
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 27 October 2021
Sec. Pediatric Rheumatology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.760517
This article is part of the Research TopicInsights in Pediatric Rheumatology: 2021View all 13 articles
Systemic lupus erythematosus (SLE) is an autoantibody-related disease that affects multiple organs. Stercoral colitis (SC) is a rare type of inflammatory colitis with a high mortality rate. Here, we report the first case of pediatric-onset lupus in a case complicated by stercoral colitis. We also conducted a literature review of patients with SC under 30 years old to provide useful clues for rapid diagnosis at a young age. A 28-year-old female with a history of lupus and neuropsychiatric SLE was admitted with severe abdominal pain. She was found to have stercoral colitis during surgery. Two years later, the patient underwent Hartman's operation due to ischemia of the colon. In addition, 10 patients younger than 30 years old with a diagnosis of SC were analyzed based on clinical presentation, physical examination, laboratory exam, imaging and treatment. All cases had a favorable outcome without mortality. Stercoral colitis is a rare but lethal complication, emphasizing the importance of a multidisciplinary approach. Differential diagnosis should include stercoral colitis for patients with SLE developing unexplained sharp abdominal pain.
Systemic lupus erythematosus (SLE) is an autoantibody-related disease that affects multiple organs. In addition, some SLE patients have gastrointestinal presentations or complications, including 6% of those with chronic constipation (1). Such involvement may be due to lupus enteritis, lupus mesenteric vasculitis, inflammatory bowel disease or adverse reactions of drugs used to treat SLE (2). Furthermore, patients with pediatric-onset lupus, accounting for 15–20% of lupus cases, have severe presentations, with 19% having gastrointestinal manifestations (3, 4).
Stercoral colitis is a rare type of inflammatory colitis with a high mortality rate ranging from 32 to 57%; it occurs mainly in elderly individuals with a history of chronic constipation, and it presents in a moribund state (5). Chronic constipation may lead to fecaloma formation in the large bowel and cause an increase in intraluminal pressure, eventually inducing bowel wall necrosis and perforation, known as stercoral perforation. Most patients are diagnosed during emergency laparotomy or post-mortem (6).
To our knowledge, this is the first reported pediatric-onset lupus case complicated by stercoral colitis. The importance of the possibility of the occurrence of this disease in patients with SLE should be highlighted, even in young adults and especially in those with early childhood-onset disease. Moreover, due to the high mortality rate of stercoral colitis, awareness is promptly required for immediate diagnosis and treatment.
A 28-year-old female was diagnosed with pediatric-onset lupus at the age of 8, with initial presentations of fever, malar rash, arthritis, positive antinuclear antibodies (ANAs), and elevated anti-dsDNA antibodies. In addition, she had episodes of neuropsychiatric SLE (NPSLE) at the ages of 19, 22, and 24, with seizures, cranial neuropathies of the facial nerve and transverse myelitis with neurogenic bladder, respectively, and treated with one dose of 0.5 g/m2 intravenous cyclophosphamide pulse therapy. Furthermore, she experienced a brain abscess at the age of 25 years. After the above presentations, she regained clear consciousness without cognitive dysfunction or neurologic deficits. Daily medication was maintained with 5 mg prednisolone (i.e., cumulative dose of ~144 g) and 100 mg azathioprine daily, with regular follow-up at our pediatric rheumatology clinic.
Later, she presented to our emergency department with severe abdominal pain and fever for 1 day. She had a history of chronic constipation and urinary tract infection for 2 weeks before admission, and her last bowel movement was 3 days prior. On arrival, she showed tachycardia (123 beats per min) and a body temperature of 38°C but no hypertension or hypotension (121/70 mmHg). Physical examination revealed a distended abdomen, severe tenderness in the lower abdomen, positive peritoneal signs, rebounding pain and decreased bowel sounds; rectal examination was not performed. Blood laboratory tests showed elevated inflammation markers, a C-reactive protein (CRP) level of 77.35 mg/L, a white blood cell count of 7,900/μL, and normal platelet count, serum creatinine, electrolyte and liver function test results. There were no obvious symptoms of lupus attacks, such as discoid rash, oral ulcer or arthralgia. SLE activity remained stable. C3 and C4 levels were 63.4 and 19.1 mg/dL, respectively, and her anti-dsDNA antibody level was 382.0 U/mL, lower than those over the previous 6 months. Disease activity, as quantified by the SLEDAI-2K score (7), was calculated to be 6, which indicated that a lupus flare was unlikely.
The initial plain abdominal radiograph in Figure 1 shows a non-specific intestinal gas distribution without abnormal calcification or pneumoperitoneum. Ultrasound examination revealed a dilated colon, accompanied by air-fluid levels and ascites with a large amount of residual urine. A Foley catheter was inserted, but her abdominal pain did not improve. As peritonitis was suspected, she was given 1,000 g ceftriaxone and 500 mg metronidazole. However, at 6 hour after admission, the abdominal pain worsened, and repeated blood tests revealed the progression of inflammatory markers, with a CRP level of 197 mg/L. A subsequent computed tomography (CT) scan indicated a large number of fecal impactions and colon dilations, along with colon wall thickening and pneumatosis coli, as depicted in Figure 2. Laparoscopic exploration indicated poor perfusion of the upper rectum with pericolonic fibrin and turbid ascites in the pelvic cavity, but no perforation was found. Rigid sigmoidoscopy showed ischemia of the rectal mucosa. The ischemic rectum was removed, and primary anastomosis of the descending colon and rectum was performed, with a diverting loop transverse colostomy over the right quadrant of the abdomen (Figure 3). The pathology illustrated in Figure 3 showed focal ischemic changes, severe mucosal damage and focal transmural necrosis of the large intestine, which were evidence for diagnosis. All cultures were negative, and the patient was discharged at 46 days after surgery. However, 2 years later, the patient experienced abdominal pain and shock, and CT revealed inferior mesenteric artery occlusion and ischemia of the descending sigmoid colon. During the second operation, we observed ischemic colitis with gangrene changes, and the Hartmann procedure was performed. The patient recovered smoothly under our outpatient follow-up.
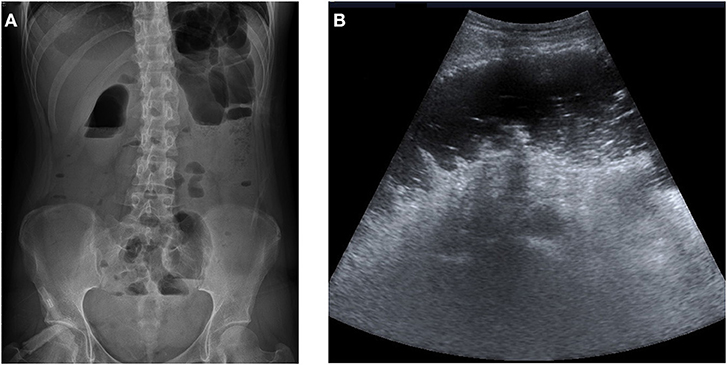
Figure 1. (A) The initial abdominal plain film showed non-specific bowel gas distribution without abnormal calcification or free air. (B) Ultrasonography of the abdomen revealed colon dilatation with air-fluid levels and ascites.
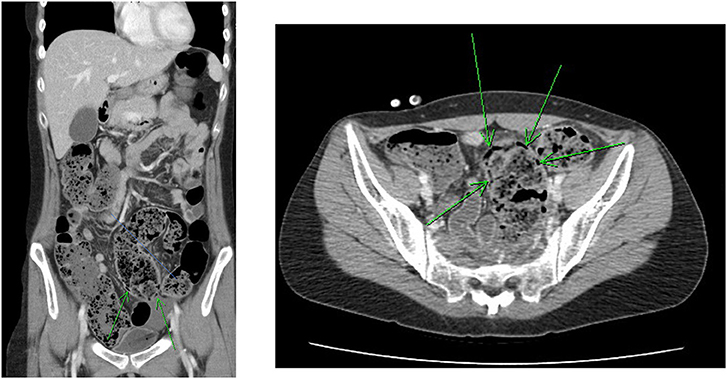
Figure 2. Abdominal CT revealed much fecal impaction and colon dilatation along with colon wall thickening and pneumatosis intestinalis (arrow).
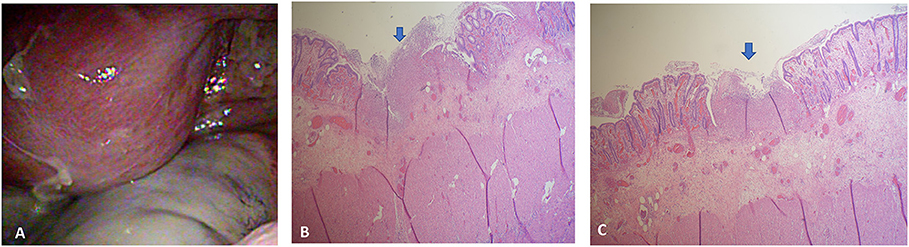
Figure 3. (A) Exploratory laparoscopy revealed poor perfusion of the upper rectum with pericolonic fibrin and turbid ascites in the pelvic cavity. (B) The section showed ischemic change, intensive mucosal injury, and focal transmural necrosis of the large intestine (arrow). (C) The specimen showed focal ischemic change and transmural necrosis of the large intestine.
Medical records, including clinical records, physical examination results, laboratory results and imaging series, were reviewed by Lin LL and Gau CC of Taiwan's tertiary center. This study was approved by the Ethics Committee on Human Studies at Chang Gung Memorial Hospital in Taiwan, R.O.C. (IRB 201601678A3C501). Informed consent was obtained from the patient. We report this case according to CARE (for Case Reports) guidelines (8).
In a review of the literature by Lin LL and Gau CC, clinical manifestations, imaging findings, management strategies and outcomes were analyzed. We used the Medline subheading keywords “systemic lupus erythematosus (SLE),” “stercoral colitis,” and “stercoral perforation” to search for studies in PubMed published between 1965 and August 2020 in English. Relevant articles and additional references were found by checking the citations in the articles retrieved.
To date, fewer than 200 cases of stercoral colitis or stercoral perforation have been reported, and only 10 patients [3 males (9–11) and 7 females (12–17)] younger than 30 years old are reported in PubMed. The analysis of patients aged 2–28 years old is shown in Table 1. Except for the present patient, all of the young adult or adolescent patients experienced psychiatric problems or were under substance use. According to the presentations, most patients (8 of 10) complained of abdominal pain, but only 40% had fever. According to physical examinations, signs of peritonitis were detected only in six cases (cases 1, 3, 5, 8, 9, and 10). Laboratory testing showed obvious inflammatory processes in 6 patients, with 2 having metabolic acidosis and one patient both anemia and acute kidney injury. According to the indications of Kumar et al. (18), 7 cases showed diagnostic signs of stercoral perforation and CT features of fecal protrusion through the colonic wall; extraluminal air was found in half of the cases. Seven of the patients underwent surgery, and all of them were alive.
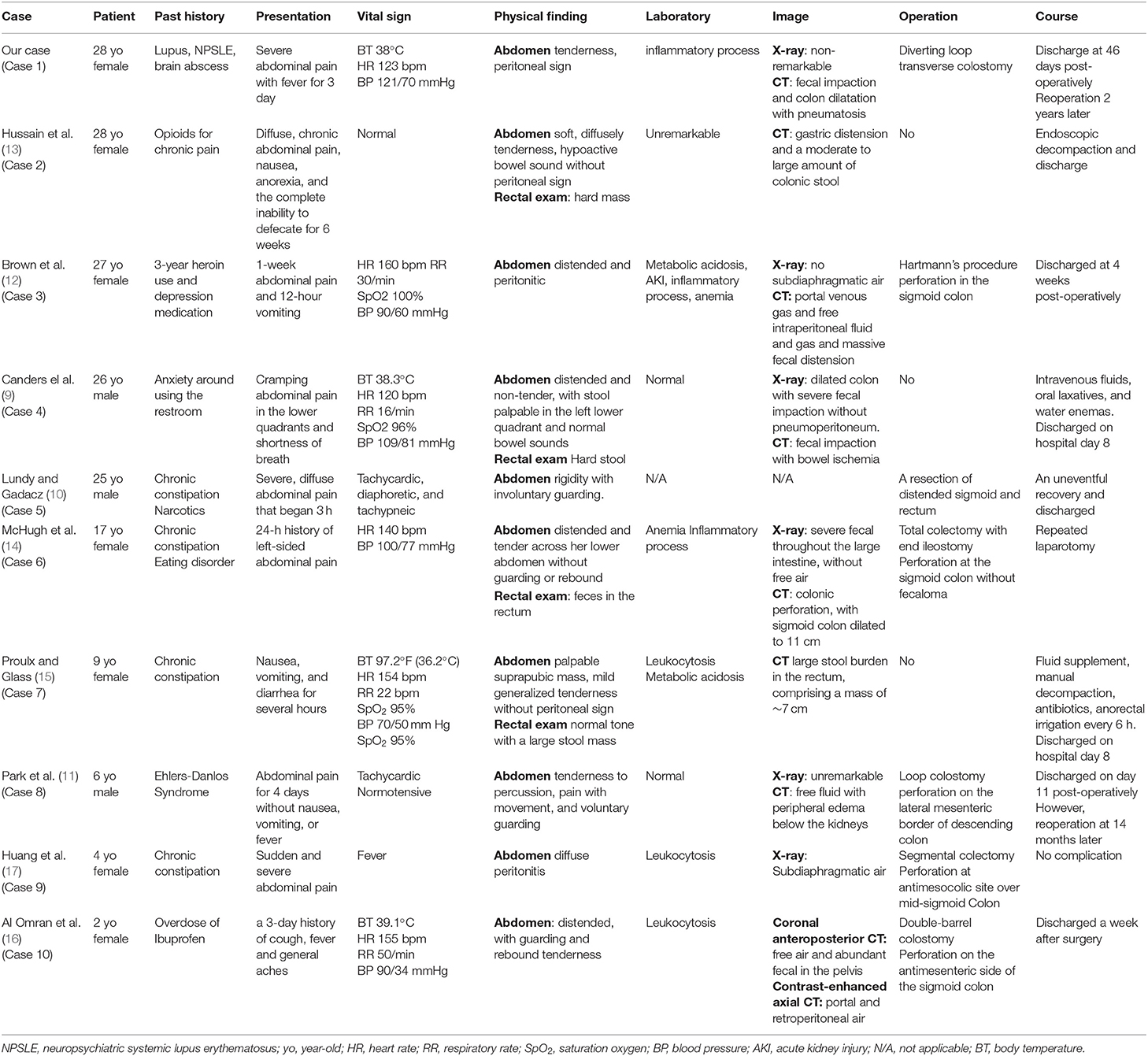
Table 1. Comparison of our patient with published data on stercoral colitis or perforation at young ages.
Stercoral perforation is a rare but fatal complication of constipation and fecal impaction. Before our patient, fewer than 200 cases have been reported since the first report in 1894 (19). Maurer et al. asserted that 1.2% of all emergency colorectal procedures and 3.2% of all colonic perforations involve stercoral perforation (6). In a retrospective study, stercoral perforation occurred in 81% of patients, especially with relation to a long-term history of chronic constipation with a median age of 62 (19). In general, patients taking medication such as non-steroidal anti-inflammatory drugs (NSAIDs) have a higher risk of stercoral perforation (20, 21) due to the propensity of these drugs to travel slowly and transiently through the bowel.
By exploring the published English literature (PubMed search from 1965 through August 2020), we found only two cases of stercoral colitis in patients with SLE (22, 23). In one case report, a 45-year-old woman with SLE presented with epigastric pain for 12 hours, and the author believed that the cause of stercoral perforation was related to NSAIDs (22). Another case report involved a 44-year-old SLE woman presenting with stercoral perforation due to long-term steroid use (23). Our patient is the first pediatric-onset (onset age at the 8) lupus case complicated by stercoral colitis without a disease flare or NSAID medication use. Although there is a reports that ischemia colitis is associated with antiphospholipid syndrome (24), but reviewing her 20-year history of lupus, no antiphospholipid antibodies were found. In addition, it is reported that some anticonvulsants can induce stercoral colitis (25), but only one short-term benzodiazepines was used 9 year ago to control a seizure. Importantly, we mainly considered the four neurological events (seizure, cranial neuropathy, transverse myelitis, and brain abscess) lead to impaired nerve innervation and cause neurogenic bladder and chronic constipation, which resulted in this catastrophic stercoral colitis. The sigmoid colon and rectum, especially the rectosigmoid junction, are the most vulnerable parts of the colon for stercoral colitis development. An insufficient blood supply at the rectosigmoid junction is specifically defined as Sudeck's point with insufficient or absent anastomosis between the superior rectal artery and inferior mesenteric artery branch at the watershed area (17, 26). Although her lupus condition was stable, bowel inflammation may also have contributed to poor tissue recovery in this insufficient blood supply area and the need for a second operation. In terms of the multiple manifestations of lupus, a correct diagnosis is difficult in this population.
Various causes of abdominal pain in lupus may indicate misdiagnosis, such as lupus enteritis, primary peritonitis and chronic lupus peritonitis (27, 28). Lupus enteritis, including mesenteric arteritis or vasculitis, gastrointestinal vasculitis and intestinal vasculitis, is a rare but life-threatening complication of SLE. Furthermore, postprandial abdominal pain can be insidious due to chronic mesenteric ischemia (27). Primary peritonitis secondary to SLE can develop rapidly, but the simultaneous use of immunosuppressive agents might mask the symptoms. In view of the aforementioned challenges of misleading diagnosis, stercoral colitis is easily overlooked, but prompt treatment is required due to its rapid progression.
According to Maurer et al.'s definition (6), there are three diagnostic criteria: (1) a round or ovoid colonic perforation more than 1 cm in diameter on the antimesenteric side; (2) fecalomas observed in the colon, protruding through the perforation site or lying within the abdominal cavity; and (3) pressure necrosis or ulceration with chronic inflammatory reactions around the perforation site. In addition, it has been reported that the mortality rate is 32–60%, regardless of age (5). In our analysis, all patients met the diagnostic criteria, were still alive and had been discharged from the hospital; thus, a young age may result in favorable outcomes.
Early diagnosis of stercoral colitis remains a dilemma. According to a previous review, not all cases involve fever, peritoneal signs, elevation of inflammatory markers, metabolic acidosis, or electrolyte imbalance (16, 29). In addition, urinary retention, incontinence or frequency may be early signs of fecal obstruction (30). A tubular mass may be palpable in the lower left quadrant because the fecal-filled rectosigmoid and rectal exam can reveal feces located at the sigmoid colon and obstruction (31, 32). One of the patients in our review was at the age of two and unable to express abdominal discomfort; therefore, diagnosis may be difficult to confirm. Our lupus patient had urinary retention, which should be one of the early signs of chronic constipation. Our review is also in line with previous research showing that peritoneal signs and inflammation laboratory data were observed in only 60% of cases.
Although previous experts have mentioned that pain during an upright abdominal X-ray examination can provide clues about stercoral colitis or perforation, including colonic swelling at the impaction site, calcified fecaloma, or free air, fecal matter may obscure these findings (6, 33). The pivotal diagnostic role of CT radiologic investigation includes a large fecaloma with distention >6 cm of the affected colon, wall thickening >3 mm of the affected colon, pericolonic fat stranding, mucosal discontinuity, free fluid, pericolonic abscess and extraluminal gas bubbles (18, 33–35). However, in our review of patients at a young age, plain abdominal X-ray did not reveal remarkable findings for most patients, with only half displaying perforation on CT images.
According to Wu et al.'s research, 52% of patients can be treated non-operatively through an intestinal regimen (36). However, if peritonitis occurs, emergency laparotomy should be performed to rule out situations that may be related to stercoral perforation. Stercoral colitis can be diagnosed through pathology and intraoperatively (6). The usual surgery is Hartmann's procedure, which removes possible lesions (37). Intraoperative colonoscopy should be applied to determine the presence of additional stercoral ulcers, and a specimen of the altered or dilated colon should be taken to prevent recurrence of stercoral perforation (17). Additionally, pathology can reveal mucosal hemorrhage, submucosal congestion, and sharp demarcation without undermining the ulcer margins and transmural necrosis on the perforated side (29, 36). In our young age case analysis, 30% of patients received conservative treatment with good prognosis but two cases need re-operation. One was our patient and the other was a patient with Ehlers-Danlos syndrome, a connective tissue disease, and both underlying diseases may cause poor wound healing.
It is important to identify SLE patients with severe abdominal pain who may be at risk of stercoral colitis or perforation. Stercoral colitis is a rare yet lethal complication that can occur in patients as young as 28 years old, and differential diagnosis should highlight the importance of a multidisciplinary approach. In addition to appendicitis, peritonitis, or vasculitis, differential diagnosis should include stercoral colitis for patients with SLE who develop unexplained sharp abdominal pain.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee on Human Studies at Chang Gung Memorial Hospital in Taiwan, R.O.C. (IRB 201800989A3). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
C-CG and L-LL carried out the case analysis, participated in the sequence alignment, and drafted the manuscript. C-YW and J-LH participated in the design of the study and performed the statistical analysis. J-LH conceived of the study and participated in its design. All authors contributed to the article and approved the submitted version.
This work was supported by the Research Fund of Chang Gung Memorial Hospital CMRPG3H1201-2, CMRPVVH0023, the National Science Council NMRPVVK6021-3 and the Ministry of Science and Technology (MOST; 109-2314-B-182A-105-MY3).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Yu-Jen Hsu, Division of Colon and Rectal Surgery, Chang Gung Memorial Hospital for permission to report the patient's surgical record and Dr. Pai-Jui Yeh, Division of Pediatric Gastroenterology, Department of Pediatrics, Chang Gung Memorial Hospital, Linkou Branch and Chang Gung University College of Medicine, Taoyuan City, Taiwan, for the critical suggestion in gastroenterology. We also thank the children and their parents for participating in the study and the assistance of medical staff.
ANAs, antinuclear antibodies; CRP, C-reactive protein; CT, computed tomography; NSAIDs, non-steroidal anti-inflammatory drugs; NPSLE, neuropsychiatric SLE; SC, stercoral colitis; SLE, systemic lupus erythematosus; WBC, white blood cell.
1. Alves SC, Fasano S, Isenberg DA. Autoimmune gastrointestinal complications in patients with systemic lupus erythematosus: case series and literature review. Lupus. (2016) 25:1509–19. doi: 10.1177/0961203316655210
2. Brewer BN, Kamen DL. Gastrointestinal and hepatic disease in systemic lupus erythematosus. Rheum Dis Clin North Am. (2018) 44:165–75. doi: 10.1016/j.rdc.2017.09.011
3. Richer O, Ulinski T, Lemelle I, Ranchin B, Loirat C, Piette JC, et al. Abdominal manifestations in childhood-onset systemic lupus erythematosus. Ann Rheum Dis. (2007) 66:174–8. doi: 10.1136/ard.2005.050070
4. Aggarwal A, Srivastava P. Childhood onset systemic lupus erythematosus: how is it different from adult SLE? Int J Rheum Dis. (2015) 18:182–91. doi: 10.1111/1756-185X.12419
5. Serpell JW, Nicholls RJ. Stercoral perforation of the colon. Br J Surg. (1990) 77:1325–9. doi: 10.1002/bjs.1800771204
6. Maurer CA, Renzulli P, Mazzucchelli L, Egger B, Seiler CA, Büchler MW. Use of accurate diagnostic criteria may increase incidence of stercoral perforation of the colon. Dis Colon Rectum. (2000) 43:991–8. doi: 10.1007/BF02237366
7. Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients The Committee on Prognosis Studies in SLE. Arthritis Rheum. (1992) 35:630–40. doi: 10.1002/art.1780350606
8. Riley DS, Barber MS, Kienle GS, Aronson JK, von Schoen-Angerer T, Tugwell P, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. (2017) 89:218–35. doi: 10.1016/j.jclinepi.2017.04.026
9. Canders CP, Shing R, Rouhani A. Stercoral colitis in two young psychiatric patients presenting with abdominal pain. J Emerg Med. (2015) 49:e99–103. doi: 10.1016/j.jemermed.2015.04.026
10. Lundy JB, Gadacz TR. Massive fecal impaction presenting with megarectum and perforation of a stercoral ulcer at the rectosigmoid junction. South Med J. (2006) 99:525–7. doi: 10.1097/01.smj.0000215762.71272.8b
11. Park KY, Gill KG, Kohler JE. Intestinal perforation in children as an important differential diagnosis of vascular Ehlers-Danlos syndrome. Am J Case Rep. (2019) 20:1057–62. doi: 10.12659/AJCR.917245
12. Brown CD, Maxwell F, French P, Nicholson G. Stercoral perforation of the colon in a heroin addict. BMJ Case Rep. (2017) 2017:bcr2016218875. doi: 10.1136/bcr-2016-218875
13. Hussain ZH, Whitehead DA, Lacy BE. Fecal impaction. Curr Gastroenterol Rep. (2014) 16:404. doi: 10.1007/s11894-014-0404-2
14. McHugh S, Todkari N, Moloney T, Leahy A. Stercoral perforation in a 17-year old. Ir J Med Sci. (2011) 180:581–2. doi: 10.1007/s11845-009-0329-1
15. Proulx E, Glass C. Constipation-associated stercoral colitis. Pediatr Emerg Care. (2018) 34:e159–60. doi: 10.1097/PEC.0000000000001600
16. Al Omran Y, Al Hindi S, Alarayedh S, Hawash A. Stercoral perforation in a child: a rare complication of NSAID use. BMJ Case Rep. (2014) 2014:bcr2014203652. doi: 10.1136/bcr-2014-203652
17. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. Management of patients with stercoral perforation of the sigmoid colon: report of five cases. World J Gastroenterol. (2006) 12:500–3. doi: 10.3748/wjg.v12.i3.500
18. Kumar P, Pearce O, Higginson A. Imaging manifestations of faecal impaction and stercoral perforation. Clin Radiol. (2011) 66:83–8. doi: 10.1016/j.crad.2010.08.002
19. Chakravartty S, Chang A, Nunoo-Mensah J. A systematic review of stercoral perforation. Colorectal Dis. (2013) 15:930–5. doi: 10.1111/codi.12123
20. Wang SY, Sutherland JC. Colonic perforation secondary to fecal impaction: report of a case. Dis Colon Rectum. (1977) 20:355–6. doi: 10.1007/BF02586438
21. Abella ME, Fernández AT. Large fecalomas. Dis Colon Rectum. (1967) 10:401–4. doi: 10.1007/BF02617028
22. Patel VG, Kalakuntla V, Fortson JK, Weaver WL, Joel MD, Hammami A. Stercoral perforation of the sigmoid colon: report of a rare case and its possible association with nonsteroidal anti-inflammatory drugs. Am Surg. (2002) 68:62–4.
23. Latif E, Musthafa S, Ahmed A, Abu Amr A. Stercoral perforation of sigmoid colon in systemic lupus erythematosus: a rare cause of peritonitis. Cureus. (2020) 12:e9495. doi: 10.7759/cureus.9495
24. Choobi Anzali B, Bahreini M, Habibi B, Sharifi Sistani N. Ischemic colitis due to antiphospholipid antibody syndrome. Turk J Emerg Med. (2019) 19:36–8. doi: 10.1016/j.tjem.2018.10.001
25. George J, Hotham R, Melton W, Chapple K. Clozapine-induced stercoral colitis: a surgical perspective. BMJ Case Rep. (2019) 12:e227718. doi: 10.1136/bcr-2018-227718
26. Naseer M, Gandhi J, Chams N, Kulairi Z. Stercoral colitis complicated with ischemic colitis: a double-edge sword. BMC Gastroenterol. (2017) 17:129. doi: 10.1186/s12876-017-0686-6
27. Chng HH, Tan BE, Teh CL, Lian TY. Major gastrointestinal manifestations in lupus patients in Asia: lupus enteritis, intestinal pseudo-obstruction, and protein-losing gastroenteropathy. Lupus. (2010) 19:1404–13. doi: 10.1177/0961203310374337
28. Ogbu EA, Rouster-Stevens K, Vega-Fernandez P. Lupus mesenteric vasculitis: a rare initial presentation of pediatric systemic lupus erythematosus. J Clin Rheumatol. (2021) 27:e181. doi: 10.1097/RHU.0000000000001317
29. Wu CH, Wang LJ, Wong YC, Huang CC, Chen CC, Wang CJ, et al. Necrotic stercoral colitis: importance of computed tomography findings. World J Gastroenterol. (2011) 17:379–84. doi: 10.3748/wjg.v17.i3.379
30. McWilliams WA, Khauli RB, Zein TA. Ureteral obstruction due to massive fecal impaction. South Med J. (1984) 77:275–6. doi: 10.1097/00007611-198402000-00043
31. Wright BA, Staats DO. The geriatric implications of fecal impaction. Nurse Pract. (1986) 11:53–8, 60, 4–6. doi: 10.1097/00006205-198610000-00004
32. Eitan A, Bickel A, Katz IM. Fecal impaction in adults: report of 30 cases of seed bezoars in the rectum. Dis Colon Rectum. (2006) 49:1768–71. doi: 10.1007/s10350-006-0713-0
33. Saksonov M, Bachar GN, Morgenstern S, Zeina AR, Vasserman M, Protnoy O, et al. Stercoral colitis: a lethal disease-computed tomographic findings and clinical characteristic. J Comput Assist Tomogr. (2014) 38:721–6. doi: 10.1097/RCT.0000000000000117
34. Heffernan C, Pachter HL, Megibow AJ, Macari M. Stercoral colitis leading to fatal peritonitis: CT findings. AJR Am J Roentgenol. (2005) 184:1189–93. doi: 10.2214/ajr.184.4.01841189
35. Ünal E, Onur MR, Balci S, Görmez A, Akpinar E, Böge M. Stercoral colitis: diagnostic value of CT findings. Diagn Interv Radiol. (2017) 23:5–9. doi: 10.5152/dir.2016.16002
36. Wu CH, Huang CC, Wang LJ, Wong YC, Wang CJ, Lo WC, et al. Value of CT in the discrimination of fatal from non-fatal stercoral colitis. Korean J Radiol. (2012) 13:283–9. doi: 10.3348/kjr.2012.13.3.283
Keywords: pediatric-onset systemic lupus erythematosus, stercoral colitis, stercoral perforation, case report, neuropsychiatric SLE (NPSLE)
Citation: Gau C-C, Lin L-L, Wu C-Y and Huang J-L (2021) Stercoral Colitis in a Patient With Pediatric-Onset Systemic Lupus Erythematosus: Case Analysis and Review of the Literature. Front. Pediatr. 9:760517. doi: 10.3389/fped.2021.760517
Received: 18 August 2021; Accepted: 28 September 2021;
Published: 27 October 2021.
Edited by:
Deborah Levy, Hospital for Sick Children, CanadaReviewed by:
Rabia Miray Kisla Ekinci, Ministry of Health, TurkeyCopyright © 2021 Gau, Lin, Wu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Long Huang, bG9uZ0BhZG0uY2dtaC5vcmcudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.