
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Pediatr. , 20 December 2021
Sec. Pediatric Rheumatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.783745
This article is part of the Research Topic COVID-19 and Hyper Inflammation Syndrome: Different Presentation and Management View all 13 articles
 Giacomo Brisca1*
Giacomo Brisca1* Alessandro Consolaro2,3
Alessandro Consolaro2,3 Roberta Caorsi2
Roberta Caorsi2 Daniela Pirlo1
Daniela Pirlo1 Giulia Tuo4
Giulia Tuo4 Claudia Campanello3
Claudia Campanello3 Elio Castagnola5
Elio Castagnola5 Andrea Moscatelli1
Andrea Moscatelli1 Marco Gattorno2,3
Marco Gattorno2,3 Angelo Ravelli3,6
Angelo Ravelli3,6In this observational study, we report the clinical, therapeutics and outcome features of 23 patients with multisystem inflammatory syndrome (MIS-C) who have been treated in Gaslini Children Hospital (Genoa, Italy) with a multistep antinflammatory treatment protocol, based on disease severity at admission. Patients were initially assigned to four severity classes on admission and treated accordingly. The therapeutic options ranged from IV immunoglobulin alone to a combination of IVIG plus pulses of methylprednisolone plus anakinra for patients with marked cardiac function impairment or signs of macrophage activation syndrome, with rapid treatment escalation in case of inadequate therapeutic response. With the application of this therapeutic strategy, no patient required admission to Intensive Care Unit (ICU) or invasive mechanical ventilation, and no inotropic drugs administration was required. Early aggressive treatment of MIS-C, with therapeutic interventions modulated based on the severity of clinical manifestations may help to prevent the progression of the inflammatory process and to avoid the need of admission to the ICU. A timely intervention with anti-IL-1 blockers can play a pivotal role in very severe patients that are at risk to have an incomplete response to immunoglobulins and steroids.
In the spring of 2020, a multisystem inflammatory syndrome in children (MIS-C) emerged in countries mostly hit by COVID-19 (1–4). Strong viral and epidemiological evidence suggested that SARS-CoV-2 was the trigger of the syndrome. However, the observation of a time lag of 2–6 weeks between the peak of SARS-CoV-2 infection and the onset of MIS-C indicated that the virus acted as a trigger of a post-infectious inflammatory process. (5).
All patients with MIS-C present with fever, and a sizeable proportion of them display some of the typical manifestations of Kawasaki disease (KD), especially rash, cheilitis, and bilateral non-secretive conjunctival injection. However, most cases develop symptoms that are uncommonly seen in KD, such as gastrointestinal complaints (abdominal pain, vomiting and diarrhea), myocarditis associated with cardiogenic-vasoplegic shock, and neurological abnormalities (meningitis-like symptoms or encephalitis) (6). Impaired cardiac function and shock necessitate admission to the Intensive Care Unit (ICU) for 60–80% of patients, and half of them require inotropes and/or fluid resuscitation (5, 7). The fatality rate is approximately 2% (8, 9).
Most patients with MIS-C have been treated with intravenous immunoglobulin (IVIG), alone or in combination with glucocorticoids (10–12). In severe or refractory cases, particularly when myocarditis was present, biologic response modifiers, particularly tumor necrosis factor or interleukin-1 inhibitors, were given (13, 14). However, despite the publication of various therapeutic recommendations (15–17) the treatment of MIS-C remains empirical and little information is available about the effectiveness of proposed strategies.
We describe herein our experience with a therapeutic protocol based on an early aggressive multi-step therapy, which enabled quick control of the inflammatory process and avoided admission to the ICU of all patients with MIS-C seen at our hospital.
All consecutive patients seen at the Gaslini Institute of Genoa, Italy from 1st April 2020 to 1st June 2021 who met the case definition of MIS-C (18, 19) were included in the study.
This study was reviewed and approved by the Regione Liguria Ethical Board (IRB# 370/2020). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
All children were treated according to an internal multistep treatment protocol originally devised for KD by expert pediatric rheumatologists (AC, RC, MG, AR) and infectious disease specialists (EC).
Patients were initially assigned to four severity classes developed in our Institute on the basis of their clinical features on admission, the severity of cardiac involvement expressed by echocardiographic findings or cardiac enzymes levels and/or the occurrence of blood tests abnormalities suggestive for macrophage activation syndrome (Table 1). The severity classes' descriptors were progressively adapted to the evolving scenario and the growing body of evidence on the new clinical entity.
The steps of the protocol, which is shown in Figure 1, were applied as follows. (1) MIS-C patients presenting the American Heart Association (AHA) criteria for complete or incomplete KD (20) with normal left ventricular (LV) ejection fraction (EF>55%) and absence of major abdominal complaints or signs of impending macrophage activation syndrome (MAS) (class I) were given IVIG alone at the standard dosage of 2 gr/kg, as recommended for KD. (2) MIS-C patients with one or more ipokinetic or diskinetic LV segments at the initial echocardiographic evaluation but in the context of a preserved LV global systolic function (EF>50%), and in the absence of major abdominal complaints or signs of impending macrophage activation syndrome (MAS) (class II) were given IVIG plus glucocorticoids upfront by administering intravenous methylprednisolone at 2–3 mg/kg/day in two to four daily doses. (3) MIS-C patients with signs of LV global systolic dysfunction (i.e. EF between 50 and 35%, and/or increased cardiac troponin T level or N-terminal B-type natriuretic peptide level > 1,000/pg/ml) (21), significant abdominal involvement, or impending MAS (class III) were given IVIG plus glucocorticoids as pulses of 10–30 mg/kg/day (maximum 1 gr/day) for 1–5 days (4) MIS-C patients with severe LV global systolic dysfunction (LV EF lower than 35%) and/or arrhythmias (i.e advanced atrioventricular block), hypotension or overt MAS (class IV) were simultaneously started with anakinra at 5–10 mg/kg/day, administered subcutaneously or, in the sickest patients, intravenously.
In patients who did not improve within 24–48 h with the initially selected therapeutic intervention, treatment was escalated throughout the steps outlined above.
For patients with severely depressed cardiac function, we closely monitored for fluid overload, considering adjunctive use of diuretics with IVIG administration possibly given in divided doses (1 g/kg daily over 2 days).
According to our Institute policy, class I patients were managed in the general ward or in the Rheumatology Unit. Patients assigned to class II-IV or patients in class I with worsening of clinical conditions, were admitted to high-dependency unit until stabilization and then transferred to cardiology unit or rheumatology clinics depending on the characteristics of the cardiac involvement (Figure 2).
From 1st April 2020 to 1st June 2021 23 patients (11 females, 48, 2%) with MIS-C were treated according to the multistep therapeutic protocol illustrated above. The median age at fever onset was 5, 8 (range 2, 4–12, 3) years and the median time between the onset of fever and hospital admission was three (range 2–4) days. No patients had comorbidities.
In patients with confirmed SARS-CoV-2 infection (i.e. patients who had nasopharyngeal swab positive for SARS-CoV-2), the median time between the infection and MIS-C onset was 4 weeks.
The main clinical and laboratory features of the 23 patients at the time of admission are reported in Table 2. Five and 7 patients met the AHA criteria for complete or incomplete KD, respectively.
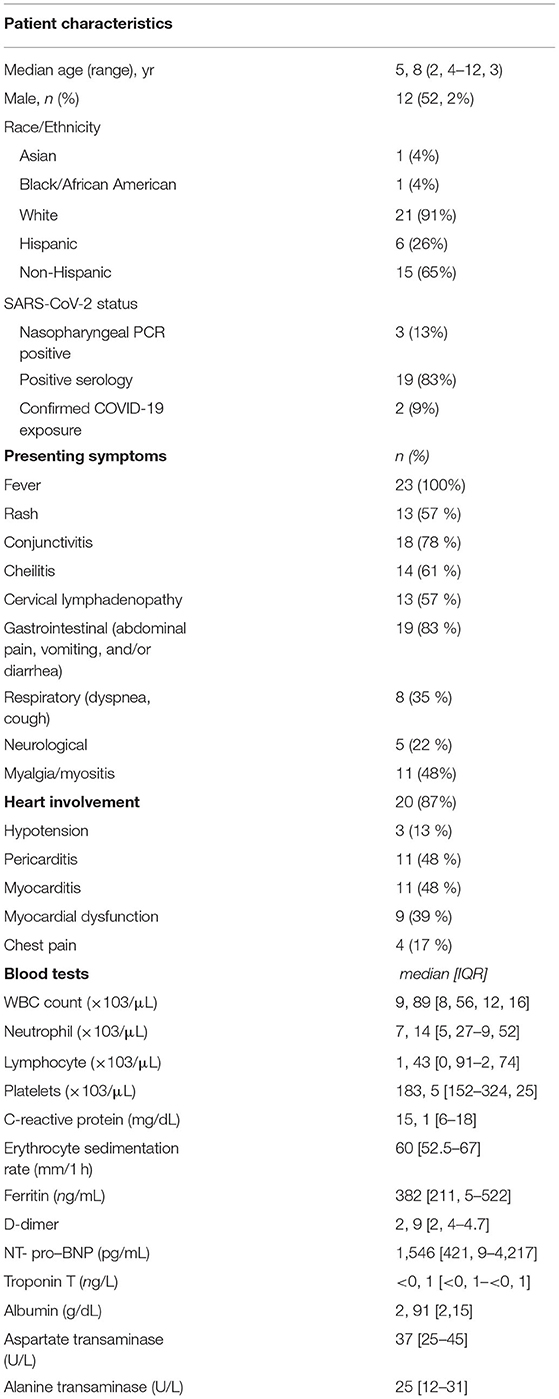
Table 2. Main clinical features and laboratory studies at presentation of children treated for MIS-C.
With the application of our severity assessment tool, six patients were assigned to class I, 9 to class II, 5 to class 3, and 3 to class IV.
According to this, in the first 48 h after admission, six patients were given IVIG alone, 14 IVIG plus intravenous methylprednisolone (2–3 mg/kg/day in 9 patients and pulses of 30 mg/kg/day in five patients) and three IVIG, methylprednisolone and anakinra.
In 18 (78%) patients, the treatment allocated by the severity score was able to prevent disease progression and to achieve a rapid control of fever, inflammatory markers (Figure 3) and cardiac involvement.
In 5 (22%) patients a subsequent therapeutic escalation was required, due to persistence of fever or worsening of cardiac function. Two patients received methylprednisolone (3 mg/kg/day) after IVIG monotherapy and 3 patients required second-line treatment with anakinra (2–3 mg/kg twice a day IV) for lack of improvement after 3 to 6 days.
In these five patients a full normalization of inflammation markers and cardiac function was rapidly obtained.
Altogether, 23 patients were treated with IVIG, 19 with glucocorticoids, and 6 with anakinra. Patients in severity class I received antiplatelet prophylaxis with acetylsalicylic acid; all remaining patients received low molecular weight heparin prophylaxis.
No patient required admission to the ICU or invasive mechanical ventilation, extracorporeal circulatory and respiratory support and no patients needed administration of inotropic drugs.
The median time of normalization of C reactive protein levels was 9.5 (range 6–16) days (Figure 3).
Five patients were found to have prolonged QTc interval on EKG during hospitalization. Two of them were temporarily treated with beta blockers therapy. Arrhythmia resolved in all patients.
Eleven patients (48%) had coronary involvement: six patients (26%) were found with coronary artery dilations, four patients (17%) with small coronary artery aneurysm and one patient (4%) with medium coronary artery aneurysm.
All cardiac manifestations normalized prior to hospital discharge without developing any sequelae in all but one patient who showed multiple coronary artery aneurysms at last echocardiogram.
All patients were discharged after a median of 20 (range 14, 5–26, 5) days after admission.
The safety of the treatment was good, with only three patients experiencing transient hypertension related to glucocorticoid treatment. No patient had infections related to the immunosuppressive treatment.
We have described our experience with the first 23 patients with MIS-C treated in our Hospital with an early multi-step anti-inflammatory treatment protocol, based on therapeutic interventions tailored based on the severity of the initial clinical manifestations, and rapidly escalated in case of insufficient improvement within the first 24–48 h. Moreover, an integrated multi-specialist organization centered on the presence of a pediatric high-dependency unit closely coordinated with pediatric rheumatologists and cardiologists allowed a similar logistic multi-step approach according to the different disease severity.
With the application of this therapeutic strategy, none of our patients required ICU admission and the outcome was uneventful in all but one patient, a 1-year-old girl assigned to class I on admission, who developed multiple coronary artery aneurysms 2 weeks after receiving treatment with IGIV. The treatment was overall well tolerated, with the exception of the transient occurrence of glucocorticoid-related hypertension in three patients. Although our findings are limited by the small size of our series, they compare favorably with most literature data, which report a significant frequency of ICU admission, need for vasoactive medications and mechanical ventilation, ranging from 60 to 80%, 12 to 47% and 5 to 49% respectively (5, 6, 8, 9, 21, 22).
Of note, in most of our patients, hospital admission and initiation of anti-inflammatory treatment occurred soon after the beginning of symptoms.
Although the lack of fine details about timing of treatment in published case series does not allow us to make comparisons, this observation supports that early identification and prompt initiation of anti-inflammatory therapy may be key factors to obtain a favorable course of the disease and to prevent use of inotropic drugs, mechanical ventilation or ICU admission.
Most of the largest studies available in the literature are multi-centric. It is therefore likely that, beside the different therapeutic strategies, also the different functional organization of each center could have dramatically influenced the results on disease progression and final outcome. In our hospital patients with signs of cardiac involvement or need for close clinical monitoring, were admitted to high-dependency unit until clinical stability was achieved and then transferred to rheumatology or cardiology unit depending on the characteristics of cardiac involvement (Figure 2).
This likely contributed to avoid ICU admissions resulting in significant cost and resource savings and less disruption for patients and their families.
Moreover, since the initial reports of the inflammatory complications related to SARS-COV2 infection, in our hospital, the immuno-rheumatologists raised an internal alert for severe COVID-19 pneumonia (23–25) and, subsequently, for MIS-C (16). Multi-specialist educational meetings with the aim of raising awareness of the disease among emergency and family pediatricians, likely leading to earlier recognition of our cases, were also organized.
On the basis of our experience, considering the wide variability of clinical signs and symptoms at presentation of MIS-C, and the need to induce rapid immunomodulation, we propose a clinical severity stratification tool that allows the clinician to identify the most appropriate anti-inflammatory therapeutic regimen.
So far, the most common therapeutic approach reported in large cohorts of patients with MIS-C, consists in IVIG and systemic glucocorticoids (15).
However, previous studies in adult patients with severe form of COVID-19 pneumonia and evidence of hyperinflammation, have suggested the potential efficacy and safety of the early use of high doses of intravenous anakinra (24, 25), and subsequently confirmed by an extensive metanalysis data (26) and a recent randomized placebo-control study (27).
Moreover, anakinra is becoming increasingly more popular also for the management of KD after failure of IVIG and its efficacy in KD is being scrutinized in a phase IIa trial (28).
More recently, case reports and case series of patients with MIS-C reported the efficacy of treatment with anakinra, highlighting its role particularly in those children who have insufficient response to IVIG and systemic glucocorticoids (29–31).
At the time of admission, patients who meet the case definition criteria for MIS-C are assigned to four severity classes, on the basis of their clinical features, the severity of cardiac involvement expressed by echocardiographic findings and cardiac enzymes levels and the occurrence of blood tests abnormalities suggestive for macrophage activation syndrome, and treated accordingly.
The therapeutic options ranged from IV immunoglobulin alone for patients in class 1 with no sign of cardiac dysfunction to a combination of IVIG plus pulses of methylprednisolone plus anakinra for those in class four with severe cardiac function impairment or signs of macrophage activation syndrome.
We suggest that the treatment allocated by this severity assessment tool may be able to prevent disease progression and to achieve a rapid control of fever, inflammatory markers and cardiac impairment.
Patients who do not respond to initial treatment within the first 48 h enter in the following therapeutic step and receive a more aggressive anti-inflammatory treatment with the aim of rapidly limiting the course of the illness.
Other authors have proposed similar therapeutic algorithms for the management of MIS-C. Schlapbach et al. (32) suggested a disease stratification based on the presence of shock and supported the use of anakinra in severe patients who do not respond to IVIG and methylprednisolone pulses.
Similarly Handerson et al. (15) recommended IVIG and adjunctive low-to moderate dose of glucocorticoids in patients with shock or presenting with concerning features supporting the use of anakinra only for patients with refractory disease.
Differently, we suggest that early identification of more severe patients (i.e. severity class IV of our assessment tool) and initiation of anakinra as first-line therapy may be decisive in turning off the hyperinflammation which underlies the disease and preventing the need for escalation of care.
In our experience only one patient showed residual cardiac lesion at last ultrasound evaluation before hospital discharge, highlighting the potential for medium and long-term complications. Therefore, it is essential to guarantee an adequate follow-up to these patients scheduling both Echocardiograms and EKG at regular intervals for evaluation of ventricular function and coronary artery dimensions after initial diagnosis. Moreover, in patients with a history of ventricular dysfunction, cardiac magnetic resonance imaging (MRI) may be considered 2–6 months after initial diagnosis for evaluation of ventricular function, edema, diffuse fibrosis, and scar (33).
Our findings should be interpreted in the light of some limitations, which include the retrospective nature of the analysis, the relatively low sample size, the absence of a control group and the short-term outcome.
In conclusion, our experience suggests that early aggressive treatment of MIS-C, with therapeutic interventions modulated based on the severity of clinical manifestations and rapidly escalated in case of inadequate therapeutic response may help to prevent the progression of the inflammatory process and to avoid the need of escalation of care and admission to the ICU. The optimal therapeutic approach to MIS-C should be established through multinational consensus initiatives and, whenever possible, randomized controlled trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Regione Liguria Ethical Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
GB, RC, and AC conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. DP, GT, and CC collected data, carried out the initial analyses, and reviewed and revised the manuscript. EC, MG, AM, and AR conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MIS-C, multisystem inflammatory syndrome in children; KD, Kawasaki disease; ICU, intensive care unit; IVIG, intravenous immunoglobulin; LV, left ventricular; EF, ejection fraction; MAS, macrophage activation syndrome.
1. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) 395:1607–8. doi: 10.1016/S0140-6736(20)31094-1
2. Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. (2020) 395:1771–8. doi: 10.1016/S0140-6736(20)31103-X
3. Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, et al. SARS-CoV-2-Induced Kawasaki-Like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. (2020) 146:e20201711. doi: 10.1542/peds.2020-1711
4. Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, et al. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. (2020) 142:429–36. doi: 10.1161/CIRCULATIONAHA.120.048360
5. Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. (2020) 25:2001010. doi: 10.2807/1560-7917.ES.2020.25.22.2001010
6. Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) 324:259–69. doi: 10.1001/jama.2020.10369
7. Davies P, Evans C, Kanthimathinathan HK, Lillie J, Brierley J, Waters G, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. (2020) 4:669–77. doi: 10.1016/S2352-4642(20)30215-7
8. Godfred-Cato S, Bryant B, Leung J, Oster ME, Conklin L, Abrams J, et al. California MIS-C Response Team. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. (2020) 69:1074–80. Erratum in: MMWR Morb Mortal Wkly Rep. (2020) 69:1229. doi: 10.15585/mmwr.mm6932e2
9. Hoste L, Van Paemel R, Haerynck F. Multisystem inflammatory syndrome in children related to COVID-19: a systematic review. Eur J Pediatr. (2021) 180:2019–34. doi: 10.1007/s00431-021-03993-5
10. Belhadjer Z, Auriau J, Méot M, Oualha M, Renolleau S, Houyel L, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation. (2020) 142:2282–4. doi: 10.1161/CIRCULATIONAHA.120.050147
11. Davies P, Lillie J, Prayle A, Evans C, Griffiths B, du Pré P, et al. Association between treatments and short-term biochemical improvements and clinical outcomes in post-severe acute respiratory syndrome coronavirus-2 inflammatory syndrome. Pediatr Crit Care Med. (2021) 22:e285–93. doi: 10.1097/PCC.0000000000002728
12. Ouldali N, Toubiana J, Antona D, Javouhey E, Madhi F, Lorrot M, et al. French Covid-19 paediatric inflammation consortium. association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA. (2021) 325:855–64. Erratum in: JAMA. (2021) 326:90. doi: 10.1001/jama.2021.0694
13. DeBiasi RL, Harahsheh AS, Srinivasalu H, Krishnan A, Sharron MP, Parikh K, et al. Children's National Hospital MIS-C taskforce. Multisystem inflammatory syndrome of children: subphenotypes, risk factors, biomarkers, cytokine profiles, and viral sequencing. J Pediatr. (2021) 237:125-135.e18. doi: 10.1016/j.jpeds.2021.06.002
14. Cole LD, Osborne CM, Silveira LJ, Rao S, Lockwood JM, Kunkel MJ, et al. Compared to IVIG plus infliximab in multisystem inflammatory syndrome in children. Pediatrics. (2021) 21:e2021052702. doi: 10.1542/peds.2021-052702
15. Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, et al. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 2. Arthritis Rheumatol. (2021) 73:e13–29. doi: 10.1002/art.41616
16. Cattalini M, Taddio A, Bracaglia C, Cimaz R, Paolera SD, Filocamo G, et al. Rheumatology Study Group of the Italian Society of Pediatrics. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): a diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital J Pediatr. (2021) 47:24. doi: 10.1186/s13052-021-00980-2
17. Harwood R, Allin B, Jones CE, Whittaker E, Ramnarayan P, Ramanan AV, et al. PIMS-TS National Consensus Management Study Group. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health. (2021) 5:133–41. doi: 10.1016/S2352-4642(20)30304-7
18. Centers for Disease Control and Prevention. Emergency preparedness and response: multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Health advisory. https://emergency.cdc.gov/~han/2020/han00432.asp
19. WHO/2019, nCoV/Sci_Brief/Multisystem_Syndrome_Children/2020,.1. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed July 15, 2021).
20. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on cardiovascular disease in the young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. (2017) 135:e927–99. doi: 10.1161/CIR.0000000000000484
21. Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H. Overcoming COVID-19 Investigators. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA. (2021) 325:1074–87. doi: 10.1001/jama.2021.2091
22. McArdle AJ, Vito O, Patel H, Seaby EG, Shah P, Wilson C, et al. BATS Consortium. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. (2021) 385:11–22. doi: 10.1056/NEJMoa2102968
23. Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, et al. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol. (2020) 72:1059–63. doi: 10.1002/art.41285
24. Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. (2020) 146:213–5. doi: 10.1016/j.jaci.2020.05.002
25. Pontali E, Volpi S, Signori A, Antonucci G, Castellaneta M, Buzzi D, et al. Efficacy of early anti-inflammatory treatment with high doses of intravenous anakinra with or without glucocorticoids in patients with severe COVID-19 pneumonia. J Allergy Clin Immunol. (2021) 147:1217–25. doi: 10.1016/j.jaci.2021.01.024
26. Kyriazopoulou E, Huet T, Cavalli G, Gori A, Kyprianou M, Pickkers P, et al. International Collaborative Group for Anakinra in COVID-19. Effect of anakinra on mortality in patients with COVID-19: a systematic review and patient-level meta-analysis. Lancet Rheumatol. (2021) 3:e690–7. doi: 10.1016/S2665-9913(21)00216-2
27. Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. (2021) 27:1752–60. doi: 10.1038/s41591-021-01499-z
28. Koné-Paut I, Tellier S, Belot A, Brochard K, Guitton C, Marie I, et al. Phase II open label study of anakinra in intravenous immunoglobulin-resistant Kawasaki disease. Arthritis Rheumatol. (2021) 73:151–61. doi: 10.1002/art.41481
29. Della Paolera S, Valencic E, Piscianz E, Moressa V, Tommasini A, Sagredini R, et al. Case Report: use of anakinra in multisystem inflammatory syndrome during COVID-19 pandemic. Front Pediatr. (2021) 8:624248. doi: 10.3389/fped.2020.624248
30. Dove ML, Jaggi P, Kelleman M, Abuali M, Ang JY, Ballan W, et al. Multisystem inflammatory syndrome in children: survey of protocols for Early Hospital Evaluation and Management. J Pediatr. (2021) 229:33–40. doi: 10.1016/j.jpeds.2020.10.026
31. Bhat CS, Shetty R, Ramesh D, Banu A, Ramanan AV. Anakinra in refractory multisystem inflammatory syndrome in children (MIS-C). Indian Pediatr. (2021) 58:994–6. doi: 10.1007/s13312-021-2340-1
32. Schlapbach LJ, Andre MC, Grazioli S. PIMS-TS working group of the Interest Group for Pediatric Neonatal Intensive Care (IGPNI) of the Swiss Society of Intensive Care and the Pediatric Infectious Diseases Group Switzerland (PIGS). Best Practice Recommendations for the Diagnosis and Management of Children With Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2 (PIMS-TS; Multisystem Inflammatory Syndrome in Children, MIS-C) in Switzerland. Front Pediatr. (2021) 9:667507. doi: 10.3389/fped.2021.667507
Keywords: anakinra, multi-step anti-inflammatory treatment, SARS-CoV-2, pediatric COVID-19, immunoglobulins, kawasaki disease, multisystem inflammatory syndrome in children, intensive care unit
Citation: Brisca G, Consolaro A, Caorsi R, Pirlo D, Tuo G, Campanello C, Castagnola E, Moscatelli A, Gattorno M and Ravelli A (2021) Timely Recognition and Early Multi-Step Antinflammatory Therapy May Prevent ICU Admission of Patients With MIS-C: Proposal for a Severity Score. Front. Pediatr. 9:783745. doi: 10.3389/fped.2021.783745
Received: 26 September 2021; Accepted: 30 November 2021;
Published: 20 December 2021.
Edited by:
Dimitri Poddighe, Nazarbayev University School of Medicine, KazakhstanReviewed by:
Lauren Henderson, Boston Children's Hospital and Harvard Medical School, United StatesCopyright © 2021 Brisca, Consolaro, Caorsi, Pirlo, Tuo, Campanello, Castagnola, Moscatelli, Gattorno and Ravelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacomo Brisca, Z2lhY29tb2JyaXNjYUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.