- 1Department of Neonatology, Children's Hospital of Chongqing Medical University, National Clinical Research Center for Child Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing, China
- 2Department of Neonatology, The General Hospital of Ningxia Medical University, Ningxia, China
- 3Department of Neonatology, First Affiliated Hospital of Harbin Medical University, Harbin, China
- 4Department of Neonatal Surgery, Children's Hospital of Chongqing Medical University, Chongqing, China
Background: Information regarding the localization of gastrointestinal perforation is crucial for the following surgical procedure. This study was to determine the key indicators and develop a prediction model for the localization in neonates with gastrointestinal perforation.
Methods: A nomogram to predict the location of neonatal gastrointestinal perforation was developed using a cohort of patients who underwent surgery between July 2009 and May 2021. Baseline variables were analyzed using logistics regression and nomogram developed using significant predictors. The predictive performance of the nomogram was assessed by the concordance index (C-index), calibration curve, and area under the receiver operating characteristic (ROC) curve (AUC). The nomogram was further validated in an integrated external cohort.
Results: We investigated the data of 201 patients, of which 65 (32.3%) were confirmed with upper gastrointestinal perforation by surgery. Multivariate logistic regression analysis identified the following as independent predictors: preterm [OR: 5.014 (1.492–18.922)], time of onset [OR: 0.705 (0.582–0.829)], preoperative hemoglobin [OR:1.017 (1.001–1.033)], bloody stool: No [OR: 4.860 (1.270–23.588)], shock [OR: 5.790 (1.683–22.455)] and sepsis: No [OR 3.044 (1.124–8.581)]. Furthermore, the nomogram was effective in predicting the perforation site, with an AUC of 0.876 [95% confidence interval (CI): 0.830–0.923]. Internal validation showed that the average AUC was 0.861. Additionally, the model achieved satisfactory discrimination (AUC, 0.900; 95% CI, 0.826–0.974) and calibration (Hosmer-Lemeshow test, P = 0.4802) in external validation.
Conclusions: The nomogram based on the six factors revealed good discrimination and calibration, suggesting good clinical utility. The nomogram could help surgeons predict the location of gastrointestinal perforation before surgery to make a surgical plan.
Introduction
Neonatal gastrointestinal perforation (GIP) has significant morbidity and mortality, which poses a significant challenge for the pediatric surgical team (1–6).
Gastrointestinal perforations commonly result in generalized secondary peritonitis (7, 8). Despite advances in neonatal intensive care, antimicrobial therapy, parenteral nutrition, operative, and anesthetic techniques, neonatal mortality remains high (9). A prompt diagnosis is life-saving, and surgical source control must be placed on top of the therapeutic priority list for all patients with generalized secondary peritonitis (10–12). Byun et al. have found that the time between symptoms and surgical intervention was the only prognostic factor for survival (13). Predicting the location of the perforation before surgery will help shorten the time from the onset to the surgical source control. This leads to a reduction in the total cost of treatment and reduces the operation time caused by preoperative planning, therefore, the time of anesthesia and the risk of infection.
Imaging methods such as abdominal X-ray, ultrasound, and computerized tomography (CT) are commonly used to diagnose GIP. However, most of these methods have some limitations. The detection of free intraperitoneal gas on abdominal X-rays is the most definitive sign of perforation (14). However, abdominal X-rays have limited ability to distinguish the location of perforation. Some studies have proved that ultrasound has a certain value in predicting the location of gastrointestinal perforation. However, the accuracy is closely dependent on the experience of sonographers and the quality of ultrasound equipment. CT has become the preferred method for predicting the location of gastrointestinal perforation (15, 16). However, CT still has limitations, such as CT having a potential risk of radiation exposure and is relatively expensive and time-consuming.
In this study, we aimed to build a diagnostic tool that incorporated the clinical factors and serological biomarkers for early identification of the location of gastrointestinal perforation. This could provide useful information to clinicians for the appropriate management and surgical planning.
Materials and Methods
Study Design and Participants
A retrospective review of the clinical records of neonates who underwent surgical treatment for GIP at the Children's Hospital of Chongqing Medical University between July 2009 and May 2021 was performed and used to develop the prediction model. The validation data set comprised neonates with GIP between January 2015 and March 2021 at the First Affiliated Hospital of Harbin Medical University and General Hospital of Ningxia Medical University. All patients received standard of care therapy. This study was approved by the institutional review board of each hospital (No. 2020289). All data were encoded and used anonymously. Verbal informed consent and written informed consent were obtained from all participants' parents/guardians.
Inclusion and Exclusion Criteria
Patients were first screened for eligibility based on the following criteria: (1) Age at onset ≤ 30 d; (2) Gastrointestinal perforation was found during the operation.
Exclusion criteria: (1) The treatment before entering our hospital is unknown; (2) Neonates with major congenital structural or chromosomal anomalies; (3) Neonates without complete records.
Data Collection
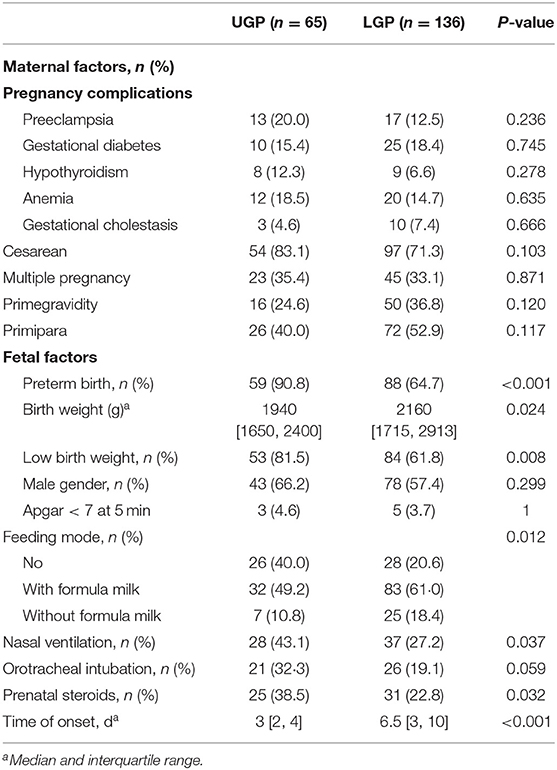
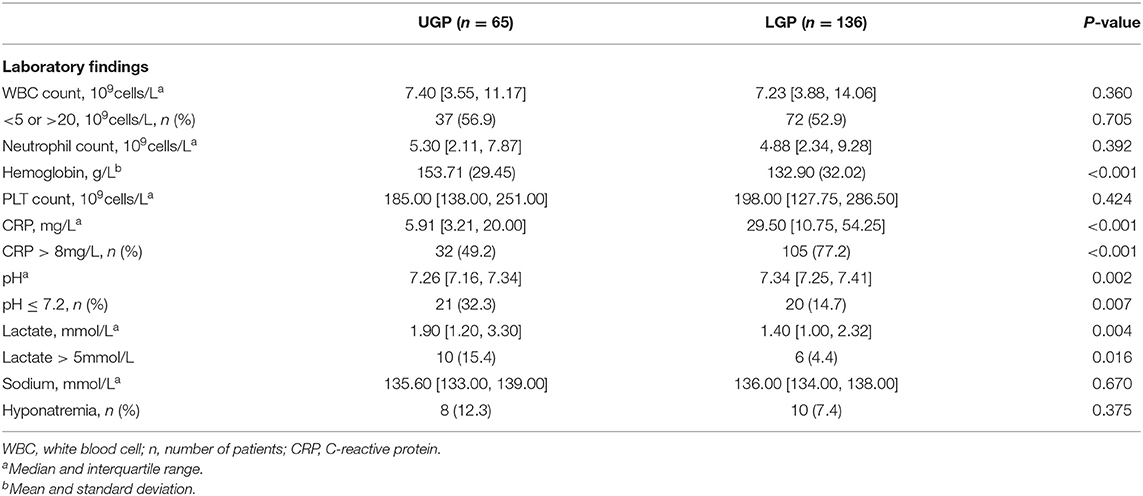
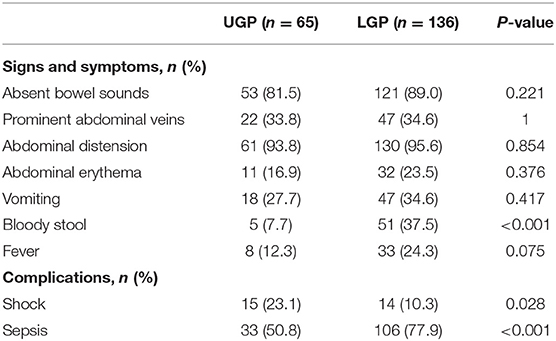
Data on patient demographics (gestational age, sex, birth weight, pregnancy complications, mode of delivery, multiple pregnancies, number of pregnancies, number of deliveries, Apgar scores at 1 min or 5 min, feeding mode, and time of onset), preoperative laboratory findings (leukocyte count, neutrophil count, hemoglobin, platelet count, C-reactive protein, blood pH, serum lactic acid, and sodium concentration), clinical manifestations (absent bowel sounds, prominent abdominal veins, abdominal distension, abdominal erythema, vomiting, bloody stool, and fever), preoperative complications (shock and sepsis), and the location of gastrointestinal perforation were abstracted from each patient record. For this study, complete-case analysis was undertaken; patients who had data missing for any of the predictive factors in the nomogram were excluded.
Definitions
GIP was defined as the destruction of integrity of the digestive tract. Patients were divided into upper and lower GIP groups according to the perforation site (above or below Treitz ligament). Preterm- and term-born were defined as birth at <37 and ≥37 weeks' gestation, respectively. Fever was defined as an axillary temperature of at least 37.3°C. Hyponatremia was defined as a serum sodium concentration (Na+) < 130 mEq/L (17). Bloody stool was defined as visible blood in the stool. In this study, sepsis and shock were defined according to Pediatric Sepsis Consensus (PSC) criteria (18).
Statistical Analysis
Categorical variables were presented as frequency rates and percentages, and continuous variables were expressed as mean ± standard deviation (SD) if they were normally distributed or median (interquartile range [IQR]) if they were not. Proportions for categorical variables were compared using the χ2 test or Fisher's exact test. Means for continuous variables were compared using independent group t-test when the data were normally distributed.
To develop a nomogram with well-calibration and discrimination for prediction for location of GIP before surgery, a model was built in a training set and then validated in another data set. A logistic regression model was used to construct the nomogram. Variables with P < 0.05 in the multivariable analysis were included in the nomogram. The nomogram is based on proportionally converting each regression coefficient in multivariate logistic regression into 0-100 points. The scores of each variable can be added to derive the total score, which corresponds to the prediction probability. The predictive performance of the nomogram was measured by concordance index (C-index) and calibrated with 1,000 bootstrap samples to reduce the overfit bias. An AUC > 0.80 was considered to be an acceptable value. The optimal cut-off value determined by the maximum Youden index was calculated by receiver operating characteristic curve analysis. Calibration curves were plotted to assess the calibration of the model, accompanied by the Hosmer-Lemeshow test. The prediction model was validated in an independent external cohort. All analyses were performed using R version 3.6.1. A p-value < 0.05 (two-tailed) was considered to be statistically significant.
Results
In our study, 14 neonates were excluded because there was no clear record of the perforation site. Data were collected on 201 patients from the Southwest center (Chongqing, China) and 69 from the Northwest center (Ningxia, China) and Northeast center (Harbin, China). Finally, a cohort of 69 patients from North centers was used for external validation of the nomogram. Demographic, preoperative laboratory findings, clinical characteristics and complications of the patients in primary cohorts are summarized in Tables 1–3.
Upper gastrointestinal perforations were present in 65 of 201 patients (32.3%) and 20 of 69 patients (29.0%) in the train and validation cohorts, respectively. There was no statistically significant difference in upper gastrointestinal perforation rate between the two cohorts (P = 0.605).
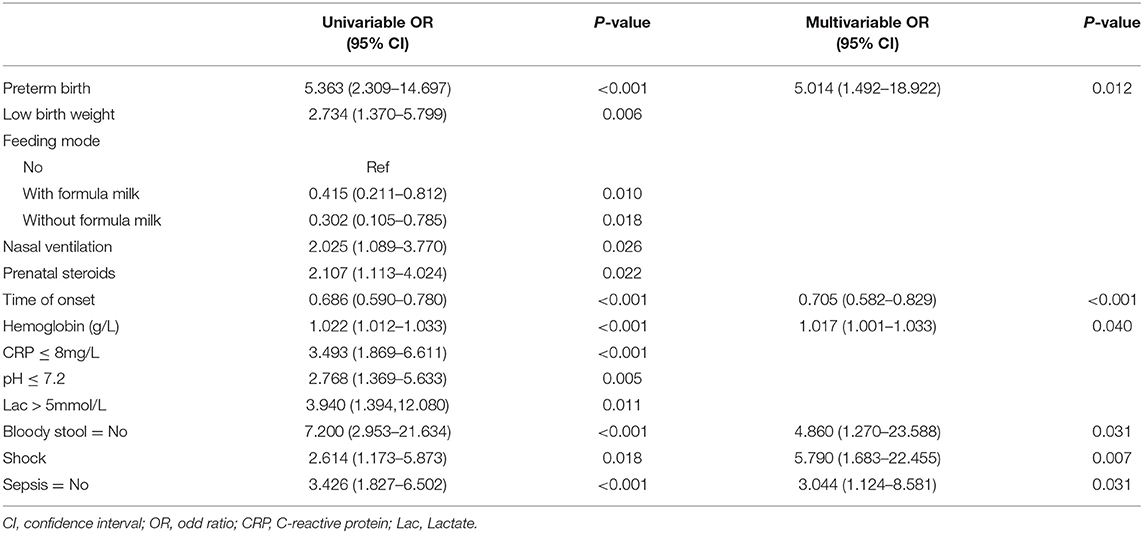
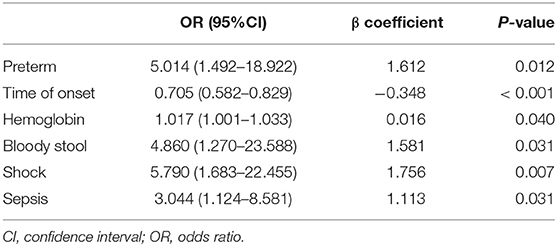
In multivariable analysis, preterm, time of onset, hemoglobin level, bloody stool, shock, and sepsis were predictors of gastrointestinal perforation sites (Table 4).
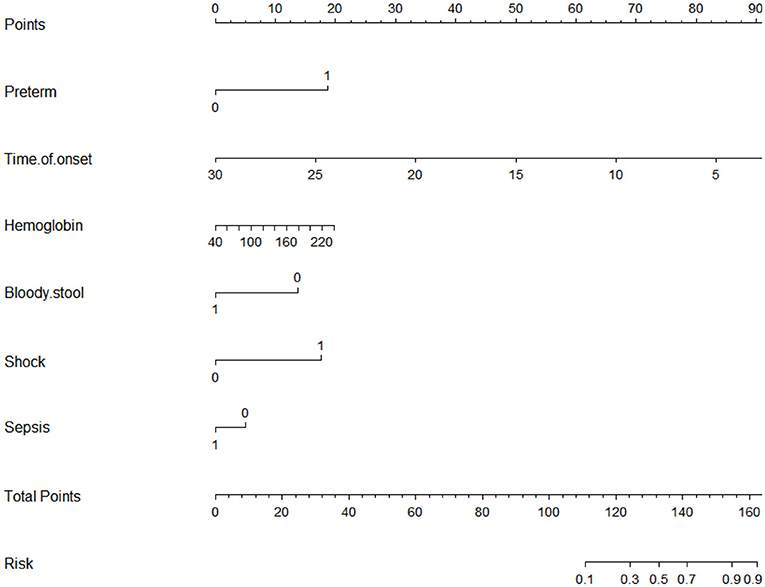
A nomogram that incorporated these factors was constructed (Figure 1). The C-index for the prediction nomogram was 0.876 (95% CI: 0.804–0·890) for the training cohort and 0.861 via bootstrapping validation (Figure 2). The calibration curve for the model showed good agreement between prediction and observation in the training cohort (Figure 3). The Hosmer-Lemeshow test yielded a P-value of 0.8977, indicating that the model was well-fitted.
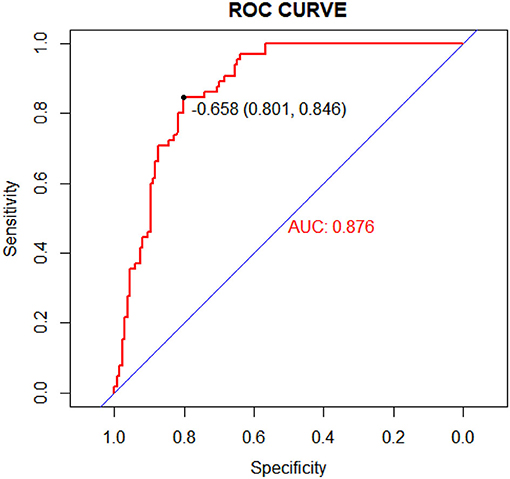
Figure 2. Receiver operating characteristic (ROC) curves of the nomogram model in the training cohort.
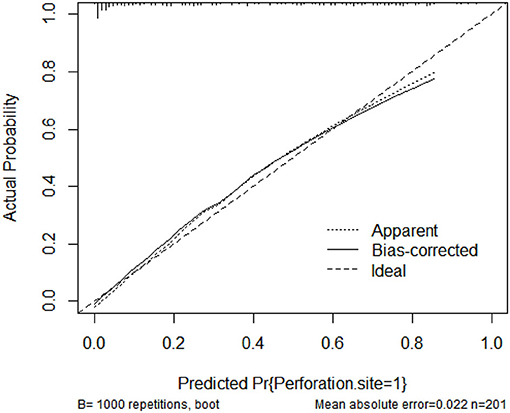
Figure 3. Calibration curves of the nomogram model showing the predicted vs. actual probability for upper GIP (gastrointestinal perforation) in the training cohort.
Development of Nomogram
Logistic regression modeling identified six variables that were associated with upper gastrointestinal perforation: preterm [OR: 5.014 (1.492–18.922); P = 0.012], time of onset [OR: 0.705 (0.582–0.829); P < 0.001], preoperative hemoglobin [OR: 1.017 (1.001–1.033); P = 0.040], bloody stool: No [OR: 4.860 (1.270–23.588); P = 0.031], shock [OR: 5.790 (1.683–22.455); P = 0.007] and sepsis: No [OR 3.044 (1.124–8.581); P = 0.031] (Table 5).
Validation of Nomogram
The C-index for the prediction nomogram was 0.900 (95% CI: 0.826–0.974) (Figure 4). The calibration curve showed good agreement between prediction and observation for the risk of upper GIP in the validation cohort (Figure 5). The Hosmer-Lemeshow test yielded a non-significant P-value of 0.4802.
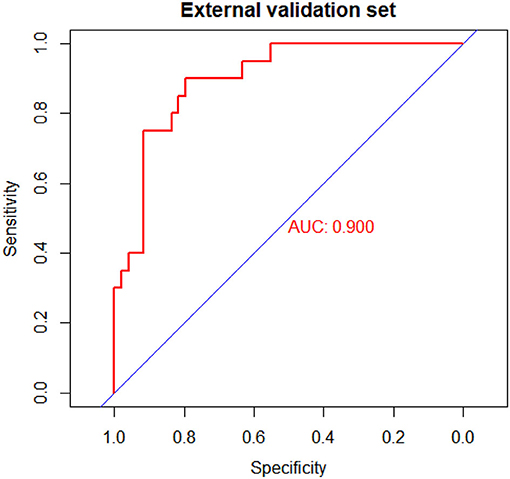
Figure 4. Receiver operating characteristic (ROC) curves of the nomogram model in the validation cohort.
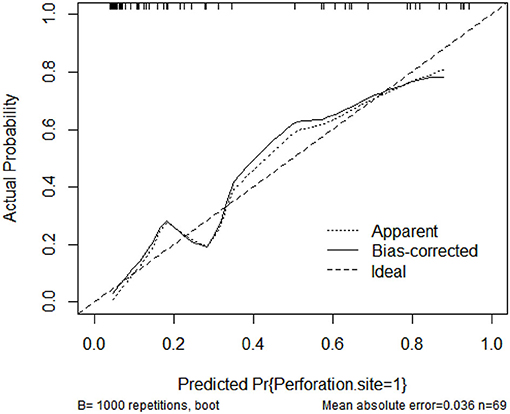
Figure 5. Calibration curves of the nomogram model showing the predicted vs. actual probability for upper GIP (gastrointestinal perforation) in the validation cohort.
Discussion
We developed and validated a prediction nomogram for preoperative prediction of the location of gastrointestinal perforation. The nomogram incorporates six items, including preterm, time of onset, preoperative hemoglobin, bloody stool, sepsis, and shock. The nomogram performed very well to predict the location of GIP in both the training (c-index 0.876) and external validation (c-index 0.900) cohorts. In particular, the calibration plots demonstrated good agreement between the estimated and observed perforation sites. As such, the proposed nomogram may be a helpful clinical tool to clinicians for the appropriate management and surgical planning. This result indicates that this nomogram is expected to be applied to neonates with GIP nationwide.
All predictors included in the nomogram were easy to readily available. It requires no advanced medical equipment and is particularly practical for medical-lacking or developing countries. This approach is less time-consuming, cheaper, and does not require any radiation exposure to the patient.
In the current study, several predictors have been found to be useful in predicting the location of neonatal gastrointestinal perforation.
In our study, the earlier the onset, the greater the possibility of upper GIP. Upper GIP is mostly caused by congenital defects in the musculature of the stomach, so it tends to have an early onset. Saracli et al. have reported that NGP mainly occurs between 2 and 7 days of age (19). Lower GIP is mostly caused by NEC and SIP (20). Preterm perforated-NEC usually occurs between 2 and 8 weeks after birth. And SIP usually presents as an isolated perforation within 10 days of birth (6). Calisti et al. have reported that the mean age of intestinal perforation was 10 days (21).
In our study, we have found that preterm birth was associated with upper GIP. The upper GIP is mostly gastric perforation, and duodenal perforation is extremely rare in newborns (22). In a previous systematic review, 47% of gastric perforation cases were considered idiopathic (23). Neonatal idiopathic gastric perforation is associated with the development of the gastric muscularis and is more likely to occur in premature infants (3). NEC is another major cause of gastric perforation, and it is also related to premature delivery (23).
In our study, a significant decrease in hemoglobin level often indicates intestinal perforation. Lower GIP is often accompanied by primary intestinal lesions, leading to obstacles to the intestinal absorption function. On the other hand, upper GIP is more likely to develop sepsis, causing sepsis-related anemia (24).
The results of this study suggest that the occurrence of preoperative sepsis was associated with lower GIP. Due to the difference in perforation sites and contents, the bacterial load was higher in lower GI than upper GI. When the intestinal integrity is impaired, the microbiota can come in contact with the intestinal mucosa and stimulate inflammation through the immune system (25).
In our study, the occurrence of preoperative shock was associated with upper GIP. Upper GIP is mainly caused by congenital defects in the musculature of the stomach, so the diameter of the perforation is often larger than that of lower GIP. Byun et al. reported that the length of gastric perforation was up to 10 cm in diameter (13). We speculate that this can easily cause a large amount of gastrointestinal fluid to enter the abdominal cavity in a short time, causing chemical peritonitis and even toxic shock.
In our study, we found that bloody stool often indicated lower GIP. Patients with lower GIP often have primary intestinal lesions, which can cause bleeding when the lesions accumulate blood vessels. Lower GI bleeding often manifests as bloody stools, while upper GI bleeding often manifests as melena. This is in line with our observations.
While knowledge of predictors associated with the location of GIP may be helpful, the practical utilization of this information can be challenging in the clinical setting. In turn, predictive model (nomogram) has gained popularity as they are relatively easy to use with a simple graphic that enables the incorporation of multiple relevant clinical predictors that can be applied to individual patients. In addition, in an era of personalized medicine, nomograms directly quantify individual patient risk based on statistically derived predictive variables. The variables used in our predictive nomogram are readily and routinely available (26–28). Importantly, the proposed nomogram to predict the location of GIP performed very well, with a c-index of 0.876 in the training cohort and 0.900 in the validation cohort, as well as excellent calibration. The proposed nomogram may help surgeons predict the location of gastrointestinal perforation before surgery to make a surgical plan.
Our study has some limitations: (1) Although this nomogram was based on Chinese patients alone and may not apply well to Western populations; (2) Although we undertook external validation, this was also performed in a small cohort; (3) The retrospective design and the reliability of the electronic patient records are always associated with limitations; (4) Our hospital is the largest children's medical center in the Southwest China, which may lead to selection bias because we have a higher proportion of critically premature infants; (5) Our model is constructed by comparing data in newborns with UGI and LGI perforations. Therefore, it is not suitable for neonates with no perforations found during surgery. Clearly, our results should be further validated by prospective studies in multicenter clinical trials. Other predictive variables may be included to improve performance of this model.
Conclusion
Mortality of neonatal gastrointestinal perforation has decreased since development in intensive medical care, but how to predict the location of GIP is still a challenge for surgeons. This study identified predictors for the location of GIP. The nomogram for predicting the location of neonatal gastrointestinal perforation has thus been developed in this study to permit surgeons to make appropriate management and surgical planning.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. Requests to access these datasets should be directed to Yao Huang, aHVhbmcxOTk2NjUmI3gwMDA0MDtxcS5jb20=.
Ethics Statement
This study received approval from the Ethics Committee of Children's Hospital of Chongqing Medical University, First Affiliated Hospital of Harbin Medical University, and the General Hospital of Ningxia Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
YH and QL are the guarantors and take responsibility for the manuscript, including the data and analysis and drafted the manuscript. QL, YH, YW, and DJ conceived and designed the study. YH, YW, DJ, QT, and PY acquired the data. YH and YW analyzed the data. All authors approved the final version for submission.
Funding
This study was supported by Chongqing Health Committee and Chongqing Science and Technology Bureau [No. 2021ZY023801] and National Special Fund for the Development of Local Science and Technology.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank our patients for entrusting us with their care and for giving us the opportunity to learn and understand their disease and hopefully help others who may suffer from this disease in the future. We also thank all participants and staff of this study and the physicians at the Children's Hospital of Chongqing Medical University, the General Hospital of Ningxia Medical University, and First Affiliated Hospital of Harbin Medical University.
References
1. St-Vil D, LeBouthillier G, Luks F, Bensoussan A, Blanchard H, Youssef S. Neonatal gastrointestinal perforations. J Pediatr Surg. (1992) 27:1340–2. doi: 10.1016/0022-3468(92)90292-F
2. Tan CEL, Kiely EM, Agrawal M, Brereton RJ, Spitz L. Neonatal gastrointestinal perforation. J Pediatr Surg. (1989) 24:888–92. doi: 10.1016/S0022-3468(89)80589-5
3. Lin CM, Lee HC, Kao HA, Hung HY, Hsu CH, Yeung CY, et al. Neonatal gastric perforation: report of 15 cases and review of the literature. Pediatr Neonatol. (2008) 49:65–70. doi: 10.1016/S1875-9572(08)60015-7
4. Kawase Y, Ishii T, Arai H, Uga N. Gastrointestinal perforation in very low-birthweight infants. Pediatr Int. (2010) 48:599–603. doi: 10.1111/j.1442-200X.2006.02282.x
5. Shah J, Singhal N, Silva OD, Rouvinez-Bouali N, Seshia M, Lee SK, et al. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol. (2015) 35:595–600. doi: 10.1038/jp.2015.41
6. Clyman RI, Jin C, Hills NK. A role for neonatal bacteremia in deaths due to intestinal perforation: spontaneous intestinal perforation compared with perforated necrotizing enterocolitis. J Perinatol. (2020) 40:1662–70. doi: 10.1038/s41372-020-0691-4
7. Pehlivanl F, Aalar F, Aalar C, Saygun O, Ahiner T. The value of CRP, IL-6, leptin, cortisol, and peritoneal caspase-3 monitoring in the operative strategy of secondary peritonitis. Ulusal travma ve acil cerrahi dergisi. (2011) 17:390–5. doi: 10.5505/tjtes.2011.03443
8. Sartelli M, Abu-Zidan FM, Labricciosa FM, Kluger Y, Coccolini F, Ansaloni L, et al. Physiological parameters for prognosis in abdominal sepsis (PIPAS) Study: a WSES observational study. World J Emerg Surg. (2019) 14:34. doi: 10.1186/s13017-019-0253-2
9. Grosfeld JL, Molinari F, Chaet M, Engum SA, West KW, Rescorla FJ, et al. Gastrointestinal perforation and peritonitis in infants and children: experience with 179 cases over ten years. Surgery. (1996) 120:655–6. doi: 10.1016/S0039-6060(96)80012-2
10. Strobel O, Werner J, Büchler M. Chirurgische therapie der peritonitis. Der Chirurg. (2011) 82:242–8. doi: 10.1007/s00104-010-2015-2
11. Wittmann DH, Schein M, Condon RE. Management of secondary peritonitis. Ann Surg. (1996) 224:10–8. doi: 10.1097/00000658-199607000-00003
12. Agalar F, Sayek I, Agalar C, Cakmakçi M, Kavuklu B. Factors that may increase morbidity in a model of intra-abdominal contamination caused by gallstones lost in the peritoneal cavity. Eur J Surg. (1997) 163:909–14.
13. Byun J, Kim H, Noh S, Kim S, Jung S, Lee S, et al. Neonatal gastric perforation: a single center experience. World J Gastrointest Surg. (2014) 6:151–5. doi: 10.4240/wjgs.v6.i8.151
14. Okuyama H, Kubota A, Oue T, Kuroda S, Ikegami R, Kamiyama M. A comparison of the clinical presentation and outcome of focal intestinal perforation and necrotizing enterocolitis in very-low-birth-weight neonates. Pediatr Surg Int. (2003) 18:704–6. doi: 10.1007/s00383-002-0839-7
15. Singh J, Steward M, Booth T, Mukhtar H, Murray D. Evolution of imaging for abdominal perforation. Ann Royal Coll Surgeons of Engl. (2010) 92:182–8. doi: 10.1308/003588410X12664192075251
16. Furukawa A, Sakoda M, Yamasaki M, Kono N, Tanaka T, Nitta N, et al. Gastrointestinal tract perforation: CT diagnosis of presence, site, and cause. Abdom Imaging. (2005) 30:524–34. doi: 10.1007/s00261-004-0289-x
17. Noah Z, Budek C, De WM, Weesemayer DE. Nelson Textbook of Pediatrics. 20th ed. Philadelphia, PA: Elsevier Saunders (2015).
18. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Critic Care Med. (2005) 6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6
19. Saracli T, Mann M, French DM, Booker CR, Scott RB. Rupture of the stomach in the newborn infant: report of three cases and review of the world literature. Clin Pediatr. (1967) 6:583–8. doi: 10.1177/000992286700601014
20. Torikai M, Ibara S, Ieiri S, Hamada T, Noguchi H, Sueyoshi K, et al. Prophylactic efficacy of enteral miconazole administration for neonatal intestinal perforation and its potential mechanism. Pediatr Surg Int. (2016) 32:953–7. doi: 10.1007/s00383-016-3946-6
21. Calisti A, Perrelli L, Nanni L, Vallasciani S, Maragliano G. Surgical approach to neonatal intestinal perforation. An analysis on 85 cases (1991-2001). Minerva Pediatrica. (2004) 56:335–9.
22. Hamada Y, Kato Y, Takada K, Sato M, Sanada T, Tsuji M, et al. Neonatal spontaneous duodenal perforation : a report of two cases and review of the Japanese literatures. J Jpn Soc Pediatr Surg. (1994) 30:1303–9.
23. Iacusso C, Boscarelli A, Fusaro F, Bagolan P, Morini F. Pathogenetic and prognostic factors for neonatal gastric perforation: personal experience and systematic review of the literature. Front Pediatr. (2018) 6:61. doi: 10.3389/fped.2018.00061
24. Jiang Y, Jiang F-Q, Kong F, An M-M, Jin B-B, Cao D, et al. Inflammatory anemia-associated parameters are related to 28-day mortality in patients with sepsis admitted to the ICU: a preliminary observational study. Ann Intensive Care. (2019) 9:67. doi: 10.1186/s13613-019-0542-7
25. Gao Y, Yang L, Chin Y, Liu F, Li R, Yuan S, et al. Astaxanthin n-octanoic acid diester ameliorates insulin resistance and modulates gut microbiota in high-fat and high-sucrose diet-fed mice. Int J Mol Sci. (2020) 21:2149. doi: 10.3390/ijms21062149
26. Kim Y, Spolverato G, Ejaz A, Squires MH, Poultsides G, Fields RC, et al. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma. Ann Surg Oncol. (2015) 22:1828–35. doi: 10.1245/s10434-014-4230-4
27. Bischof DA, Kim Y, Behman R, Karanicolas PJ, Quereshy FA, Blazer DG, et al. A nomogram to predict disease-free survival after surgical resection of GIST. J Gastrointest Surg. (2014) 18:2123–9. doi: 10.1007/s11605-014-2658-2
Keywords: neonate, gastrointestinal perforation, nomogram model, perforation site, shock, sepsis
Citation: Huang Y, Wu Y, Jin D, Tang Q, Yuan P and Lu Q (2021) Development and Validation of a Nomogram for Preoperative Prediction of Localization of Neonatal Gastrointestinal Perforation. Front. Pediatr. 9:754623. doi: 10.3389/fped.2021.754623
Received: 06 August 2021; Accepted: 06 October 2021;
Published: 02 November 2021.
Edited by:
Pedro Gutierrez-Castrellon, Hospital General Dr. Manuel Gea Gonzalez, MexicoReviewed by:
Jose Francisco Gonzalez-Zamora, National Institute of Pediatrics, MexicoAnkur Mandelia, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGI), India
Copyright © 2021 Huang, Wu, Jin, Tang, Yuan and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Lu, cWlsdV9xaSYjeDAwMDQwO2hvdG1haWwuY29t
 Yao Huang
Yao Huang Yuhua Wu2
Yuhua Wu2