
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 09 February 2022
Sec. Pediatric Immunology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.738215
Kawasaki disease (KD), also called mucocutaneous lymph node syndrome, is a febrile multisystem vasculitis mainly affecting children younger than 5 years. KD typically manifests as skin lesions and in the lymph nodes and oral and conjunctival mucosa. It may induce coronary artery abnormalities, such as aneurysms, but gastrointestinal and hepatobiliary involvement are not common. We reviewed 32 cases of patients with a diagnosis of KD with hepatobiliary involvement between 2000 and 2021 and present the case of a 4-year-old girl who received a diagnosis of KD with combined cholestatic hepatitis and Mycoplasma pneumoniae infection. In the 33 cases reviewed, in addition to the classical clinical findings of KD, the most common clinical presentations were jaundice and abdominal pain. Moreover, abnormal laboratory results indicating hyperbilirubinemia, cholestasis, and hepatitis, among other conditions, were noted. Abdominal ultrasonography revealed abnormal findings in more than half children with KD with hepatobiliary involvement. Furthermore, cardiac involvement was noted in a high proportion of the patients. In particular, we noted the case of a 4-year-old girl with a rare presentation of 3-day fever combined with abdominal pain and jaundice. Her levels of aspartate aminotransferase, alanine aminotransferase, total bilirubin, direct bilirubin, alkaline phosphatase, and γ-glutamyl transpeptidase were 489 (15–50) U/L, 253 (5–45) U/L, 4.3 (<1.5) mg/dl, 2.4 (<0.2) mg/dl, 337 (134–315) U/L, and 145 (5–32) U/L, respectively. These results were indicative of cholestatic hepatitis. Furthermore, her serological test results for mycoplasma infection were positive. KD was diagnosed because the patient had high fever for more than 5 days and presented with lymphadenopathy on the left side of neck, a polymorphic skin rash, redness of oral mucosa with strawberry tongue, and nonpurulent conjunctival congestion. After intravenous immunoglobulin injection (IVIG) and acetylsalicylic acid administration, the fever subsided rapidly and clinical manifestations, such as jaundice and abdominal pain, were mitigated. The laboratory parameters gradually returned to within normal ranges. Echocardiography revealed no aneurysm. In conclusion, KD with cholestatic hepatitis should be considered when pediatric patients present with fever combined with abdominal pain and jaundice. Early treatment with IVIG and aspirin is recommended and can effectively relieve cholestatic hepatitis.
Kawasaki disease (KD) is a multisystem inflammatory disease encompassing medium vessel vasculitis, potentially including the coronary arteries. The prevalence of KD among children in Japan between 1994 and 2002 and Taiwan between 1997 and 2010 was 119.6–151.2 and 48.5–82.8 per 100,000 person-years, respectively (1, 2). The average male–female ratio of KD was 4:1, with onset before the age of 5 years in 80% of cases (1). The etiology of KD remains unclear; one study reported immunologic abnormalities, but further investigation is required (3). KD diagnosis is based on various clinical measures provided by the American Heart Association and Japanese Ministry of Health (4, 5). Typically, KD symptoms include fever lasting at least 5 days combined with at least four of the following five manifestations: (i) conjunctival congestion in both eyes; (ii) changes in the lips and oral cavity, such as reddening of the lips, strawberry tongue, or diffuse injection of oral and pharyngeal mucosa; (iii) polymorphous exanthema; (iv) changes in the peripheral extremities; and (v) acute nonpurulent cervical lymphadenopathy and the exclusion of other diseases with similar findings (1). Alternatively, patients may present with three or fewer of these criteria along with coronary abnormalities or abnormal laboratory data, and this condition is classified as incomplete KD (4–6). Gastrointestinal and hepatobiliary involvement are rare clinical presentations in KD (7), and respiratory, neurological, genitourinary, and musculoskeletal involvement are also relatively uncommon clinical manifestations (1). The most common reason for sudden death in KD is cardiovascular involvement (1), but the administration of oral aspirin and intravenous immunoglobulin (IVIG) within 10 days of symptom onset can reduce the risk of coronary abnormalities (6).
Herein, we report the case of a girl presenting with an unusual initial onset of KD characterized by fever, acute abdominal pain, jaundice, and lymphadenopathy on the left side of the neck. This patient received early treatment with aspirin and IVIG. We also review 32 other cases of KD with hepatobiliary involvement reported between 2000 and 2021; thus, a total of 33 cases, including our case, were reviewed.
We conducted a literature search for relevant articles published in English between January 1, 2000, and November 1, 2021, in PubMed, Google Scholar, Crossref, and Web of Science by using the following keywords: (Kawasaki disease) AND (jaundice), (Kawasaki disease) AND (cholestasis), (Kawasaki disease) AND (cholestatic), (Kawasaki disease) AND (hepatitis). An article was excluded if no laboratory hepatobiliary data or intact image report were available or the patient was older than 18 years. Initially, we included 26 articles plus the article on the case reported herein, but three articles were then excluded. Finally, we reviewed 24 articles involving 33 cases (Table 1).
The patient was a girl aged 4 years and 2 months who was otherwise healthy before presenting with KD symptoms; she had achieved normal developmental milestones and received regular vaccinations. She did not have any relevant medical, psychosocial, or family history. She was admitted to our hospital because she had persistent high fever (≤ 38.5°C) for 3 days. Her parents stated that she had first developed diffuse abdominal pain and swelling on the left side of the neck and had initially been taken to a local general practitioner, who suspected acute gastroenterocolitis. Because of the persistent fever and subsequent development of jaundice, she was brought to our hospital for further evaluation. When admitted, she had poor appetite and vomiting and had developed symptoms of respiratory tract infection, such as rhinorrhea and coughing with sputum. Sore throat, dyspnea, diarrhea, constipation, dysuria, and reductions in urine output were not noted.
A physical examination revealed that the patient had a 39°C fever, scleral icterus in both eyes, nonpurulent conjunctival congestion, oral mucosa redness, swelling on the left side of the neck, diffuse abdominal tenderness in the periumbilical area, and crackles in the lungs. Chest X-ray revealed an elevated level of pulmonary infiltrates in both lungs (Figure 1A). Her laboratory data were as follows: hemoglobin = 10.2 (normal range = 11.0–14.5) g/dl, leukocytes = 24,700 (4,000–12,000) 106/L, thrombocytes = 250,100 (130,000–400,000) 106/L, C-reactive protein (CRP) = 14.17 (<1) mg/dl, erythrocyte sedimentation rate = 95 (0–20) mm/h, prothrombin time = 17.3 (11.0–14.5) s, international normalized ratio = 1.32 (<1.20), activated partial thromboplastin time = 45.8 (32.0–45.0) s, aspartate aminotransferase = 489 (15–50) U/L, alanine aminotransferase = 253 (5–45) U/L, total bilirubin = 4.3 (<1.5) mg/dl, direct bilirubin = 2.4 (<0.2) mg/dl, alkaline phosphatase = 337 (134–315) U/L, γ-glutamyl transpeptidase = 145 (5–32) U/L, amylase = 112 (26–115) U/L, lipase = 58 (22–51) U/L, blood urea nitrogen = 11 (5–18) mg/dl, serum creatinine = 0.49 (0.20–0.60) mg/dl, albumin = 3.9 (3.5–5.6) g/dl, ceruloplasmin = 24.2 (20–60) mg/dl, and Cu = 860 (700–1,500) μg/L. Her serological test results revealed positivity for Mycoplasma pneumoniae [M. pneumoniae; immunoglobulin G = 1,810.79 (<100.00) 103U/L and immunoglobulin M = 3,381.64 (<770.00) 103U/L] but negativity for Epstein–Barr virus, cytomegalovirus, and hepatitis A, B, and C. Furthermore, tests for antinuclear antibodies, rheumatoid factor, antineutrophil cytoplasmic antibodies, and antistreptolysin O were negative, as were the results of the influenza A/B and group A streptococcus antigen screening test and pneumococcal urinary antigen testing. Nasal swab and stool tests revealed negative results for adenovirus, and results for rotavirus were also negative. The stool microscopy results were normal. Samples were collected for culture, and the patient was treated with 80 mg/kg ceftriaxone for enteric fever and cholestatic hepatitis and with azithromycin for bronchopneumonia (induced by the M. pneumoniae infection). Treatment response was poor, and the fever persisted. Abdominal ultrasonography indicated acute gastroenterocolitis with ileus and pelvic ascites but without cystic duct or bile duct dilatation. Neck tissue ultrasonography revealed multiple lymphadenopathies on the left side of the neck (largest dimensions: 2.08 cm × 1.49 cm; Figure 1B).
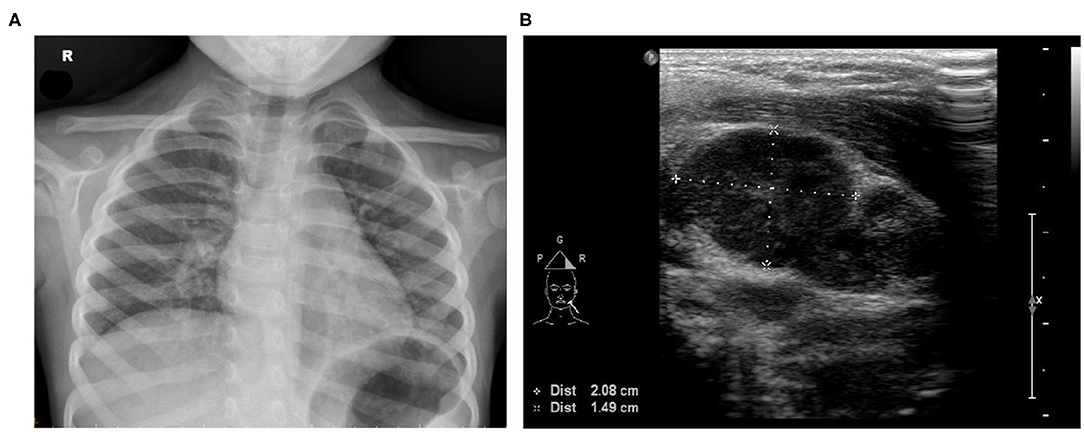
Figure 1. (A) Chest X-ray image exhibiting increased bronchoalveolar infiltration over the right lung field. (B) Neck tissue echography revealing multiple lymphadenopathy on the left side of the neck (largest dimensions: 2.08 × 1.49 cm).
The high fever persisted for 2 days after admission (>5 days total), and a polymorphic skin rash developed, accompanied by lymphadenopathy on the left side of the neck, oral mucosa redness with strawberry tongue, and nonpurulent conjunctival congestion. The patient received a diagnosis of KD, and a single dose of IVIG as a 12-h 2 g/kg intravenous infusion and acetylsalicylic acid (50 mg/kg/day, divided into four doses) was administered. Echocardiography revealed no coronary artery involvement. After the administration of IVIG and aspirin, the fever and abdominal pain subsided and the jaundice and skin rash gradually resolved. Acetylsalicylic acid was reduced to one dose of 3–5 mg/kg at 48 h after the fever had subsided, and liver function improved gradually. Further laboratory examination results are presented in Table 2. The patient was discharged and followed up at our outpatient department. Abnormal laboratory data gradually returned to normal, and the echocardiography results for the coronary arteries in the outpatient department follow-up were normal.
In KD, hepatobiliary involvement is an atypical presentation. Our patient was a girl aged 4 years and 2 months with KD. In addition to fever, a classical symptom of KD, our patient had lymphadenopathy on the left side of the neck, a polymorphic skin rash, redness of the oral mucosa with strawberry tongue, and nonpurulent conjunctival congestion. Echocardiography indicated no coronary artery involvement. Jaundice with abdominal pain was diagnosed early, and febrile cholestatic hepatitis was also noted. Studies have reported different rates of diarrhea, vomiting, abdominal pain, hepatic dysfunction, and bile duct hydrops as nonspecific symptoms of KD, although these symptoms are not among the diagnostic criteria (4, 8, 9). In the present case, we observed the development of cholestatic hepatitis. Our findings indicate that cholestatic hepatitis can be an atypical early sign of KD, and early treatment with IVIG can rapidly improve liver function, cholestasis, and other conditions and also prevent cardiac complications.
In this study, in addition to the reported case, we reviewed another 32 cases of KD with hepatobiliary involvement reported between 2000 and 2021 in studies retrieved from PubMed, Google Scholar, Crossref, and Web of Science; the details of these studies are presented in Tables 3, 4 (7, 9–30). The mean age of the pediatric patients in the reports was 5.26 years. The age at the onset of KD with hepatobiliary involvement was later than the average age at KD onset, with 73 and 27% patients presenting with classical and incomplete KD, respectively. In addition to the classical clinical findings, the most common clinical presentations in these patients were jaundice and abdominal pain. In total, 76% of the patients presented with jaundice and 48% presented with abdominal pain. Laboratory data indicated hyperbilirubinemia, cholestasis, hepatitis, and other abnormalities. For five patients, direct bilirubin data were not available, and for two, total bilirubin data were not available; however, all the other patients exhibited an increase in direct bilirubin and total bilirubin. Furthermore, all the patients except three (for whom serum inflammatory marker data were not available) exhibited an increase in serum inflammatory markers. In total, 91% of the patients had increased levels of liver enzymes. However, γ-glutamyl transpeptidase activity was examined in 24 patients, all of whom had high cholestatic indexes. The 33 patients were all prescribed IVIG and oral acetylsalicylic acid when KD was diagnosed. After treatment, fever subsidence was noted for all these patients, and rapid regression of jaundice was noted in 96% of these patients. Although jaundice was identified in most of the cases, not all had notable results for abdominal ultrasonography and echocardiography. Moreover, 33% of the patients had an enlarged liver, 18% had a thickened gallbladder wall, and one patient was revealed to have intrahepatic bile duct stasis through abdominal ultrasonography. Despite the diverse abdominal ultrasonography results, only one of the patients had autoimmune sclerosing cholangitis; the others all had negative abdominal ultrasonography results after follow-up. In addition, 39% of the patients had dilated coronary arteries, resulting in aneurysms. Preventing coronary artery aneurysm is crucial because it may cause stenosis of the vessels, often resulting in coronary artery obstruction and myocardial ischemia (1). Furthermore, 30% of the patients had other comorbidities. Overall, abnormal abdominal ultrasonography findings were obtained for 61% of the patients, and echocardiography revealed cardiac involvement in 45% of the patients. A high proportion of children with KD with hepatobiliary involvement appear to have cardiac involvement, but the physiopathology of hyperbilirubinemia and cholestatic liver damage in KD remains undetermined. Vasculitis-associated inflammation and obstruction in the liver and gallbladder are considered the cause of increased transaminase levels and cholestasis (9). Increased serum γ-glutamyl transpeptidase activity is a marker of cholangiocyte injury and is indicative of bile duct damage. Acute hydrops of the gallbladder in some patients suggests that inflammatory cholangiocyte injury may extend from small to large bile ducts (17). However, hydrops of the gallbladder is an atypical sign of KD (1). Children with KD develop isolated gall bladder hydrops without bilirubin or liver transaminase elevation, which should also be considered. Abdominal ultrasonography in 91% of the patients reviewed did not indicate hydrops of the gallbladder, and cholestasis in 96% of these patients was mitigated after IVIG treatment.
Regarding the pediatric diagnosis of KD, a differential diagnosis may be required given that KD is diagnosed on the basis of clinical criteria; no specific diagnostic test has been developed. Various organ systems are involved in atypical KD, including the central nervous system and ocular, renal, joint, and gastrointestinal systems. Abdominal manifestations are sometimes acute, and severe abdominal pain similar to that associated with appendicitis or pancreatitis may lead to a surgical intervention (28). Such atypical manifestations of KD may delay treatment. Prompt KD diagnosis is crucial because IVIG administration within 10 days of the onset of fever results in a lower rate of coronary abnormalities and lowering of the risk of coronary artery aneurysm formation from 20–25% to 3–5% in those who are appropriately treated (9, 31, 32).
The etiology of KD remains unclear. Immunological responses are reported to be associated with KD, but the immunological profile associated with KD has yet to be defined (1). Furthermore, an infection-induced immunologic response may be considered a predisposing factor; according to some case reports, KD may be associated with M. pneumoniae infection (33–35). M. pneumoniae has several arrays for immunomodulation, including T cell and B cell activation and cytokine oversecretion, which are thought to trigger KD (36). M. pneumoniae infection can have a wide spectrum of extrapulmonary manifestations (including dermatological) that may mimic KD (37). A study reported that acute hepatitis can be included in the heterogeneous group of extrapulmonary diseases related to M. pneumoniae infection (38). The pathogenesis of hepatitis related to M. pneumoniae infection is likely to be immune mediated (38), but the clinical manifestations of KD, including strawberry tongue and jaundice, are not a clinical extrapulmonary manifestation of M. pneumoniae infection (1, 37). M. pneumoniae infection may be a trigger for KD leading to hepatobiliary problems. The exact pathological mechanisms and whether M. pneumoniae infection is related to KD warrant further investigation. Notably, KD combined with M. pneumoniae infection may present as a more severe clinical condition; thus, early treatment of the infection is essential.
This study has limitations. Not all the cases that we reviewed had complete hepatobiliary data. The child in the reported case was not tested for hepatitis E, and complete work-up for autoimmune hepatitis was not performed. However, the presence of these conditions was unlikely.
Jaundice is a critical differential diagnosis of KD, a disease requiring prompt treatment with IVIG and aspirin to alleviate symptoms and prevent fatal cardiac complications. Even if coronary abnormalities are not initially observed, cardiac involvement is highly likely in patients with KD and hepatobiliary involvement and must be treated without delay. Physicians should consider the presence of this condition when examining children presenting with febrile cholestatic hepatitis.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Taipei Medical University-Joint Institutional Review Board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
S-WH cared the patient, drafted the initial manuscript, and approved the final manuscript as submitted. S-CL diagnosed, cared and treated the patient, also conceived, drafted, reviewed, and revised the manuscript, and approved the final manuscript as submitted. S-YC and K-SH are consultants and approved the final manuscript as submitted. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
KD, Kawasaki disease; IVIG, intravenous immunoglobulin.
1. Kawasaki T. Kawasaki disease. Proc Jpn Acad Ser B Phys Biol Sci. (2006) 82:59–71. doi: 10.2183/pjab.82.59
2. Lin MC, Lai MS, Jan SL, Fu YC. Epidemiologic features of Kawasaki disease in acute stages in Taiwan, 1997–2010: Effect of different case definitions in claims data analysis. J Chin Med Assoc. (2015) 78:121–6. doi: 10.1016/j.jcma.2014.03.009
3. Matsubara T, Ichiyama T, Furukawa S. Immunological profile of peripheral blood lymphocytes and monocytes/macrophages in Kawasaki disease. Clin Exp Immunol. (2005) 141:381–7. doi: 10.1111/j.1365-2249.2005.02821.x
4. Singh S, Jindal AK, Pilania RK. Diagnosis of Kawasaki disease. Int J Rheum Dis. (2018) 21:36–44. doi: 10.1111/1756-185X.13224
5. Jindal AK, Pilania RK, Prithvi A, Guleria S, Singh S. Kawasaki disease: characteristics, diagnosis, and unusual presentations. Expert Rev Clin Immunol. (2019) 15:1089–104. doi: 10.1080/1744666X.2019.1659726
6. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. (2004) 110:2747–71. doi: 10.1161/01.CIR.0000145143.19711.78
7. Valentini P, Ausili E, Schiavino A, Angelone DF, Focarelli B, Rosa GD, et al. Acute cholestasis: atypical onset of Kawasaki disease. Dig Liver Dis. (2008) 40:582–4. doi: 10.1016/j.dld.2007.10.010
8. Baker AL, Lu M, Minich LL, Atz AM, Klein GL, Korsin R, et al. Associated symptoms in the ten days before diagnosis of Kawasaki disease. J Pediatr. (2009) 154:592–5.e2. doi: 10.1016/j.jpeds.2008.10.006
9. Kiliç BO, Baysun S, Gökşen TC, Akinbingöl I, Arslan Z. An unusual presentation of Kawasaki disease: gallbladder hydrops and acute cholestatic hepatitis. Case Rep Med. (2018) 2018:4930234. doi: 10.1155/2018/4930234
10. Anjani G, Deglurkar R, Pilania R, Chaudhary H, Vaiphei K, Vignesh P, et al. Fulminant acute liver failure as an unusual presentation of Kawasaki disease. Scand J Rheumatol. (2021) 50:327–9. doi: 10.1080/03009742.2020.1812711
11. Morita A, Imagawa K, Ishiodori T, Tagawa M, Takada H. Kawasaki disease with dilatation of the common bile duct: A case report and review of literature. Int J Rheum Dis. (2021) 24:1325–30. doi: 10.1111/1756-185X.14208
12. Menon J, Shanmugam N, Vasudevan A, Kumar N, Rammohan A, Rela M. Kawasaki disease in a pediatric liver transplant patient. Transplant Immunology. (2021) 67:101416. doi: 10.1016/j.trim.2021.101416
13. Paglia P, Nazzaro L, Anseris D, Elena AG, Lettieri M, Colantuono R, et al. Atypically protracted course of liver involvement in Kawasaki disease. Case Report and Literature Review Pediatric Reports. (2021) 13:357–62. doi: 10.3390/pediatric13030044
14. Sarkar S, Bhattacharyya B, Agarwal A. Jaundice at the onset: A rare event in Kawasaki disease. Indian J Pediatr. (2021) 88:379–80. doi: 10.1007/s12098-020-03559-7
15. Bylund W, Zarow GJ, Ponce DM. Acute jaundice in a six-year-old: an unusual presentation of atypical Kawasaki disease. Clin Pract Cases Emerg Med. (2020) 4:142–5. doi: 10.5811/cpcem.2019.12.45180
16. Martínez Vázquez JA, Sánchez García C, Rodríguez Muñoz L, Martínez Ramírez RO. Acute kidney injury and cholestasis associated with Kawasaki disease in a 9-year-old: Case report. Reumatol Clin (Engl Ed). (2019) 15:e114–5. doi: 10.1016/j.reumae.2017.11.003
17. Koca T, Aslan N, Akaslan Kara A, Pektas A, Ozen M, Akcam M. Kawasaki disease in a 9-year old girl presenting with febrile cholestasis: case report and review of literature. Int J Rheum Dis. (2018) 21:2046–9. doi: 10.1111/1756-185X.12700
18. Goknar N, Demir AD, Ataman Y, Gokalp S, Oktem F, Kasapcopur O, et al. case of Kawasaki disease with initial presentation of arthritis and icterus. Bezmialem sci. (2017) 5:86–9. doi: 10.14235/bs.2016.939
19. Kaman A, Aydin-Teke T, Gayretli-Aydin ZG, Öz FN, Metin-Akcan Ö, Eriş D, et al. Two cases of Kawasaki disease presented with acute febrile jaundice. Turk J Pediatr. (2017) 59:84–6. doi: 10.24953/turkjped.2017.01.015
20. Keeling IM, Beran E, Dapunt OE. Kawasaki disease and hepatobiliary involvement: report of two cases. Ital J Pediatr. (2016) 42:1–3. doi: 10.1186/s13052-016-0238-7
21. Rosencrantz RA, Huang T, Sonke PY, Tewari D, Chander PN. Autoimmune sclerosing cholangitis: An atypical association with kawasaki disease. Hepatology. (2016) 64:2253–6. doi: 10.1002/hep.28694
22. Perera PJ, Samarasinghe D, Pathirana D, Randeni S, Samdamal LYS. An atypical case of Kawasaki disease presenting with cholestatic jaundice. Sri Lanka J Child Health. (2015) 44:58–60. doi: 10.4038/sljch.v44i1.7965
23. Talebian A, Soltani B, Rezaei MH. Jaundice as an unusual presentation of Kawasaki disease: A case report. Arch Pediatr Infect Dis. (2015) 3:e27594. doi: 10.5812/pedinfect.27594
24. Grewal A, Singh S, Suri D, Lal S, Manojkumar R, Thapa BR. Kawasaki disease masquerading as jaundice. Indian J Pediatr. (2013) 80:261–2. doi: 10.1007/s12098-012-0736-6
25. Jafari SA, Kiani MA, Ahanchian H, Khakshour A, Partovi S, Kianifar HR, et al. Kawasaki disease presenting as acute clinical hepatitis. Int J Pediatr. (2013) 1:51–3.
26. Karpathios T, Moustaki M, Yiallouros P, Sharifi F, Attilakos A, Papadopoulou A, et al. Severe jaundice in two children with Kawasaki disease: a possible association with Gilbert syndrome. J Korean med sci. (2012) 27:101–3. doi: 10.3346/jkms.2012.27.1.101
27. Ibáñez-Alcalde M, Sánchez-Forte M, Giménez-Sánchez F, Ortega-Montes Á, Martínez-Espinosa G. Cholestasis as the initial feature of Kawasaki disease. Pediatr Infect Dis J. (2012) 31:766–7. doi: 10.1097/INF.0b013e318253a1d8
28. Taddio A, Pellegrin MC, Centenari C, Filippeschi IP, Ventura A, Maggiore G. Acute febrile cholestatic jaundice in children: keep in mind Kawasaki disease. J Pediatr Gastroenterol Nutr. (2012) 55:380–3. doi: 10.1097/MPG.0b013e31825513de
29. Grech V, Buttigieg T, Portelli A, Kotes S, Attard G, Huhn P, et al. Kawasaki disease presenting as hepatitis. Ann Trop Paediatr. (2007) 27:303–6. doi: 10.1179/146532807X245706
30. Chen WT, Huang SR, Wang JK. Kawasaki disease presenting with hepatitis and prolonged fever: report of one case. Acta Paediatrica Taiwanica. (2003) 44:174–6.
31. Bal AK, Prasad D, Umali Pamintuan MA, Mammen-Prasad E, Petrova A. Timing of intravenous immunoglobulin treatment and risk of coronary artery abnormalities in children with Kawasaki disease. Pediatr Neonatol. (2014) 55:387–92. doi: 10.1016/j.pedneo.2013.11.007
32. Rife E, Gedalia A. Kawasaki Disease: an Update. Curr Rheumatol Rep. (2020) 22:75. doi: 10.1007/s11926-020-00941-4
33. Lee MN, Cha JH, Ahn HM, Yoo JH, Kim HS, Sohn S, et al. Mycoplasma pneumoniae infection in patients with Kawasaki disease. Korean J Pediatr. (2011) 54:123–7. doi: 10.3345/kjp.2011.54.3.123
34. Tang Y, Yan W, Sun L, Huang J, Qian W, Hou M, et al. Kawasaki disease associated with Mycoplasma pneumoniae. Ital J Pediatr. (2016) 42:83. doi: 10.1186/s13052-016-0292-1
35. Vitale EA, La Torre F, Calcagno G, Infricciori G, Fede C, Conti G, et al. Mycoplasma pneumoniae: a possible trigger of Kawasaki disease or a mere coincidental association? Report of the first four Italian cases. Minerva Pediatr. (2010) 62:605–7.
36. Narita M. “Mycoplasma pneumoniae as an under-recognized agent of vasculitic disorders,” in Advances in the Etiology, Pathogenesis and Pathology of Vasculitis, ed L. M. Amezcua-Guerra (Rijeka: In Tech). (2011) 37–56. Available online at: http://www.intechopen.com/books/advances-in-the-etiologypathogenesis-and pathology-of-vasculitis/mycoplasma-pneumoniae-as-anunder-recognized-agent-of-vasculitic disorders. doi: 10.5772/22875
37. Narita M. Classification of Extrapulmonary Manifestations Due to Mycoplasma pneumoniae Infection on the Basis of Possible Pathogenesis. Front Microbiol. (2016) 7:23. doi: 10.3389/fmicb.2016.00023
Keywords: Kawasaki disease (KD), cholestatic hepatitis, jaundice, abdominal pain, Mycoplasma pneumoniae infection
Citation: Huang S-W, Lin S-C, Chen S-Y and Hsieh K-S (2022) Kawasaki Disease With Combined Cholestatic Hepatitis and Mycoplasma pneumoniae Infection: A Case Report and Literature Review. Front. Pediatr. 9:738215. doi: 10.3389/fped.2021.738215
Received: 08 July 2021; Accepted: 13 December 2021;
Published: 09 February 2022.
Edited by:
Ankur Kumar Jindal, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaReviewed by:
Rakesh Kumar Pilania, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaCopyright © 2022 Huang, Lin, Chen and Hsieh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sheng-Chieh Lin, amFja2xpbm1haWxzQHlhaG9vLmNvbS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.