
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 22 October 2021
Sec. Pediatric Nephrology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.752717
Aim: To Identify association between risk factors to Chronic kidney disease (CKD) stage 5 in children with glomerular diseases in children in China.
Methods: The Hospital Quality Monitoring System database was used to extract data for the study cohort. The primary outcome included progression to CKD stage 5 or dialysis. Cox regression was used to assess potential risk factors. Patients with lower stages (CKD stage 1 and 2) and higher stages (CKD stage 3 and 4) at baseline were analyzed separately.
Results: Of 819 patients (4,089 hospitalization records), 172 (21.0%) patients reached the primary outcome during a median followed-up of 11.4 months. In the lower stages group, factors associated with the primary outcome included older age [Hazard Ratio (HR), 1.21; 95% confidence interval (CI), 1.10–1.34] and out-of-pocket payment (HR, 4.14; 95% CI, 1.57–10.95). In the higher stages group, factors associated with the primary outcome included CKD stage 4 (HR, 2.31; 95% CI, 1.48–3.62) and hypertension (HR, 1.99; 95% CI, 1.29–3.07). The medical migration rate was 38.2% in this study population.
Conclusion: There are different risk factors for progression to the primary outcome in different stages in CKD with glomerular etiology. Further prospective studies are needed to assess these risk factors. The high medical migration rate reflected the regional disparities in the accessibility of pediatric kidney care between regions.
Chronic kidney disease (CKD) is a leading global health problem with a prevalence of 8–16% that continues to increase (1, 2). In recent years, increasing attention has been paid to childhood CKD. History of childhood kidney disease was found to be associated with a 3.19-times higher risk of end-stage kidney disease (ESKD) (3). Nearly 68% of children with CKD progress to ESKD by age 20 years (4). CKD with glomerular etiology was found to be associated with a significantly higher risk of CKD progression in children compared with non-glomerular etiology (5–9). Moreover, glomerular disease is the leading cause of CKD in India, Southeast Asia, and several other districts, with a prevalence ranging from 30 to almost 60% (10–17), and Asia has the largest CKD population (1, 18). Thus, it's important to identify risk factors for CKD progression in glomerular disease, especially in Asia.
Previous studies showed that advanced CKD stage, hypertension, anemia, older age, and female gender were related to a higher risk of CKD progression in children (5–8). However, these studies were performed in Europe and America pediatric CKD population, in which the dominant cause of CKD was non-glomerular disease such as congenital anomalies of the kidney and urinary tract (CAKUT) (5–8), and glomerular disease only made up <20% (5–8). Few studies had been concerned about the risk of progression in glomerular diseases in Asia children (19, 20). Additionally, children with CKD stage 1 were rarely included in previous studies because they were believed to have a low risk of progression. Consequently, little is known about the risk and risk factors of progression in stage 1.
China has a large CKD population (1, 2, 21), therefore, research on risk factors for the progression of CKD in Chinese children is helpful to reveal the potential risk factors for children CKD progression in Asia. We, therefore, conducted a cohort study based on Hospital Quality Monitoring System (HQMS), a national inpatient database in China, to evaluate the association between risk factors and progression to CKD stage 5 in CKD children with glomerular etiology.
HQMS database is the largest national inpatient database in China, authorized by the National Health Commission of the People's Republic of China. There are over 190 million standardized inpatient records from 998 tertiary hospitals across 31 provinces in China in the HQMS database. There are up to 353 variables including patient demographics, clinical diagnosis coded according to the International Classification of Disease, 10th revision (ICD-10), medical payment methods, and information on hospitals on every record. Owing to the big data stored in the HQMS, numerous studies have been published on CKD (21–23), cardiovascular diseases (24), and other diseases (25).
Records of patients under 18 years old who were admitted between June 1, 2013, and December 31, 2018, were screened. Patients who were hospitalized more than once with a diagnosis of any stage from CKD stage 1 to stage 5 or dialysis were identified by ICD-10 disease codes. Patients with missing information on sex were excluded because sex was considered a primary parameter for evaluating the quality and completeness of records. Patients with the diagnosis of CKD stage 5 (including the diagnosis of chronic kidney failure) or dialysis on the first admitted record were excluded because these diagnoses were defined as the outcome. The details of patients excluded for this group were shown in Supplementary Data 1. Patients with the primary cause of non-glomerular diseases or unknown causes of CKD were excluded. The details of non-glomerular diseases excluded were shown in Supplementary Data 2. The study protocol was reviewed and approved by the Ethics Committee Board of Peking University First Hospital [Approval Number: 2021 (009)].
Extensive demographic and clinical data were extracted from each record, including age, sex, admission date, location of residence, location of the hospital, payment method and the hospitalization counts for each patient during study period. Stage of CKD, specific disease as the cause of CKD, comorbidity with hypertension, and comorbidity with anemia was acquired from the recorded diagnoses (including one primary diagnosis and up to nine other diagnoses). For diagnosis of hypertension, HQMS use the measurement of BP more than 95th in the same sex and age group at 3 different visits in clinical practice according to the 2010 Chinese guidelines for the management of hypertension (26), which is consistence with the pediatric hypertension recommendations in Europe (27) and United States (28). Stages of CKD were extracted from each discharge record as a time-dependent parameter. Diagnoses in all records of the same individual were analyzed to obtain the specific disease as the cause of CKD. For comorbidity classification, patients were classified as hypertension vs. non-hypertension and anemia vs. non-anemic, according to their diagnosis. Payment methods were classified as out-of-pocket or basic medical insurance according to the patient's payment information in each record. The location of residence (province-level) of each patient was identified from the address, hometown, and zip code on each record. The location of the hospital (province-level) was identified from the address of the hospital. Medical migration (province-level) was defined as a patient left their location of residence and traveled to a hospital in another province for admission. Followed-up time was calculated from the admission date of the first record to the admission date of the last record of the same person.
The primary outcome was progression to CKD stage 5, which was defined as a diagnosis of CKD stage 5 (including the diagnosis of chronic kidney failure), or dialysis (including peritoneal dialysis and hemodialysis) in the HQMS records. These diagnoses were identified by ICD-10 accordingly. The followed-up time was calculated from the admission date of the first record to the admission date of the last record of the same patient.
Patients were classified into lower stages group and higher stages group according to their first discharge record. Baseline features of different stages groups were compared using chi-squared tests for categorical data and Wilcoxon rank-sum tests for non-Gaussian continuous data.
For those who reached the primary outcome, the time-to-event was calculated from the admission date of the first record during the study period to the admission date of record when the primary outcome was reached. For those without a primary outcome in the study period, the time-to-event was calculated from the admission date of the first record to the admission date of the last record.
Cox proportional hazard models were used for multivariate analysis to evaluate independent risk factors for the primary outcome, with missing observations excluded. Variables examined in the multivariate analysis included age, sex, hypertension, anemia, medical migration, and payment methods. For analysis purposes, specific disease as the cause of CKD was classified into four groups: nephrotic syndrome, lupus nephritis, IgA nephropathy, and miscellaneous disease. Given the heterogeneity in baseline characters and interaction between CKD stages and other factors, patients in the lower stages group and higher stages group were analyzed separately. A likelihood ratio-based test was performed to test the null hypothesis that the coefficient in the regression equaled zero, with a two-tailed alpha of 0.05 considered statistically significant. Covariates within the pre-defined statistical significance level were retained in the final model. Hazard ratios (HRs) were used to measure the association with progression. The proportional hazard assumption was checked using weighted Schoenfeld residuals, with violated covariates included as time-dependent covariates.
All analyses were performed using SAS software 9.4 (SAS Inc., Cary, NC, USA), with confidence level of 95% used for interval estimates, unless otherwise specified.
There were 819 patients (4,089 hospitalization records) included in the final analysis. The study population selection process was shown in Figure 1. Demographic and clinical data pertaining to included patients are shown in Table 1. Among the 819 patients, 498 (60.8%) were boys, and the median age at enrollment was 11.9 [interquartile range (IQR), 7.9–15.6] years old. There were 549 (67.0%) patients with CKD stage 1, 59 (8.4%) with CKD stage 2, 127 (15.5%) with CKD stage 3 and 84 (10.3%) with CKD stage 4 at first admitted. The three most common specific disease causes were nephrotic syndrome (53.8%), lupus nephritis (16.7%), and immunoglobulin A (IgA) nephropathy (10.7%). Details on disease are shown in Supplementary Data 3. The prevalence of hypertension was 27.0% (148/549) in CKD stage 1, 33.9% (20/59) in stage 2, 48.8% (62/127) in stage 3, and 53.6% (46/84) in stage 4. Out-of-pocket payment made up 67.2% of the payment methods in the study population.
During a followed-up of 11.4 (IQR, 4.6–21.4) months, 172 (21.0%) patients reached the primary outcome including 43 (7.1%) patients in the lower stages group and 129 (61.1%) patients in the higher stages group. Patient characters classified by the outcome were shown in Table 2 and Supplementary Data 4, 5. Among the progressors (n = 43) in lower stages group, no statically significant difference was observed in the numbers of hospitalization between out-of-pocket payment patients than those paid by insurance [33 patients with out-of-pocket payment, total number of hospitalization = 203, median 4.0 (IQR, 2.0–9.0) vs. 8 patients with insurance, total number of hospitalization = 49, median 4.0 (IQR, 3.0–7.0), p-value for difference = 0.8542. There were 2 patients whose payment method unknown].
Multivariate proportional hazard regression analyses were used to evaluate independent risk factors for the primary outcome. The results are shown in Table 3. There were 608 patients in the lower stages group. In this group, older age (HR, 1.21; 95% CI, 1.10–1.34), and out-of-pocket payment method (HR, 4.14; 95% CI, 1.57–10.95) were significantly associated with a higher risk of the primary outcome. There were 211 patients in the higher stages group. CKD stage 4 (HR, 2.31; 95% CI, 1.48–3.62) and hypertension (HR, 1.99; 95% CI, 1.29–3.07) were significantly associated with higher risks of the primary outcome.
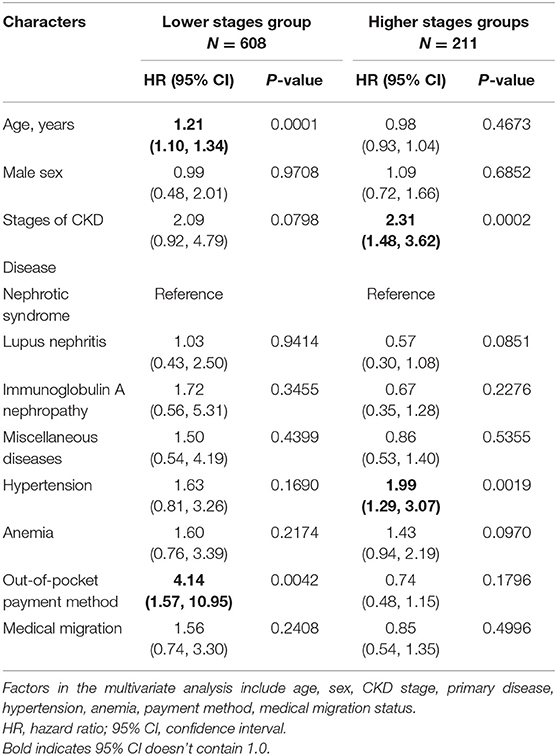
Table 3. Multivariate COX regression assessing the association between baseline characters and progression to CKD stage 5.
Sensitivity analysis was carried out by dividing the specific disease as the cause of CKD into two groups: nephrotic syndrome and others in multivariate proportional hazard regression analysis. The results of the sensitivity analyses remained generally consistent with the primary findings. The results of sensitivity analysis were shown in Supplementary Data 6.
A strength of our study is using the big data in HQMS to investigate the association between risk factors and progression of CKD with glomerular etiology in China, an area where the data of CKD in children is scarce. Our study results showed that there is significant heterogeneity of baseline characters between the lower stages group and higher stages group. Further multivariate analysis on each group revealed that risk factors associated with the primary outcome are different. In the lower stages group, older age and out-of-pocket payment methods were found to be associated with the primary outcome. In the higher stages group, CKD stage 4 and hypertension were found to be associated with adverse outcomes.
In lower stages participants, we found that children with out-of-pocket payment methods were associated with a 3.14-times higher risk of progression compared with children with basic medical insurance. However, among the progressors in this group, patients with out-of-pocket payment did not have fewer number of hospitalization compared with those insured patients. How payment method impact on the progression of disease in this group is still unknown, which might be the subject for further studies. A previous study in adults found that lower social-economic status was associated with poor kidney function (29). Children from lower social-economic backgrounds experience reduced quality of life, including less access to medical services compared with their wealthier counterparts (29). In China, basic medical insurance covers up to 70–90% of hospitalized medical costs, thus can greatly reduce economic burdens for families with CKD children. With the support of basic medical insurance, CKD children could have more treatment options in disease management, and better disease management would lead to a better disease prognosis.
Older age was associated with a higher risk of progression in the lower stages group. This result is consistent with the other studies (30). Children with an early diagnosis of CKD may have more opportunities of receiving more attention for disease management, thereby associated with a lower risk of progression. Identifying and diagnosing children with CKD stage 1 or 2 as early as possible might help to reduce the risk of disease progression (31).
In the higher stages group, CKD stage 4 and hypertension were found to be associated with a higher risk of the primary outcome. It's well-established that hypertension is associated with CKD progression in children and adults (6–8, 32). Our results support the previous studies from the childhood CKD population in China.
Even in a relatively short followed-up time, there were 21.0% of the hospitalized children reached the primary outcome. The progression speed is much faster compared to other CKD cohorts studies which mainly consisted of non-glomerular disease and were not restricted to hospitalized patients (5–9, 33). There are two reasons for this difference. Firstly, CKD children with glomerular etiology are at higher risk of progression in previous studies (6, 7, 9, 34). Secondly, compared to patients in the clinic, hospitalized patients tend to be more serious. Thus, hospitalized CKD children with glomerular etiology might have a higher risk for progression. More attention should be paid to this high-risk population. In our study, 7.1% of patients in the lower stages group progressed to stage 5 or dialysis, which has not been reported by other studies yet. This finding suggests that the risk of progression in children with lower stages of CKD cannot be ignored.
The rate of medical migration was 38.2%, which was more than 6 times higher than that of the Chinese adult population (23). This finding reflected the regional disparities of medical accessibility of CKD children in China. Therefore, optimizing the allocation of resources and enhancing the capacity and accessibility of kidney care in vulnerable areas are emerging policy priorities.
This study had several limitations. The first limitation was that the data source was discharge records from a medical quality monitoring system. Only a few clinical and epidemiological parameters were included in this database. Consequently, CKD stages were based on diagnoses only rather than measurement of estimated glomerular filtration rate (eGFR). But we still have proper reasons to applied these classifications according to diagnosis in HQMS in this study. Firstly, discharge diagnoses in HQMS are the official records with legal effect. The diagnosis in HQMS was made by well-trained doctors working in tertiary hospitals and double-checked by their superior doctors according to the current medical management policy to ensure the accuracy. Second, because specific ICD-10 codes were applied to different stages of CKD when extracting the corresponding diagnosis from the system, the results can maintain a certain degree of accuracy. Similarly, because the included database does not include very rich variables, for example, urine protein and specific information of blood pressure were not included, so our results cannot fully explain all possible risk factors and the subgroup analysis of different stages of hypertension could not be achieved. Prospective studies with more comprehensive survey information are still needed to verify our findings. The second limitation in our study is that only hospitalized records were collected in HQMS and some portion of patients with CKD would not be included. Most children with CAKUT, especially in the early stages, have an outpatient follow-up and are therefore not included in the HQMS inpatient database. However, under the current medical system in China, outpatient clinics, in most cases, are not sufficient to make an accurate diagnosis of CKD in children, especially in glomerular etiology. Therefore, it is surmised that CKD patients diagnosed in hospital account for the vast majority of all CKD patients. Nonetheless, more efforts are needed to study all CKD children in China.
Our study assessed potential risk factors according to different stages of CKD. Age and out-of-pocket payment methods were significantly associated with the primary outcome in childhood CKD stages 1 and 2. Early diagnosis of CKD and increased social medical insurance coverage in early CKD stages, may help to slow down the speed of disease progression. Hypertension was associated with higher risk of the primary outcome in CKD stage 3 and 4. More initiatives should be taken among patients with higher CKD stage and hypertension, to delay the progression process. The rate of medical migration in children is much higher than in adults. Disparities in medical resources between regions should be improved to enhance the accessibility of kidney care for children.
The datasets presented in this article are not readily available due to the requirement of the Hospital Quality Monitoring System (HQMS) database management rules. Requests to access the datasets should be directed to Jie Ding, ZGpuY181ODU1QDEyNi5jb20=.
The studies involving human participants were reviewed and approved by the Ethics Committee Board of Peking University First Hospital [Approval Number: 2021 (009)]. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Material preparation was performed by GH, HW, YS, and LG. Data collection and analysis were performed by GH, CL, XZ, FW, and JD. The first draft of the manuscript was written by GH, CL, and JD. All authors contributed to the study's conception and design, revised the manuscript critically for important intellectual content, commented on previous versions of the manuscript, and read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the Bureau of Medical Administration and Medical Service Supervision, National Health Commission of the People's Republic of China, all study participants, the original data creators, and Wuxiang Xie, Ph.D., Peking University Clinical Research Institute, Peking University First Hospital, Beijing, China.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.752717/full#supplementary-material
1. Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. (2013) 382:260–72. doi: 10.1016/S0140-6736(13)60687-X
2. Collaboration GCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study (2017). Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(19)32977-0
3. Calderon-Margalit R, Golan E, Twig G, Leiba A, Tzur D, Afek A, et al. History of childhood kidney disease and risk of adult end-stage renal disease. N Engl J Med. (2018) 378:428–38. doi: 10.1056/NEJMoa1700993
4. Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, et al. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. (2003) 111:e382–7. doi: 10.1542/peds.111.4.e382
5. Warady BA, Abraham AG, Schwartz GJ, Wong CS, Muñoz A, Betoko A, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am J Kidney Dis. (2015) 65:878–88. doi: 10.1053/j.ajkd.2015.01.008
6. Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. (2010) 5:2172–9. doi: 10.2215/CJN.07851109
7. Soares CM, Diniz JS, Lima EM, Oliveira GR, Canhestro MR, Colosimo EA, et al. Predictive factors of progression to chronic kidney disease stage 5 in a predialysis interdisciplinary programme. Nephrol Dial Transplant. (2009) 24:848–55. doi: 10.1093/ndt/gfn547
8. Litwin M. Risk factors for renal failure in children with non-glomerular nephropathies. Pediatr Nephrol. (2004) 19:178–86. doi: 10.1007/s00467-003-1329-4
9. Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. (2018) 71:783–92. doi: 10.1053/j.ajkd.2017.12.011
10. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. (2012) 27:363–73. doi: 10.1007/s00467-011-1939-1
11. Miller ME, Williams JA. Chronic renal failure in Jamaican children–an update (2001-2006). West Indian Med J. (2009) 58:231–4.
12. Huong NT, Long TD, Bouissou F, Liem NT, Truong DM. Nga do K, et al. Chronic kidney disease in children: the National Paediatric Hospital experience in Hanoi, Vietnam. Nephrology (Carlton). (2009) 14:722–7. doi: 10.1111/j.1440-1797.2009.01142.x
13. Ali el TM, Abdelraheem MB, Mohamed RM, Hassan EG, Watson AR. Chronic renal failure in Sudanese children: aetiology and outcomes. Pediatr Nephrol. (2009) 24:349–53. doi: 10.1007/s00467-008-1022-8
14. Vachvanichsanong P, Dissaneewate P, McNeil E. Childhood chronic kidney disease in a developing country. Pediatr Nephrol. (2008) 23:1143–7. doi: 10.1007/s00467-008-0775-4
15. Mong Hiep TT, Janssen F, Ismaili K, Khai Minh D, Vuong Kiet D, Robert A. Etiology and outcome of chronic renal failure in hospitalized children in Ho Chi Minh City, Vietnam. Pediatr Nephrol. (2008) 23:965–70. doi: 10.1007/s00467-008-0752-y
16. Bhimma R, Adhikari M, Asharam K, Connolly C. The spectrum of chronic kidney disease (stages 2-5) in KwaZulu-Natal, South Africa. Pediatr Nephrol. (2008) 23:1841–6. doi: 10.1007/s00467-008-0871-5
17. Gulati S, Mittal S, Sharma RK, Gupta A. Etiology and outcome of chronic renal failure in Indian children. Pediatr Nephrol. (1999) 13:594–6. doi: 10.1007/s004670050750
18. Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. (2015) 385:1975–82. doi: 10.1016/S0140-6736(14)61601-9
19. Powe NR. The pathogenesis of race and ethnic disparities: targets for achieving health equity. Clin J Am Soc Nephrol. (2021) 16:806–8. doi: 10.2215/CJN.12640820
20. Bignall ONR II, Crews DC. Stony the road we trod: towards racial justice in kidney care. Nat Rev Nephrol. (2021) 17:79–80. doi: 10.1038/s41581-020-00389-w
21. Shi X, Shi Y, Zhang L, Gan L, Zhong X, Huang Y, et al. Analysis of chronic kidney disease among national hospitalization data with 14 million children. BMC Nephrol. (2021) 22:195. doi: 10.1186/s12882-021-02383-1
22. Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in chronic kidney disease in China. N Engl J Med. (2016) 375:905–6. doi: 10.1056/NEJMc1602469
23. Zhang L, Zhao MH, Zuo L, Wang Y, Yu F, Zhang H, et al. China kidney disease network (CK-NET) 2016. Annual data report. Kidney Int Suppl (2011). (2020) 10:e97–185. doi: 10.1016/j.kisu.2020.09.001
24. Zhang L, Li J, Li N, Sun N, Xie L, Han Q, et al. Trends in cause-related comorbidities in hospitalized patients with secondary hypertension in China from 2013 to 2016: a retrospective analysis of hospital quality monitoring system data. J Hypertens. (2021) 39:2015–2021. doi: 10.1097/HJH.0000000000002891
25. Shen L, Wang S, Chen W, Fu Q, Evans R, Lan F, et al. Understanding the function constitution and influence factors on communication for the wechat official account of top tertiary hospitals in China: cross-sectional study. J Med Internet Res. (2019) 21:e13025. doi: 10.2196/13025
26. Liu LS. 2010 Chinese guidelines for the management of hypertension. Zhonghua Xin Xue Guan Bing Za Zhi. (2011) 39:579–615. doi: 10.3760/cma.j.issn.0253-3758.2011.07.002
27. Lurbe E, Cifkova R, Cruickshank JK, Dillon MJ, Ferreira I, Invitti C, et al. Management of high blood pressure in children and adolescents: recommendations of the European Society of Hypertension. J Hypertens. (2009) 27:1719–42. doi: 10.1097/HJH.0b013e32832f4f6b
28. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. (2004) 114(Suppl. 2):555–76. doi: 10.1542/peds.114.2.S2.555
29. Ritte RE, Lawton P, Hughes JT, Barzi F, Brown A, Mills P, et al. Chronic kidney disease and socio-economic status: a cross sectional study. Ethn Health. (2020) 25:93–109. doi: 10.1080/13557858.2017.1395814
30. Coppo R, Lofaro D, Camilla RR, Bellur S, Cattran D, Cook HT, et al. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. (2017) 32:139–50. doi: 10.1007/s00467-016-3469-3
31. Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. (2016) 9:583–91. doi: 10.1093/ckj/sfw047
32. Wühl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. (2009) 361:1639–50. doi: 10.1056/NEJMoa0902066
33. Behnisch R, Kirchner M, Anarat A, Bacchetta J, Shroff R, Bilginer Y, et al. Determinants of statural growth in european children with chronic kidney disease: findings from the cardiovascular comorbidity in children with chronic kidney disease (4C) study. Front Pediatr. (2019) 7:278. doi: 10.3389/fped.2019.00278
Keywords: chronic kidney disease, dialysis, progression, risk factor, hypertension
Citation: He G, Li C, Zhong X, Wang F, Wang H, Shi Y, Gan L and Ding J (2021) Risk Factors for Progression of Chronic Kidney Disease With Glomerular Etiology in Hospitalized Children. Front. Pediatr. 9:752717. doi: 10.3389/fped.2021.752717
Received: 03 August 2021; Accepted: 28 September 2021;
Published: 22 October 2021.
Edited by:
Constantinos J. Stefanidis, “Mitera” Children's Hospital, GreeceReviewed by:
Tracy E. Hunley, Monroe Carell Jr. Children's Hospital at Vanderbilt, United StatesCopyright © 2021 He, Li, Zhong, Wang, Wang, Shi, Gan and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Ding, ZGpuY181ODU1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.