range 2.7–22.2 years) serially during long-term follow-up of median 8 years (range 0.4–19.3 years). Evaluations comprised of patient and transplant characteristics, precisely reviewed details of National Institute of Health (NIH)-defined cGvHD, and outcome data such as relapse, overall survival (OS) and mortality. Analysis of these clinical parameters was performed to identify possible associations.
Results: Autoantibodies were detected in 65% (48/74) of patients. Anti-nuclear antibodies were the most common, occurring in 75% (36/48) of patients with autoantibodies. When comparing demographic data and transplant characteristics, there were no significant differences between patients with and without autoantibody expression; 5-year OS was excellent, at 96.4 and 95.8%, respectively. Neither the expression of autoantibodies nor the occurrence of cGvHD correlated with significantly worse OS or relapse rate. Furthermore, there was no significant association between autoantibody profiles and the incidence, overall severity or organ involvement of cGvHD. Patients with autoantibodies showed significantly better immune reconstitution, with overall higher numbers of T cells, B cells, and serum immunoglobulins. In autoantibody-positive patients with cGvHD, autoantibody production positively correlated with the expansion of CD56+ NK cells (236.1 vs. 165.6 × 103 cells/mL, respectively; p = 0.023) and with signs of B-cell perturbation, such as higher CD21low B cells (23.8 vs. 11.8 × 103 cells/mL, respectively; p = 0.044) and a higher ratio of CD21low B cells/CD27+ memory B cells (1.7 vs. 0.4, respectively; p = 0.006) in comparison to autoantibody-positive patients without cGvHD. Furthermore, when assessing the correlation between autoantibody positivity and the activity of cGvHD at time of analysis, indicators of aberrant B-cell homeostasis were substantiated by a lower proportion of CD27+ memory B cells (9.1 vs. 14.9%, respectively; p = 0.028), a higher ratio of class-switched CD27+IgD−/CD27+ memory B cells (3.5 vs. 5.1%, respectively; p = 0.013), significantly elevated numbers of CD21low B cells (36.8 vs. 11.8 × 103 cells/mL, respectively; p = 0.013) and a higher ratio of CD21lowB cells/CD27+ memory B cells (2.4 vs. 0.4, respectively; p = 0.034) in the active vs. the no cGvHD group. We then assessed the potential role of autoantibody expression in the context of elevated CD19+CD21low B cells (cutoff >7%), a well-known marker of cGvHD. Surprisingly we found a significant higher proportion of those cases where elevated CD21low B cells correlated with active cGvHD in samples from the autoantibody-negative group vs. the antibody-positive group (82 vs. 47%, respectively; p = 0.0053).
When comparing immune parameters of the large proportion of survivors (89%) with the small proportion of non-survivors (11%), data revealed normalisation within the B-cell compartment of survivors: there were increased numbers of CD27+ memory B cells (54.9 vs. 30.6 × 103 cells/mL, respectively; p = 0.05), class-switched CD27+IgD− B cells (21.2 vs. 5.0 × 103 cells/mL, respectively; p < 0.0001), and immunoglobulin G4 (40.9 vs. 19.4 mg/dL, respectively; p < 0.0001). Overall mortality was significantly associated with an elevated proportion of CD21low B cells (13.4 vs. 8.8%, respectively; p = 0.039) and CD56+ NK cells (238.8 vs. 314.1 × 103 cells/mL, respectively; p = 0.019). In multivariate analysis, better OS was significantly associated with lower numbers of CD56+ NK cells [hazard ratio (HR) 0.98, p = 0.041] and higher numbers of CD27+ memory B cells [(HR) 1.62, p = 0.014].
Conclusion: Our data shows that autoantibody profiles are not suitable biomarkers for diagnosing cGvHD in children or for predicting cGvHD severity, disease course and outcome. We identified a number of indicators of aberrant immune homeostasis associated with active cGvHD in paediatric ALL patients after HSCT. These findings confirm published results and suggest that candidate B cell subpopulations may serve as a surrogate measure for characterisation of cGvHD in paediatric HSCT for malignant diseases, and warrants confirmation in larger, multicentre studies.
Allogeneic haematopoietic stem cell transplantation (HSCT) is a potentially curative treatment for acute lymphoblastic leukaemia (ALL) in paediatric and adolescent patients. Recently, it has been shown to have excellent outcomes with low treatment-related mortality (1). However, successful long-term outcomes may be limited by chronic graft-versus-host disease (cGvHD), a serious and complex, multisystem immunological complication of HSCT and major cause of late non-relapse morbidity and mortality (2, 3). The incidence of cGvHD is ~50% in adults while the incidence in paediatric patients is lower (5–30%) (4, 5). The clinical presentation of cGvHD may resemble those seen in autoimmune disorders and nearly every organ system may be affected resulting in poor physical functioning and disability (2, 3). Regarding diagnosis and staging of cGvHD, a major advancement has been made by the publication and validation of the National Institutes for Health (NIH) Consensus Criteria in 2005 with revision in 2014 (6, 7). A paediatric adaption of the NIH documentation forms for daily clinical use has been published in the European Society for Blood and Marrow Transplantation (EBMT) handbook (3). Recent advances in understanding the pathophysiology of cGvHD demonstrated that the disease is characterised by a combination of allogeneic and autoimmune dysregulation (8) with prolonged immunodeficiency (9, 10). It is well-known that alloreactive CD8+ T cells play a crucial role in the development of a graft-versus-leukaemia (GVL) effect and GvHD and that, amongst other cell types, CD4+ T cells stimulate the production of autoantibodies after HSCT (11–13). The role of impaired B-cell homeostasis in cGvHD has been shown by many groups (9, 14, 15), in adults, and was recently observed in the paediatric population recently (16). Along those lines our centre observed that both cGvHD and its activity were associated with B-cell perturbation including low numbers of CD19+CD27+ memory B cells and increased frequencies of circulating CD19+CD21low B cells in a paediatric population (n = 146) (17). Chronic GvHD shares many similarities with de novo autoimmune disorders: presence of autoantibodies leads to target tissue damage, immune complex formation, and tissue deposition (15). An association between cGvHD and autoantibody expression has been described (18–21). Indeed, autoantibodies may be detectable before the onset of clinical manifestation of cGvHD (15). However, data on the role and clinical implication of autoantibodies in paediatric HSCT recipients are limited.
In this single-centre retrospective study of paediatric patients with ALL, our objectives were to determine autoantibody expression levels following HSCT and investigate whether there was correlation between occurrence of autoantibodies and development of cGvHD, immune reconstitution and survival using serially monitored parameters during long-term follow-up care.
A total of 74 paediatric and adolescent patients with ALL who underwent HSCT at the St. Anna Children's Hospital between February 1993 and June 2020 were included in this retrospective study. Inclusion criteria included: being alive on day +100 after HSCT, complete remission (CR) of the underlying disease, complete multi-lineage donor cell engraftment and no prior treatment with rituximab. Patients' parents' and/or guardians' written informed consent was obtained in accordance with the Declaration of Helsinki and the institutional review board of the Medical University of Vienna and the St. Anna Children's Hospital. All patients underwent HSCT according to standard of care or institutional review board-approved protocols including standard GvHD, antimicrobial and antifungal prophylaxis according to institutional guidelines.
Outpatient post-HSCT care is a calendar-driven at our institution with additional incidence-driven visits, in the event of complications. During routine follow-up visits, where all of these patients were seen in the HSCT Outpatient Clinic of our institution, clinical parameters were collected regarding patient and transplant characteristics and details of GvHD, and peripheral blood samples were analysed for cellular and humoral parameters of immune reconstitution, including analysis of autoantibody panels. Evaluations (clinical and laboratory) were performed at day +100 and every 3–6 months in the first year, every 6 months in the second year, and once a year thereafter and/or as clinically indicated. Acute GvHD (aGvHD) was scored according to the modified Glucksberg criteria (22) and chronic GvHD was graded according to the NIH consensus criteria 2005 (6) and revised 2014 NIH criteria (7). Per definitions, classic cGvHD included classic and overlap subtypes (the presentation of symptoms both of acute and chronic GvHD) and late aGvHD. All outcome data such as overall survival (OS), non-relapse mortality (NRM) and relapse of ALL were retrospectively reviewed for accuracy.
Each patient's serum was screened for the presence of autoantibodies, with testing performed either by enzyme-linked immunosorbent assay and/or immunofluorescence. Details of methodologies for antibody assessment are listed in Table 1. For immunoassay-based methods used to screen antibodies, patient values were compared to the relevant reference interval as provided by the manufacturer. Serum titre of ≥1:100 was considered positive and samples were titred at 1:320, 1:640, and 1:1,000. In-house ELISA antibody testing was performed on the DSX ELISA processing system. Patients positive for antinuclear antibody (ANA) were further screened for Smith/anti-ribonucleoprotein (anti-Sm/RNP), anti-Sjögren syndrome-related antigen A (SSA) and anti-Sjögren syndrome type B (SSB) autoantibodies.
The following assessments on serum were performed during routine follow-up examinations of patients longitudinally: leukocyte subpopulations; blood counts; concentrations of total immunoglobulin (Ig) G, and IgG subclasses 1–4, IgM, IgA, IgE; numbers of specific T-cell subpopulations (CD3+, CD4+, and CD8+ and the ratio of CD4+/CD8+), natural killer (NK) cells (CD3−CD56+CD16+), and specific B-cell subsets (CD19+, CD19+CD27+ memory, CD19+CD27+IgD+ non-class-switched memory, CD19+CD27+IgD− class-switched memory, CD19+CD21low, and the ratio of CD19CD21low/CD19+CD27+). Flow cytometry with gating strategy was described previously and involved the isolation of blood cells, immunophenotyping, flow cytometry, and fluorescence in situ hybridisation of sorted cells (9, 17, 23). Optimal concentrations of directly conjugated monoclonal antibodies were added to 50 μL of patients' whole blood and incubated at room temperature for 20 min. ADG lysis solution (An der Grub, Vienna, Austria) was used to remove red blood cells according to the manufacturer's recommendations followed by acquisition of 5 × 103 cells in the lymphogate for leukocyte subpopulations and 4–8 × 103 CD19+ B cells for B-cell subset analysis as previously described (9, 17, 23). Serum levels of IgG, IgM, and IgA were quantified by nephelometry using Beckman Coulter IMMAGE (Beckman Coulter Inc., Brea, CA).
Patients were divided into subgroups: autoantibody positive and autoantibody negative (based on absolute values or titres), and patients with or without cGvHD. Fisher's exact test was used to compare differences in categorical variables. For univariate analyses, different subpopulations and detailed clinical cGvHD characteristics at study points throughout long-term follow-up were selected and compared using the student's t-test or the Mann-Whitney U-test for continuous variables. Covariates with a p < 0.05 were entered into the multiple logistic regression analysis. If absolute values or percent values of a covariate were available as different variables, then these covariates were entered into multivariate logistic regression analysis. OS was calculated from day 0 of HSCT to the day of death from any cause, relapse or last follow-up to 1 January 2021. Patients were censored at the date of last contact. OS was analysed using the Kaplan-Meier test, and both groups were compared using a log-rank test or a Breslow test. NRM was defined as death due to causes unrelated to the underlying disease. Disease relapse and cGvHD were considered competing risks in this analysis. Statistical analyses were performed with SPSS 20.0 software (IBM Company, Chicago, IL, USA). Differences were considered statistically significant at a p < 0.05.
Between February 1993 and June 2020, 74 patients who received HSCT for ALL were enrolled in the study, yielding 440 serum samples for analysis. Median age was 10.9 years (range 2.7–22.2 years) and median follow-up was 8 years (range 0.4–19.3 years). In this homogenous cohort the distribution of patient and transplant characteristics such as age, sex, conditioning regimen, donor type, stem cell source, and GvHD prophylaxis was similar between patients who did and did not express autoantibodies during follow-up (Table 2).
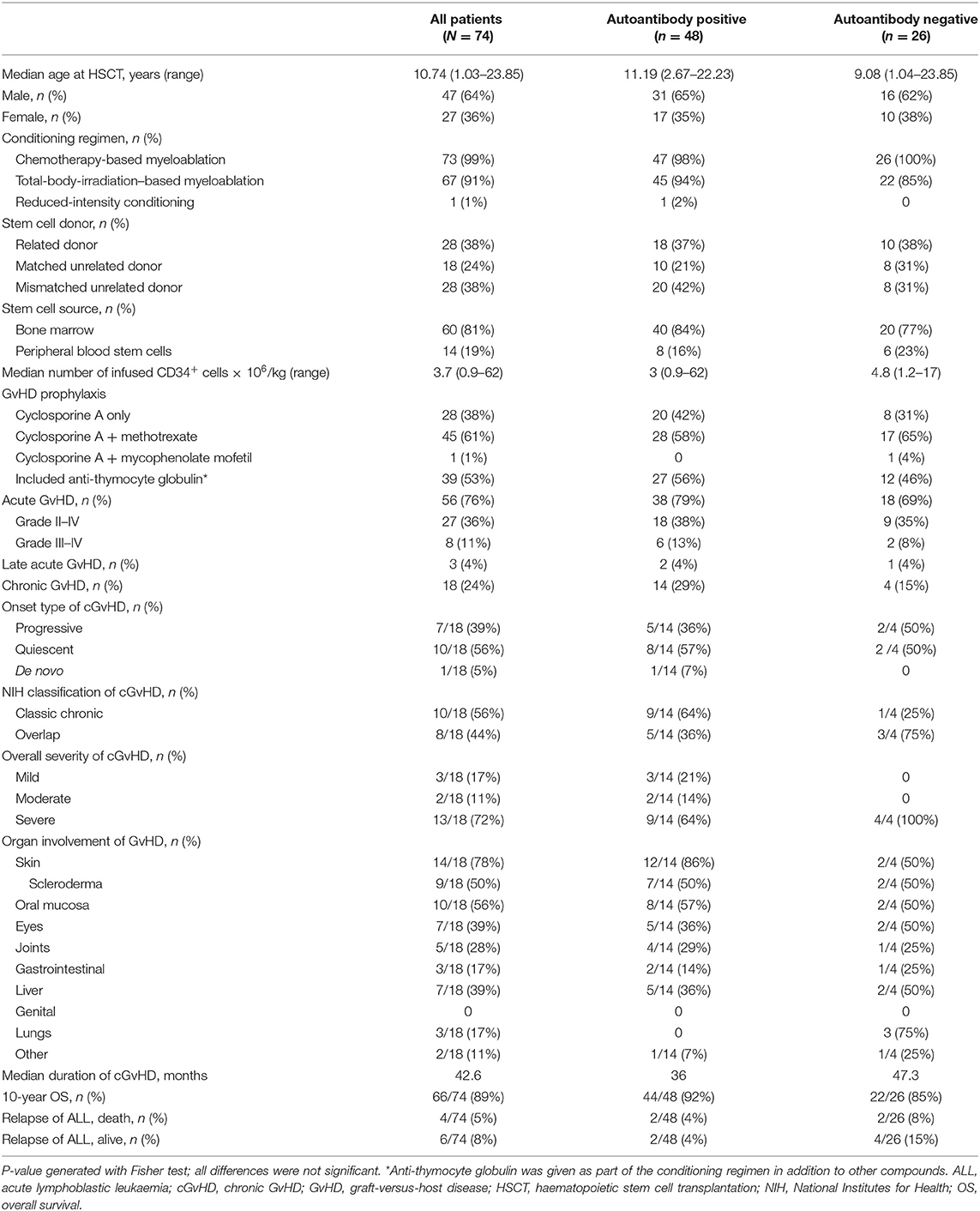
Table 2. Demographic and transplant characteristics and outcomes of paediatric ALL patients after HSCT.
In the cohort, 81% (60/74) of patients received a bone marrow graft, 62% (46/74) received stem cells from an unrelated donor, and 53% (39/74) received anti-thymocyte globulin as part of their conditioning. aGvHD of grade II–IV and grade III–IV was diagnosed in 36.5% (27/74) and 11% (8/74) of patients, respectively. cGvHD was diagnosed in 24% (18/74) of patients; severe in 72% (13/18), progressive onset in 39% (7/18), and evidence of overlap cGvHD in 44% (8/18). The majority of patients with cGvHD (93%) had a history of aGvHD. The most frequent organs affected by cGvHD were skin (78%), oral mucosa (56%) and eye (39%). Over 90% of patients suffered from multiorgan involvement, identified as cGvHD in ≥2 organs. The relapse rate of ALL was low, at 13.5% (10/74). The 10-year OS was excellent at 89% for the whole cohort. The overall mortality rate was 11% (8/74), with death occurring at a median of 5.5 years after HSCT. No difference in 5-year OS was seen when comparing the antibody-positive and the antibody-negative group (96.4 vs. 95.8%, respectively) and no influence of cGvHD was observed (95.3 vs. 93.8%, respectively) (Figures 1A,B). Causes of death were relapse of ALL in 4 patients, cGvHD in 1 patient, secondary malignancy in 1 patient, infection in 1 patient, and sudden death with epilepsy and brain oedema as determined by autopsy in 1 patient. Of note, a history of cGvHD was evident in 4 of 8 patients, but none of the patients who died of ALL relapse had a history of cGvHD.
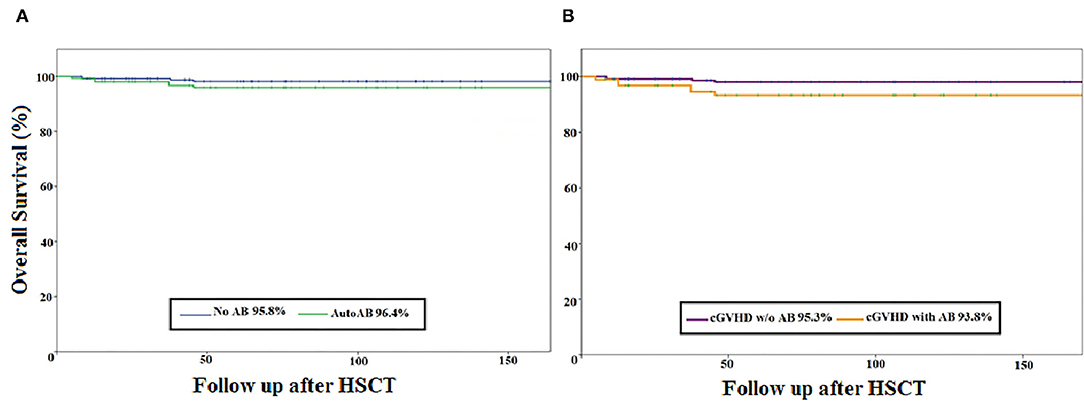
Figure 1. Kaplan–Meier curve of the overall survival of paediatric ALL patients after HSCT (N = 74). (A) overall survival (percentage) of the autoantibody-negative (n = 26) vs. the autoantibody-positive (n = 48) patient group. (B) Overall survival (percentage) of the patient group with cGvHD (n = 21) vs. the patient group without GvHD (n = 53). AB, antibody; cGvHD, chronic graft-versus-host disease; HSCT, haematopoietic stem cell transplantation; w/o, without; Follow-up after HSCT: in months.
At least one type of autoantibody was detected in 65% (48/74) patients during the follow-up period. Antinuclear antibodies were the most frequently detected antibody type, occurring in 75% (36/48) of those patients with autoantibodies (Table 2). The incidence of aGvHD was similar between groups, even when analysed by grade. Likewise, the incidence of cGvHD was comparable between groups, indicating that cGvHD was not associated with autoantibody production in our cohort. Although not statistically significant due to the low sample sizes, NIH-defined classic cGvHD was overrepresented in the autoantibody-positive group vs. the autoantibody-negative group (64 vs. 25%, respectively). Conversely, overlap manifestations of cGvHD at onset were more often diagnosed in the autoantibody-negative group than the autoantibody-positive group (75 vs. 36%, respectively). No significant correlation was found between autoantibody positivity and NIH-defined overall severity of cGvHD; however, all 4 patients (100%) in the autoantibody-negative group with cGvHD had severe cGvHD in comparison to 9 out of the 14 (64%) patients in the autoantibody-positive group with cGvHD. With regard to the organ involvement of cGvHD, no association with autoantibody expression was observed.
There were no significant differences in mortality between the autoantibody-positive group (8%, 4/47) and the autoantibody-negative group (15%, 4/47; p = 0.44). Neither the expression of autoantibodies nor the occurrence of cGvHD was correlated with significantly worse survival (Figures 1A,B). The relapse rate of ALL did not differ significantly between the two groups, being 8% (4/48) for the autoantibody-positive group and 23% (6/26) for the autoantibody-negative group (Table 2).
At least one type of autoantibody was detected in 65% (48/74) of patients and multiple autoantibodies were detected in 36% (27/74) of patients (Table 3). Antinuclear antibodies were the most frequently detected antibody type, occurring in 75% (36/48) of autoantibody-positive patients. Of patients with autoantibodies, 33% (16/48) had a history of cGvHD (including 14 cases of classic chronic and 2 cases of late aGvHD, as shown in Table 2). Of patients with autoantibodies and cGvHD, the most common antibody type was antinuclear antibody (88%, 14/16), followed by anti-rheumatoid factor antibody (38%) and anti-collagen antibody (25%). There was a trend towards patients with cGvHD being more likely to express antinuclear antibody than patients without cGvHD, although this was not statistically significant [67 (14/21) vs. 41% (22/53), respectively; p = 0.07]. Due to the small number of patients, no statistical analysis of the correlation between specific autoantibodies and the organ involvement of cGvHD during long-term follow-up was possible (descriptive data are shown in Table 4).
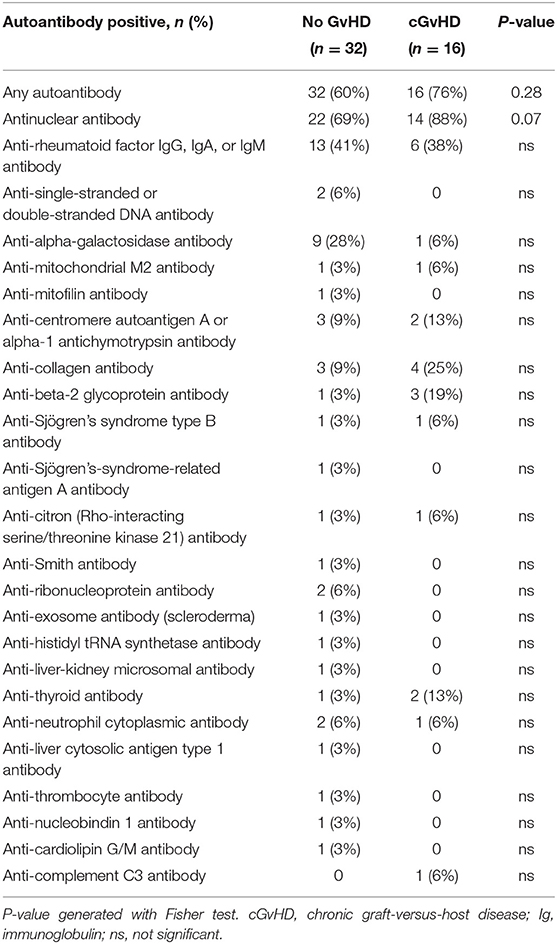
Table 3. Prevalence of autoantibodies during long-term follow-up in the patient group without (no) cGvHD and with cGvHD.
To assess immunological disparities between autoantibody-positive and negative groups, we compared the humoral and cellular parameters of 440 blood samples collected during long-term follow-up at consecutive time points alongside concurrent clinical data. In autoantibody-positive patients we found significantly increased mean numbers of leukocytes (6,410 vs. 5,815 × 103 cells/mL, respectively; p = 0.039), granulocytes (3,736 vs. 3,341 × 103 cells/mL, respectively; p = 0.034), lymphocytes (2,160 vs. 1,910 × 103 cells/mL, respectively; p = 0.024), and monocytes (504.2 vs. 437.7 × 103 cells/mL, respectively; p = 0.005), as shown in Table 5. Furthermore, the prevalence of autoantibodies was associated with significantly higher numbers of T cells and B cells including CD3+ T cells (1,508 vs. 1,276 × 103, respectively; p = 0.023), CD8+ T cells (720.8 vs. 616.6 × 103, respectively; p = 0.05), and CD19+ B cells (507.2 vs. 335.6 × 103, respectively; p = 0.006). Similarly, significantly higher mean immunoglobulin concentrations–such as IgG (1,080 vs. 896.1 mg/dL, respectively; p < 0.001), IgG3 (77.3 vs. 63.9 mg/dL, respectively; p = 0.021), IgG4 (43.8 vs. 36.7 mg/dL, respectively; p = 0.05), and IgM (114.9 vs. 86.5 mg/dL, respectively; p = 0.009) were observed in autoantibody-positive vs. autoantibody-negative patients.
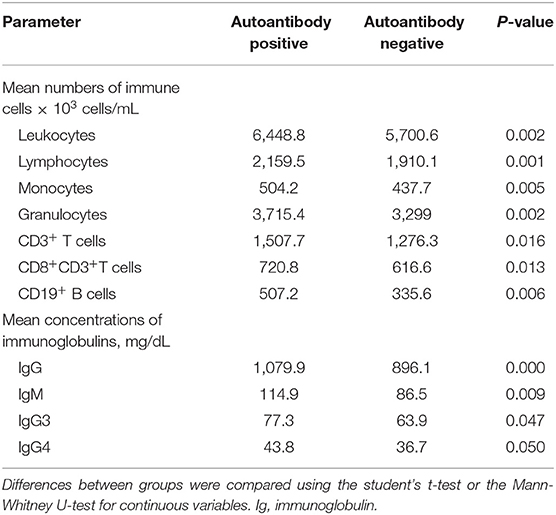
Table 5. Longitudinal assessment of humoral and cellular parameters of patients with autoantibodies vs. those without autoantibodies.
In samples positive for autoantibodies, mean numbers of leukocytes (6.534 vs. 4.852 × 103 cells/mL, p < 0.001), granulocytes (3.739 vs. 3.010 × 103 cells/mL, respectively; p = 0.018), lymphocytes (2.225 vs. 1.422 × 103 cells/mL, respectively; p = 0.024), and monocytes (504.1 vs. 363.0 × 103 cells/mL, respectively; p = 0.001) were higher for patients with cGvHD vs. those without cGvHD (Table 6). The significantly higher numbers of immune cells in cGvHD patients with autoantibodies involved CD4+ T cells (mean 632.6 vs. 384.0 × 103 cells/mL, p < 0.0001), CD8+ T cells (759.3 vs. 447.2 × 103 cells/mL, respectively; p < 0.001), CD19+ B cells (539.6 vs. 276.3 × 103 cells/mL, p = 0.003) and CD56+ NK cells (236.12 vs. 165.6 × 103 cells/mL, p = 0.023). Moreover, autoantibody production in cGvHD patients was associated with significantly higher mean numbers of CD21low B cells (23.8 vs. 11.8 × 103 cells/mL, p = 0.044), and a distorted ratio of CD21low B cells/CD27+ memory B cells (1.7 vs. 0.4 respectively; p = 0.006). Elevated levels of immunoglobulins such as IgG (1,159 vs. 916.9 mg/dL, respectively, p = 0.009), IgG3 (77.4 vs. 45.8 mg/dL, respectively; p < 0.001), and IgM (129.8 vs. 88.0 mg/dL, respectively; p = 0.038). Multivariate logistical regression analysis showed that increase of CD8+ T cells (p = 0.03), CD56+ NK cells (p = 0.04), and IgG3 (p = 0.043) was significantly associated with antibody production in cGvHD patients.
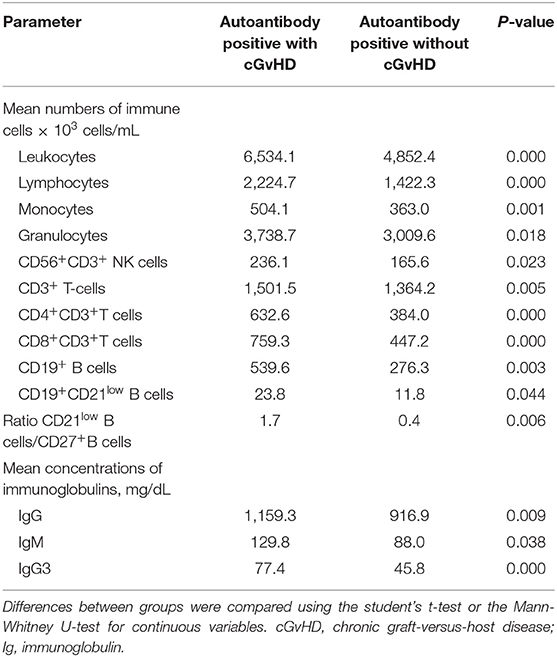
Table 6. Longitudinal assessment of humoral and cellular parameters of patients with autoantibodies both with and without cGvHD.
It has been previously demonstrated that autoantibody production may correlate with activity of cGvHD. Therefore, we assessed the correlation between autoantibody expression in patients with precisely assessed active cGvHD at the time samples were taken. Results mirrored the above-reported significant association between immunological parameters and cGvHD (data not shown). In addition, a significantly diminished proportion of CD27+ memory B cells (9.1 vs. 14.9%, respectively; p = 0.015) and an aberrantly low ratio of class-switched CD27+IgD−/CD27+ memory B cells (3.5 vs. 5.1, respectively; p = 0.013) were observed in patients with active cGvHD expressing autoantibodies, suggestive of B-cell perturbation (Table 7).
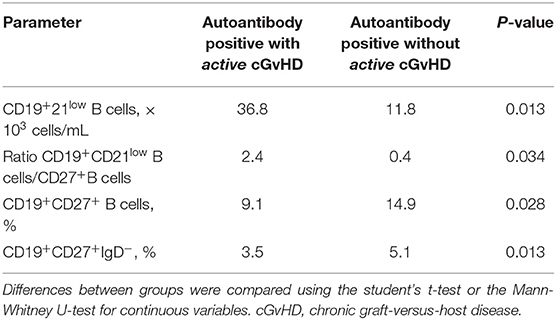
Table 7. Assessment of humoral and cellular parameters at the time of analysis in patients with autoantibodies both with and without active cGvHD.
To assess the potential association between autoantibody production and the number of CD21low B cells, which are a well-known marker of B-cell perturbation in cGvHD, we analysed the distribution of CD21low B cells using a cutoff >7% based on the publication by Wehr et al. (24). In 3 assessments the proportion of CD21low B cell was >7%. About 19 of 93 assessments were derived from the autoantibody-positive group and 74/93 assessments were derived from the autoantibody-negative group. When considering the activity of cGvHD at the time the samples were taken, 9 of the 19 CD21low B-cell samples that were derived from autoantibody-positive patients were from patients with active cGvHD (47%), while 61 of 74 CD21low B-cell samples that were derived from autoantibody-negative patients were from patients with active cGvHD (82%) (p = 0.0053).
To determine whether cGvHD is the main reason for the significant impairment of immune homeostasis regardless of the autoantibody expression, we compared cellular and humoral parameters in patients with cGvHD at any study time point to a homogenous cohort of patients without cGvHD, as shown in Table 8. This analysis was independent of autoantibody expression. cGvHD was significantly associated with increased mean numbers of leukocytes (6.289 vs. 4.878 × 103 cells/mL, p < 0.0001), granulocytes (3.603 vs. 2.922 × 103 cells/mL, respectively; p < 0.001), lymphocytes (2.154 vs. 1.476 × 103 cells/mL, respectively; p < 0.0001), and monocytes (489.9 vs. 365.4 × 103 cells/mL, respectively; p < 0.0001). cGvHD was also associated with significantly higher numbers of CD3+ T cells (1.445 vs. 1.086 × 103 cells/mL, respectively; p = 0.008), CD4+ T cells (619.7 vs. 476.1 × 103 cells/mL, respectively; p < 0.0001), CD8+ T cells (717.9 vs. 446.6 × 103 cells/mL, respectively; p < 0.001), and CD56+ NK cells (262.3 vs. 166.1 × 103 cells/mL, respectively; p < 0.001) as well as a diminished CD4/CD8 ratio (1.1 vs. 1.2, p = 0.017). In cGvHD vs. no cGvHD patients, the B-cell compartment showed significantly increased CD19+ B cells (430.9 vs. 295.5 × 103 cells/mL, respectively; p < 0.0001) and CD21low B cells (9.7 vs. 6.6%; p = 0.019), together with elevated immunoglobulin levels (IgG: 993.0 vs. 895.3 mg/dL, p = 0.022; IgG1: 687.7 vs. 580.7 mg/dL; p = 0.014; IgG3: 75.0 vs. 51.8 mg/dL; p < 0.0001, respectively).
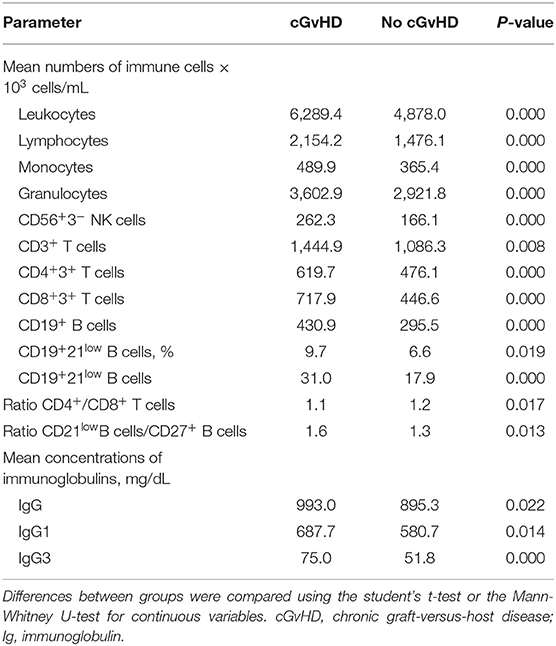
Table 8. Longitudinal assessment of humoral and cellular parameters of patients with and without (no) cGvHD independently of autoantibody expression.
When comparing survivors vs. non-survivors (Table 9) survivors showed significantly higher mean numbers of CD4+ T cells (595.1 vs. 440.0 × 103 cells/mL, respectively; p = 0.04), with a normalised CD4/CD8 ratio (1.1 vs. 0.8 × 103 cells/mL, respectively; p = 0.041). Additionally, in survivors the B-cell compartment revealed a tendency towards normalisation regarding CD19+ B cells (406.1 vs. 289.4 × 103 cells/mL respectively; p = 0.042), CD27+ B memory cells (54.9 vs. 30.6 × 103 cells/mL, respectively; p = 0.05), mainly class-switched CD27+IgD− B cells (21.2 vs. 5.0 × 103 cells/mL, respectively; p < 0.0001), the ratio of CD27+IgD+/CD27+IgD− B cells (2.2 vs. 5.9, p = 0.003), and IgG4 (40.9 vs. 19.4 mg/dL, respectively; p < 0.0001). In contrast, non-survival was associated with a significantly elevation of the proportion of CD21low B cells (13.4 vs. 8.8%, p = 0.039), and CD56+ NK cells (238.8 vs. 314.1, × 103 cells/mL, respectively; p = 0.019). In multivariate analysis, greater survival was significantly associated with lower mean numbers of CD56+ NK cells [hazard ratio (HR) 0.98, p = 0.041] and higher mean numbers of CD27+ memory B cells (HR 1.62, p = 0.014).
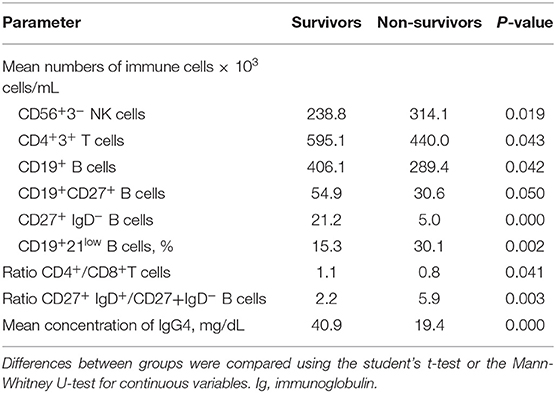
Table 9. Longitudinal assessment of humoral and cellular parameters in survivors vs. non-survivors independently of autoantibody expression.
Dysregulated immunity in cGvHD might be comparable to autoimmune diseases, where the pathogenic role of autoantibodies has been similarly shown in adult (12, 14, 15) and paediatric HSCT patients (25). This study aimed to determine the prevalence and potential value of autoantibodies as cGvHD biomarkers in the context of immune reconstitution in paediatric ALL patients after HSCT.
In this homogenous study cohort, we detected autoantibodies in 65% of patients, higher than in recently published adult cohorts (18, 21). When comparing demographic data and transplant characteristics of patients with and without autoantibody expression there were no significant differences. Although the prevalence of autoantibodies was greater in patients with cGvHD than in those without cGvHD (76 vs. 60%, respectively), this difference was not significant. This may be due to sample size. Furthermore, we did not find a significant association between the prevalence of autoantibodies and the overall severity of cGvHD, which is consistent with previous findings in adults (15, 18). Of note, in this cohort all 4 autoantibody-negative patients who had cGvHD suffered from severe cGvHD.
Consistent with previous studies (15, 18, 20), antinuclear antibodies were the most common autoantibody type detected in our patients. While cGvHD patients had a higher frequency of antinuclear autoantibodies than did patients without cGvHD (88 vs. 79%, respectively; p = 0.07), this difference did not reach statistical significance. This is in contrast to findings by Patriarca et al. (20) and Yang et al. (21) in adult HSCT patients and might possibly be explained by the lower incidence of cGvHD in paediatric patients. Further analyses regarding organ involvement of cGvHD and autoantibody profiles in our cohort were hampered by the low case numbers. In contrast to adult studies (18, 21), autoantibody profiles linked to systemic lupus erythematosus (SLE) and systemic sclerosis (such as anti-single-stranded or double-stranded DNA, anti-SSB and anti-SSA antibodies) did not correlated with cGVHD in our study. Similarly to published data (15), autoantibody positivity and profiles did not correlate with the severity, activity, or clinical characteristics of cGvHD (data not shown), indicating that autoantibodies are not suitable biomarkers for monitoring cGvHD. Besides, a correlation between explicit autoantibody profiles and specific tissue damage in cGVHD remains to be established.
In a study of 121 adolescent and adult HSCTs (mean age 35 years, range 15–68) for malignant diseases, Moon et al. showed favourable outcome regarding relapse rate and survival in patients with autoantibody positivity (19). Our data showed acceptable relapse and excellent OS with no association between presence of autoantibodies and relapse. Furthermore, neither the expression of autoantibodies nor the occurrence of cGvHD was associated with significantly worse survival (Figures 1A,B). Patients with autoantibodies showed significantly better immune reconstitution, with overall higher numbers of T cells and B cells and higher serum immunoglobulin concentrations, similarly to those reported by Patriarca et al. (20).
Notably, the prevalence of autoantibodies in patients with cGvHD correlated with better immune reconstitution and elevated numbers of NK cells. The association between elevated numbers of NK cells and cGvHD has been described by our group previously in an observational paediatric study in 146 HSCT patients (mean age 8.6 years, range 0.4–19.3 years) with 659 samples during longitudinal follow-up (17). Huenecke et al. reported a similar association between NK-cell reconstitution and cGvHD in a paediatric single centre study of 74 HSCT patients with malignant diseases (26). Among the cGvHD patients in our present study, autoantibody positivity (vs. negativity) was associated with signs of B-cell perturbation, such as significantly higher mean numbers of CD21low B cells (23.8 vs. 11.8 × 103 cells/mL, p = 0.044) and a distorted ratio of CD21low /CD27+ memory B cells (1.7 vs. 0.4, p = 0.006). When considering the activity of cGvHD in the autoantibody-positive group, we could not only confirm aberrant B-cell homeostasis but also further strengthen these findings by observing a significantly lower proportion of CD27+ memory B cells (9.1 vs. 14.9%, p = 0.015) with an altered ratio of class-switched CD27+IgD−/CD27+ memory B cells (3.5 vs. 5.13, p = 0.013) in comparison to the autoantibody-positive group without cGvHD. This is consistent with previous studies and reviews that have described disfunctional B-cell homeostasis in patients with cGvHD and the proposed a role of CD21low B cells (9, 17, 27–29). To explore this further, we assessed the potential role of autoantibody expression and CD21low B cells in cGvHD activity and, to our surprise, we found a significant correlation between active cGvHD and expanded numbers of CD21low B cells (cutoff >7%) in autoantibody-negative vs. autoantibody-positive patients (82 vs. 47%, respectively; p = 0.0053). As reported by Hao et al. from a study in 65 adult HSCT patients, these findings may further suggest a minor role of autoantibody profiles in paediatric patients with active cGvHD and signs of B-cell perturbation.
Because our results might suggest that cGvHD is the main reason for a significant impairment of immune homeostasis, we evaluated immune parameters comparing the group with and without cGvHD independently of autoantibody status. We confirmed published adult data (9, 15) that cGvHD is associated with significantly elevated T cell subsets, especially CD4+ T cells, and NK cells. We also showed that paediatric cGVHD, similar to adult cGVHD (9, 15) is associated with significantly increased CD21low B cells and elevated immunoglobulin levels as signs of B-cell perturbation. Previously, our group reported that cGVHD in children correlated with low numbers of CD27+ memory B cells in a prospective study, and we hypothesised that this subpopulation may serve as a risk factor/marker of cGvHD (17). In this here presented retrospective study we could not confirm this, emphasising on the need for further prospective multicentre studies on B-cell subpopulations in paediatric HSCT for malignant diseases in correlation with cGvHD.
The expression of circulating autoantibodies as a prognostic marker of survival or relapse has been investigated, which we could not confirm as described above. Therefore, we investigated the immune parameters of survivors vs. non-survivors. Among survivors, data revealed normalisation within the B-cell compartment with significantly higher numbers of both CD27+ memory B cells and class-switched CD27+IgD− B cells together with a mean higher concentration of IgG4 in comparison to non-survivors. Overall mortality was significantly associated with an elevated proportion of CD21low B cells and of CD56+ NK cells. In the multivariate analysis, better survival rates were significantly associated with lower numbers of CD56+ NK cells (HR 0.98, p = 0.041) and higher mean numbers of CD27+ memory B cells (HR 1.62, p = 0.014). To our knowledge these associations have not been described previously in paediatric ALL patients after HSCT.
Other strengths of the study are the homogenous cohort (paediatric ALL patients with similar HSCT characteristics) with thorough characterisation of NIH-defined cGvHD manifestations during lon-term follow-up. The assessment of cGvHD activity at the time of sample taking will allow a better understanding of the disease course of paediatric cGVHD, and may help the clinician in the future to calibrate the intensity of the immunosuppressive treatment.
Our study has several potential limitations. First, it was a retrospective study with low numbers of paediatric cGvHD patients. Secondly, details and intensity of immunosuppressive treatments that patients received were not evaluated. Data on the presence of autoantibodies both of patients and donors prior HSCT would have been informative.
In conclusion, we confirm that autoantibody profiles are not a suitable biomarker for diagnosis of paediatric cGVHD and for prediction of severe forms of this disease. However, this study identified a number of indicators of aberrant immune homeostasis associated with active cGvHD in paediatric patients with ALL who underwent HSCT, confirming results of adult studies and in line with our previous results. These indicators may serve as a surrogate measure for a better characterisation of clinical phenotypes of cGvHD. Our findings provide candidate B-cell subpopulations that could be potential targets of cGvHD treatment in paediatric HSCT for underlying malignant diseases and should be evaluated in larger, multicentre studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical University of Vienna St. Anna Children's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AL designed the study. LR, MR, NZ, and ZK analysed the data. AL, DB, LR, and ZK wrote the manuscript. WP and RG performed flow cytometric analyses. WP critically revised the manuscript. All authors approved the final version of the manuscript to be submitted.
This study was supported in part by the Jubiläumsfonds der Österreichischen Nationalbank (the Anniversary Fund of the Austrian National Bank), number 13215.
AL has served on advisory boards and received speaker's fees for participation in scientific meetings of the companies Therakos and Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with one of the authors AL.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This paper is dedicated to the memory of Andreas Heitger.
1. Peters C, Dalle JH, Locatelli F, Poetschger U, Sedlacek P, Buechner J, et al. Total body irradiation or chemotherapy conditioning in childhood ALL: a multinational, randomized, noninferiority phase III study. J Clin Oncol. (2021) 39:295–307. doi: 10.1200/JCO.20.02529
2. Inagaki J, Moritake H, Nishikawa T, Hyakuna N, Okada M, Suenobu S, et al. Long-term morbidity and mortality in children with chronic graft-versus-host disease classified by National Institutes of Health consensus criteria after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2015) 21:1973–80. doi: 10.1016/j.bbmt.2015.07.025
3. Wolff D, Lawitschka A. Chronic graft-versus-host disease. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham: Springer (2019). p. 331–46. doi: 10.1007/978-3-030-02278-5_44
4. Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. (2010) 57:297–322. doi: 10.1016/j.pcl.2009.11.003
5. Zecca M, Prete A, Rondelli R, Lanino E, Balduzzi A, Messina C, et al. Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. (2002) 100:1192–200. doi: 10.1182/blood-2001-11-0059
6. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. (2005) 11:945–56. doi: 10.1016/j.bbmt.2005.09.004
7. Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-Versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. (2015) 21(3):389-401 e1. doi: 10.1016/j.bbmt.2014.12.001
8. Williams KM, Inamoto Y, Im A, Hamilton B, Koreth J, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2020 etiology and prevention Working Group Report. Transplant Cell Ther. (2021) 27:452–66. doi: 10.1016/j.jtct.2021.02.035
9. Greinix HT, Kuzmina Z, Weigl R, Kormoczi U, Rottal A, Wolff D, et al. CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2015) 21:250–8. doi: 10.1016/j.bbmt.2014.11.010
10. Kuzmina Z, Greinix HT, Weigl R, Kormoczi U, Rottal A, Frantal S, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. (2011) 117:2265–74. doi: 10.1182/blood-2010-07-295766
11. Jia W, Poe JC, Su H, Anand S, Matsushima GK, Rathmell JC, et al. BAFF promotes heightened BCR responsiveness and manifestations of chronic GVHD after allogeneic stem cell transplantation. Blood. (2021) 137:2544–57. doi: 10.1182/blood.2020008040
12. Kremer AN, van der Griendt JC, van der Meijden ED, Honders MW, Ayoglu B, Schwenk JM, et al. Development of a coordinated allo T cell and auto B cell response against autosomal PTK2B after allogeneic hematopoietic stem cell transplantation. Haematologica. (2014) 99:365–9. doi: 10.3324/haematol.2013.086652
13. Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. (2005) 105:2973–8. doi: 10.1182/blood-2004-09-3660
14. Kuzmina Z, Eder S, Bohm A, Pernicka E, Vormittag L, Kalhs P, et al. Significantly worse survival of patients with NIH-defined chronic graft-versus-host disease and thrombocytopenia or progressive onset type: results of a prospective study. Leukemia. (2012) 26:746–56. doi: 10.1038/leu.2011.257
15. Kuzmina Z, Gounden V, Curtis L, Avila D, Rnp TT, Baruffaldi J, et al. Clinical significance of autoantibodies in a large cohort of patients with chronic graft-versus-host disease defined by NIH criteria. Am J Hematol. (2015) 90:114–9. doi: 10.1002/ajh.23885
16. Schultz KR, Kariminia A, Ng B, Abdossamadi S, Lauener M, Nemecek ER, et al. Immune profile differences between chronic GVHD and late acute GVHD: results of the ABLE/PBMTC 1202 studies. Blood. (2020) 135:1287–98. doi: 10.1182/blood.2019003186
17. Lawitschka A, Gueclue ED, Januszko A, Kormoczi U, Rottal A, Fritsch G., et al. National Institutes of Health-defined chronic graft-vs-host disease in pediatric hematopoietic stem cell transplantation patients correlates with parameters of long-term immune reconstitution. Front Immunol. (2019) 10:1879. doi: 10.3389/fimmu.2019.01879
18. Hao B, Gao S, Sang YW, Wang L, Meng XQ, You JY. Potential value of autoantibodies as biomarkers of chronic graft-versus-host disease after allogeneic stem cell transplantation. J Zhejiang Univ Sci B. (2019) 20:849–60. doi: 10.1631/jzus.B1900205
19. Moon JH, Lee SJ, Kim JG, Chae YS, Kim SN, Kang BW, et al. Clinical significance of autoantibody expression in allogeneic stem-cell recipients. Transplantation. (2009) 88:242–50. doi: 10.1097/TP.0b013e3181ac6885
20. Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. (2006) 34:389–96. doi: 10.1016/j.exphem.2005.12.011
21. Yang K, Chen Y, Qi H, Ye Y, Fan Z, Huang F., et al. Anti-Ro52 autoantibodies are related to chronic graft-vs-host disease after allogeneic hematopoietic stem cell transplantation. Front Immunol. (2020) 11:1505. doi: 10.3389/fimmu.2020.01505
22. Jacobsohn DA. Acute graft-versus-host disease in children. Bone Marrow Transplant. (2008) 41:215–21. doi: 10.1038/sj.bmt.1705885
23. Greinix HT, Pohlreich D, Kouba M, Kormoczi U, Lohmann I, Feldmann K, et al. Elevated numbers of immature/transitional CD21- B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. (2008) 14:208–19. doi: 10.1016/j.bbmt.2007.10.009
24. Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The euro class trial: defining subgroups in common variable immunodeficiency. Blood. (2008) 111:77–85. doi: 10.1182/blood-2007-06-091744
25. Fujii H, Cuvelier G, She K, Aslanian S, Shimizu H, Kariminia A, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children's Oncology Group. Blood. (2008) 111:3276–85. doi: 10.1182/blood-2007-08-106286
26. Huenecke S, Cappel C, Esser R, Pfirrmann V, Salzmann-Manrique E, Betz S, et al. Development of three different NK cell subpopulations during immune reconstitution after pediatric allogeneic hematopoietic stem cell transplantation: prognostic markers in GvHD and viral infections. Front Immunol. (2017) 8:109. doi: 10.3389/fimmu.2017.00109
27. Rozmus J, Kariminia A, Abdossamadi S, Storer BE, Martin PJ, Lee SJ, et al. Comprehensive B cell phenotyping profile for chronic graft-versus-host disease diagnosis. Biol Blood Marrow Transplant. (2019) 25:451–8. doi: 10.1016/j.bbmt.2018.11.007
28. Sarantopoulos S, Stevenson KE, Kim HT, Cutler CS, Bhuiya NS, Schowalter M, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. (2009) 113:3865–74. doi: 10.1182/blood-2008-09-177840
Keywords: paediatric, haematopoietic stem cell transplantation, acute lymphoblastic leukaemia, graft-versus-host disease, autoantibody, B cells, immune reconstitution
Citation: Lawitschka A, Ronceray L, Bauer D, Rittenschober M, Zubarovskaya N, Geyeregger R, Pickl WF and Kuzmina Z (2021) Value of Autoantibody Expression During Long-Term Follow-Up in Paediatric ALL Patients After Allogeneic Haematopoietic Stem Cell Transplantation. Front. Pediatr. 9:788360. doi: 10.3389/fped.2021.788360
Received: 02 October 2021; Accepted: 24 November 2021;
Published: 21 December 2021.
Edited by:
Peter Bader, University Hospital Frankfurt, GermanyReviewed by:
Troy Quigg, Helen DeVos Children's Hospital, United StatesCopyright © 2021 Lawitschka, Ronceray, Bauer, Rittenschober, Zubarovskaya, Geyeregger, Pickl and Kuzmina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zoya Kuzmina, em95YS5rdXptaW5hQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.