
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 22 September 2021
Sec. Pediatric Cardiology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.729198
 Hatem Rouatbi1
Hatem Rouatbi1 Nesrine Farhat1
Nesrine Farhat1 Ruth Heying2
Ruth Heying2 Jaime F. Vazquez-Jimenez3
Jaime F. Vazquez-Jimenez3 Anne-Simone Parent4
Anne-Simone Parent4 Marie-Christine Seghaye1*
Marie-Christine Seghaye1*Background: Estrogen receptors (ERs) relate to cardio-protection in adults, but their role in younger patients is not known. We aimed to assess the myocardial expression of ERα- and ERβ- mRNA in young patients with congenital cardiac disease and to analyze their putative protective role.
Patients and Methods: Twenty children and young adults (seven females and 13 males) with a median age of 13.8 years (interquartile range: 12.3 years) were enrolled in this prospective study. The myocardial expression of ER-mRNA and genes involved in inflammation, growth, and stress response was assessed by real-time PCR and was correlated to post-operative (po) outcome.
Results: ER-mRNA was detected in the myocardium of all patients, independently of gender and age. The expression of ER-mRNA correlated with that of mRNA coding for brain natriuretic peptide and for all cytokines tested. A higher ERα-mRNA expression correlated with lower troponin T concentrations at 24 h po (p = 0.032), higher PaO2/FiO2 ratio at 4 h po (p = 0.059), lower fluid retention at 4 h po (p = 0.048), and lower aspartate aminotransferase (AST) levels at 24 h po (p = 0.047). A higher ERβ-mRNA expression was also correlated with lower fluid retention at 24 h po (p = 0.048).
Patients in whom the levels of ERα- and ERβ-mRNA were >P50 had lower troponin T (p = 0.003, respectively) and lower AST concentrations at 24 h po (p = 0.043, respectively) than the others.
Conclusions: The expression of ERα- and ERβ-mRNA is present in the myocardium of children and young adults with congenital cardiac defect and is associated with lower markers of po organ damage. This suggests that ERs may provide perioperative organ protection in this population.
Estrogens are pleiotropic steroids with cardio-protective properties (1) that are related to vasodilation, anti-inflammatory, and anti-oxidant effects, inhibition of proliferation, and increased cell survival (2).
The physiological effects of estrogens are mediated by estrogen receptors (ERs) that possess a complex signaling that is not fully understood yet (3). It is admitted that the nuclear receptors ERα and ERβ are responsible for the genomic effects of estrogen and initiate ligand-activated transcription by binding estrogen receptor elements (ERE) to the promoter and regulatory regions of target genes. ERα and ERβ are encoded by two separate genes and have a different distribution within tissues and cells, including circulating cells (4). Both receptors are expressed in cardiomyocytes, smooth muscle cells, and endothelial cells and elicit different actions on the cardiovascular system (5). The ratio of their respective tissue concentrations is thought to play a crucial role in the biological response to estrogen (6). ERα and ERβ are not only located into the nucleus but also in cell membrane caveolae and exert acute effects by activating non-nuclear signaling pathways such as PI3K/Akt kinase and ERK1/2 (7). Besides nuclear receptors, estrogens also bind to a membrane receptor called G-protein coupled ER (GPER) present in cardiomyocytes that initiates rapid non-nuclear signaling (8). Estrogen activity involves a cross-talk and collaboration between nuclear and membrane signaling (9).
It is not known as yet whether the myocardium of children expresses ER, while the brain tissue of pre-pubertal children does. Indeed a role of ERs in the pathophysiology of autism in young children has been suggested (10), implying a ligand-independent activity (11) or the activation of ERs by a variety of exogenous receptor ligands, such as phytoestrogens, metallo-estrogens, and xeno-estrogens (9), that pre-pubertal individuals are naturally exposed to.
In adults, the expression of ERs is increased in pressure-loaded myocardium (12), while in children with congenital cardiac disease, hemodynamic overload induces the expression of genes involved in early stress response, inflammation, apoptosis, and fibrosis (13).
This prompted us to address the question of whether ERs would also be expressed in the myocardium of children and young adults with congenital cardiac defect and would interact with the inflammatory response to hemodynamic overload.
Our study was therefore designed to assess, as primary objective, the expression of mRNA coding for ERα and ERβ in the right atrial myocardium of children and young adults with congenital cardiac defect and to correlate this expression to that of mRNA coding for inflammatory cytokines and markers of myocardial stress involved in the pathophysiology of myocardial remodeling.
The secondary objective was to test the hypothesis that myocardial ER expression would relate to myocardial protection and influence the post-operative outcome.
After an approval by the Human Ethical Committee of the Aachen University of Technology and informed consent of the caregivers or the patients, if applicable, 20 consecutive patients (seven females and 13 male) with a median age of 13.8 years (minimum: 3 months, maximum 26.5 years; interquartile range, IQR: 12.3 years) were enrolled in this prospective study. Eight patients were younger than 12 years and pre-pubertal, 12 were older than 12 years and had reached puberty. No patient was on estrogen/progestogen combination. Table 1 summarizes the characteristics of the subjects.
All surgical procedures were performed by the same pediatric cardiac surgeon. In all cases, conventional general anesthesia consisted of isoflurane and sufentanyl. Dexamethasone (1 mg/m2 body surface area) was given before the sternotomy. Perioperative antibiotic prophylaxis was carried out with cefuroxime. Before the institution of a cardiopulmonary bypass (CPB), a right atrial biopsy was taken. After the institution of a moderate hypothermic low-flow CPB, the aorta was cross-clamped, and cardiac arrest was instituted by intra-aortal injection of 4 °C cold cardioplegic solution (Bretschneider, 30 ml/kg body weight) that was re-aspirated in the right atrium. After the intra-cardiac repair, the patient was weaned from CPB under progressive re-warming. Epicardiac pacemaker leads and pericardial and mediastinal drains were placed before chest closure.
The arterial blood pressure and central venous pressure were continuously monitored via an arterial and a central venous line, respectively.
Inotropic support, which consisted of dobutamine, was given to maintain a normal mean arterial blood pressure for age and volume therapy by injections of crystalloid solutions, if requested. The patient was transported to the intensive care unit where weaning from artificial ventilation was begun as early as possible. The ratio between the arterial partial pressure of oxygen (PaO2) and the fraction of inspired oxygen (FiO2) was used to assess oxygenation. Diuresis was continuously monitored via a bladder catheter, and water balance was calculated hourly.
The routinely performed laboratory investigations included the determination of blood gases, blood concentration of lactate, glycemia, complete blood count, serum creatinine, aspartate aminotransferase (AST), troponin T, and coagulation parameters and were measured at least 4 and 24 h post-operatively.
Biopsies taken for the detection of messenger ribonucleic acid (mRNA) were immediately snap-frozen in liquid nitrogen and stored at −80 °C until analysis.
Total ribonucleic acid (RNA) was extracted from the atrial myocardium by using the RNeasy kit (QIAGEN Inc., Hilden, Germany). The RNA (100 ng) was reverse-transcribed to complementary deoxyribonucleic acid (DNA) with random hexamers. A 2-μl cDNA sample was incubated with 20 μl of QuantiTect Mix containing fluorescence dye SYBR® Advantage® qPCR premix from Clontech (Takara Bio Inc. Otsu, Shiga, Japan).
The expression of target genes was normalized to the levels of 18S-mRNA and calculated with 2−ΔCT. Besides the expression of mRNA coding for ERα and ERβ, the expression of mRNA coding for the pro-inflammatory cytokine tumor necrosis factor-a (TNFα), interleukin (IL)-1β, for the regulator of the acute phase reaction that shares pro- and anti-inflammatory properties, IL-6, for chemokine IL-8, for the anti-inflammatory cytokine IL-10, for the growth factor and major regulator of fibrosis tissue growth factor (TGF)-β, for the main growth factor of cardiomyocytes cardiotrophin (CT)-1, and for the early marker of myocardial stress brain natriuretic peptide (BNP) was quantified. The primers used are listed in Table 2.
Results are given as median and interquartile range (median, IQR). Data analysis was done using the Statistical Product and Service Solutions program, version 25 (SPSS®, IBM, USA). The non-normal distribution of data was verified, and non-parametric tests were applied. Correlations between two independent parameters were analyzed by using Spearman's rank correlation test, and the results were reported by Spearman's rank coefficient (rs). The Mann–Whitney U-test was used to compare the clinical and biological parameters in two different groups. P ≤ 0.05 were considered significant, while p < 0.1 indicated a tendency toward significance.
The female patients were younger than the male patients [4 years (12) vs. 9.9 years (10), p = 0.047].
The myocardial expression of mRNA coding for ERα and ERβ was detected in all patients, independently of gender, age, and achieved puberty, respectively. In the whole cohort, the expression of ERβ-mRNA was significantly higher than that of ERα-mRNA [4.89 (0.49) vs. 4.22 (0.40), p < 0.0001]. The myocardial expression of mRNA coding for BNP and for all tested cytokines was also detected in all patients and was not influenced by gender or age except that of TGF-β that was significantly higher in males than in females (p = 0.024) and correlated with age (rs: 0.490, p = 0.028). TGF-β-mRNA was also higher in patients who had achieved puberty than in the others (p = 0.047).
The ERα-mRNA and ERβ-mRNA levels correlated with each other (rs: 0.922, p < 0.0001) and with the levels of mRNA coding for inflammatory cytokines (TNFα, IL-1β, IL-6, IL-8, and IL-10), growth factors (CT-1 and TGF-β), and the marker of myocardial stress (BNP), respectively (Table 3). Figure 1 shows the correlations between the myocardial levels of ERα-mRNA and IL-6-mRNA and between ERα-mRNA and IL-10-mRNA, respectively. Figure 2 shows the correlation between the myocardial levels of ERα-mRNA and BNP-mRNA.
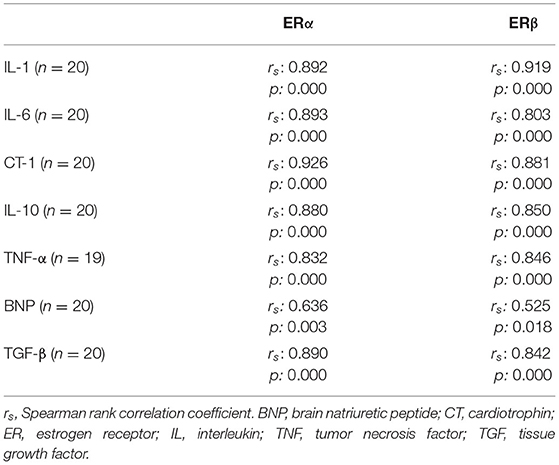
Table 3. Correlation between myocardial expression of mRNA coding for ERα and for inflammatory cytokines, growth factors and early stress response genes.
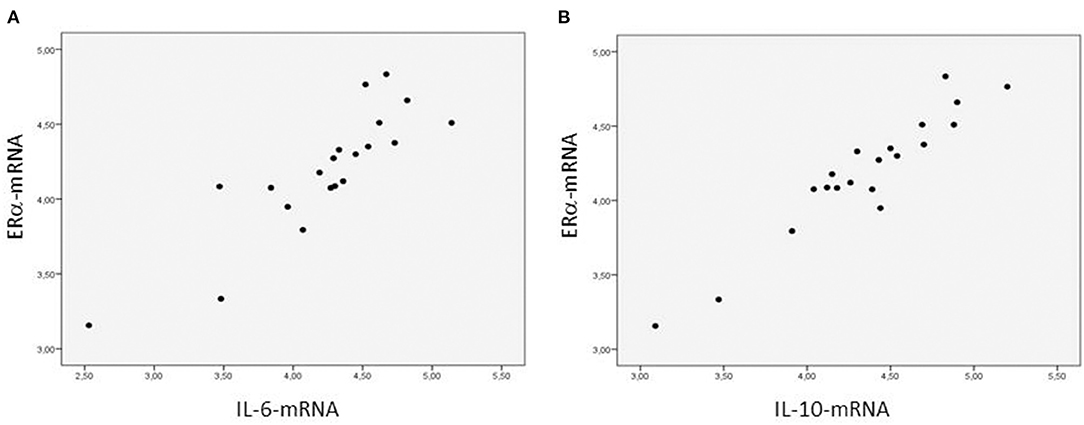
Figure 1. Relationship between the myocardial expression of ERα- and IL6-mRNA (A) and between the myocardial expression of ERα- and IL10-mRNA (B). N = 20. Spearman correlation coefficient = 0.893 (A) and = 0.880 (B), p < 0.0001, respectively. The mRNA expression of the target gene is corrected for that of 18S.
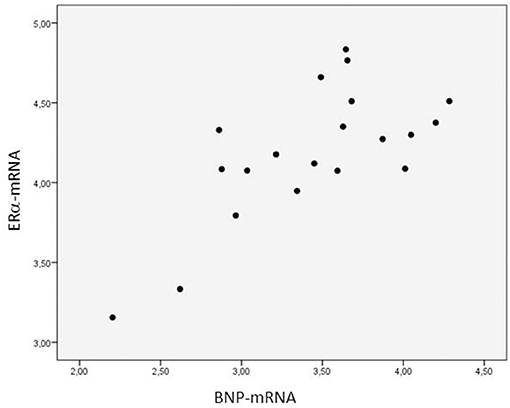
Figure 2. Relationship between the myocardial expression of ERα- and BNP-mRNA. N = 20. Spearman correlation coefficient: 0.930, p = 0.021. The expression of mRNA of the target gene is corrected for that of 18S.
The outcome variables were not different in males than in females except the creatinine concentration at 4 and 24 h post-operatively that was lower in females (p = 0.007 and p = 0.01, respectively). The outcome variables were not correlated with age except the creatinine concentration at 4 and 24 h post-operatively (rs: 0.812 and 0.804, respectively; p < 0. 0001, respectively).
The expression of ERα-mRNA correlated negatively with the troponin T concentration at 24 h after the operation (rs:−0.505, p = 0.032) (Figure 3), positively with the ratio PaO2/FiO2 calculated at 4 h post-operatively (rs: 0.453, p = 0.059), negatively with the AST levels measured 24 h post-operatively (rs: −0.489, p = 0.047), and negatively with the water balance at 4 h post-operatively (rs: −0.487, p = 0.048).

Figure 3. Relationship between the myocardial expression of ERα-mRNA and troponin T blood levels 24 h post-operatively. N = 18. Spearman correlation coefficient: −0.505, p = 0.032. The expression of mRNA of the target gene is corrected for that of 18S.
The patients were divided in two groups (n = 10 each) depending on whether their levels of ERα- and ERβ-mRNA expression was greater or less than the median value (percentile, P, 50) and were compared to each other with respect to the post-operative outcome variables. The patients with ERα- and ERβ-mRNA expression >P50 showed lower troponin T levels at 4 and 24 h po than the others [0.53 ng/ml (0.69) and 0.28 ng/ml (0.09) vs. 0.88 ng/ml (2.69) and 0.79 ng/mL (0.78); p = 0.003, respectively] and lower AST concentrations at 4 and 24 h po [51.5 IU/L (19.7) and 58 IU/L (14); p = 0.043, respectively]. Figure 4 shows the troponin T levels in patients with ERα-mRNA expression less than or greater than P50.
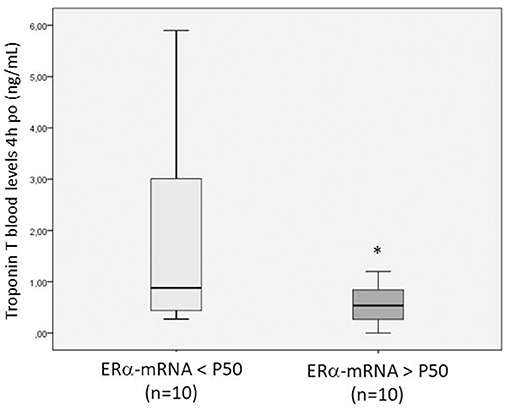
Figure 4. Box plot showing the troponin T blood levels measured 4 h post-operatively in patients with ERα-mRNA expression <P50 (light gray) or >P50 (dark gray). The box plot summarizes the minimal, maximal, and median (P50) values and interquartile range. *p = 0.043 between both groups.
We show for the first time that mRNA coding for ERα- and ERβ is expressed in the right atrium of children and young adults with congenital heart defects independently of age, gender, and achieved puberty, respectively.
We also confirm our previous results demonstrating the myocardial expression of mRNA coding for pro- and anti- inflammatory cytokines, factors regulating cell growth and fibrosis, and proteins involved in the early cellular stress responses as a consequence of the activation of inflammatory pathways by hemodynamic overload (14). In this series, the expression of TGF-β increased with age, pointing to the importance of the duration of hemodynamic load on maladaptive myocardial remodeling and myocardial fibrosis (15).
Furthermore, the concentrations of mRNA coding for the different proteins tested correlated well with each other, suggesting an interplay between ERs and the mediators of inflammation, growth, fibrosis, and stress response of myocardial cells and therefore the participation of ERs in the complex mechanisms of myocardial remodeling in patients with congenital cardiac disease (13). In particular, the relationship between the expression of ER-mRNA and BNP-mRNA might indicate a role of hemodynamic overload in the upregulation of ERs as it has been shown in adult patients with aortic stenosis (12). Moreover, the fact that healthy women have higher BNP plasma levels than healthy men of the same age group suggests that estrogens may induce BNP expression throughout ER signaling (16). This is supported by the observation of increasing BNP blood levels after estrogen replacement therapy (17).
Our results show further that the mRNA expression of ERs correlated positively with the mRNA expression of pro- and also of anti-inflammatory cytokines. This apparent contradictory result might be explained by the complexity of the induction of inflammatory cytokines by the mechanical stimulation of cardiomyocytes. Indeed the early stress response leads to a sustained induction of pro-inflammatory cytokines that, in turn, initiates the gene expression of anti-inflammatory cytokines (14). Besides this, ERs are involved in the modulation of the inflammatory response by estrogens (18) and either activate or repress gene expression depending on local estrogen concentrations (19). Thus, higher estradiol (E2) levels downregulate pro-inflammatory cytokines such as TNF-α and upregulate anti-inflammatory cytokines such as IL-10 in different cell types, whereas low E2 levels stimulate TNF-α and IL-1β expression (20). The anti-inflammatory effect of E2 might be related to ERα that blocks TNF-α-induced IL-6 synthesis by interfering with nuclear factor kappa B (NFκB) (21). ERs inhibit multiple NFκB pathways and are therefore considered anti-inflammatory proteins (22–26).
The mechanisms by which low and high physiological concentrations of estrogens differentially affect ER activity to influence the expression of inflammatory genes are unclear. One possibility is that the low and high levels of E2 induce distinct transcriptional complexes and that this activates different pathways to promote or dampen inflammation (27). Our observation that the expression of ER-mRNA was not related to gender, age, or achieved puberty and that it was present in very young infants suggests an E2-independent mechanism for the activation of ERs in the myocardium of children. Indeed a large number of substances are likely to bind ERs such as xenoestrogens, in particular, phytoestrogens present in a wide spectrum of food constituents (28).
In our series, the myocardial concentrations of ERβ-mRNA were higher than those of ERα-mRNA. While a differential expression of both ERα and ERβ in the human right atrial myocardium has not been described that far, animal studies performed in neonatal and adult female rats have shown higher ERα-mRNA concentrations in the oldest animals (29). This suggests an influence of age on ERα expression in animal cardiomyocytes. In our patients, however, ERα expression was not age dependent. The role of inflammatory cytokines induced in the myocardium by hemodynamic overload on the upregulation of ERβ and down-regulation of ERα as has been documented previously (30, 31) remains speculative in our patient cohort who was heterogeneous in terms of quality and severity of cardiac defects and hemodynamic overload (14).
Besides this, hypoxemia could also have impacted ER expression as demonstrated in human breast cancer cell lines where hypoxia represses ERα (32).
Both the repression of ERα and the upregulation of ERβ involve the activation of hypoxia-inducible factor (HIF)-1α (33, 34) that is increased in the myocardium of children with cyanotic congenital cardiac disease, as we have shown previously (35). The putative influence of pre-operative hypoxemia on ER expression in the myocardium of patients with congenital cardiac disease was not investigated in our study, owing to the fact that only three patients of this series were cyanotic.
The secondary objective of this study was to address the question of whether myocardial ER expression may provide cardio-protection to patients undergoing cardiac surgery for congenital cardiac disease and be related to better post-operative outcome.
Our results showing that a higher expression of ERα-mRNA was associated to lower myocardial damage, improved lung function, lower water retention, and lower cytolysis in the early post-operative period might support the assumed protective role of ERs in this particular patient population (22–26). The reason why the expression of ERβ-mRNA did not correlate significantly with the outcome variables but with reduced fluid retention might be related to the statistical rank correlation analysis performed on the small patient group.
Open cardiac surgery in adults and children is associated with a systemic inflammatory reaction that relates to post-operative myocardial cell damage and multiple organ dysfunction syndrome being a severe issue (36). In this context, troponin release correlates with the importance of systemic inflammation, in particular, with the levels of circulating IL-6 (37). Inflammatory proteins such as complement proteins are present in the circulation immediately after connection to the extracorporeal circuit and initiate the synthesis of pro- and anti-inflammatory cytokines by circulating and tissue cells (38). An adequate anti-inflammatory balance is thought to be necessary to limit and/or terminate inflammation and protect from organ injury (39).
The anti-inflammatory potential of myocardial ERs may provide peri-operative organ protection against operative and inflammatory stress. In an experimental sepsis model classically associated with a systemic inflammatory reaction, ERβ agonists provide increased survival and reduced tissue damage and modify the genomic sepsis signature with a decreased expression of pro-inflammatory genes (40). Besides these nuclear-mediated effects of ERβ, ERα initiates the activation of acute protective pathways via non-nuclear mechanisms involving the activation of kinases that enhance the phosphorylation of eNOS, PI3K/Akt, and ERK1/2 (41, 42).
While our results, taken together, might indicate that gender- and age-independent expression of ERα and ERβ in the myocardium of young patients undergoing cardiac surgery works as protective, more experimental data are needed to answer the question of whether the modulation of ER expression in the myocardium would participate to improve peri-operative organ protection in this patient group. For this purpose, an animal model of cardiac surgery for congenital cardiac disease with hemodynamic overload (15) involving pre-operative induction of ERs by pharmacological or genetic engineering procedures should be established.
Our study has several limitations. The small number of patients investigated and their heterogeneity in terms of cardiac diagnosis did not allow us to analyze the role of the quality of hemodynamic overload and of the degree of hypoxemia on ER-mRNA expression.
Furthermore, the limited size of the myocardial samples was insufficient to quantify protein synthesis of our target genes and prejudge the biological activity of ER induction.
Finally, we describe an association between higher myocardial ER-mRNA expression and lower clinical and biological markers of post-operative organ damage but are not able to give evidence of the organ-protective role of ERs during cardiac surgery for congenital cardiac defect at this stage. This would require experimental studies involving the modulation of ER expression in a model of cardiac surgery for congenital cardiac disease.
Our study shows, for the first time, that ERα and ERβ are expressed at the mRNA level in the myocardium of young patients with congenital cardiac defect independently of gender, age, or puberty. The correlation between ER-mRNA expression and that of pro- and inflammatory cytokines, growth factors, and early stress response genes suggests an interplay between inflammatory and ER-activating pathways. The association between a higher ER-mRNA expression and lower clinical and biological markers of post-operative organ damage might indicate a protective role of ER pathways in the setting of cardiac surgery for congenital cardiac disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Aachen University of Technology. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
HR: data analysis and manuscript redaction. NF: data collection and data analysis. RH: data collection and study design. JV-J: data collection and manuscript revision. A-SP: study design and manuscript revision. M-CS: study design, manuscript redaction and revision. All authors contributed to the article and approved the submitted version.
This work was supported by a grant of the University Hospital Liège (FIRS) to M-CS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AST, aspartate aminotransferase; BNP, brain natriuretic peptide; CPB, cardio-pulmonary bypass; CT-1, cardiotrophin-1; DNA, desoxyribonucleic acid; E2, estradiol; eNOS, endothelial nitric oxide synthase; ER, estrogen receptor; ERK1/2, extracellular signal-regulated kinases; FiO2, inspired oxygen fraction; HIF, hypoxia-inducible factor; IL, interleukin; IQR, interquartile range; mRNA, messenger ribonucleic acid; P50, percentile 50; PaO2, partial arterial oxygen pressure; PCR, polymerase chain reaction; PI3K/Akt, phosphatidylinositol 3-kinase/protein kinase B; TGFβ, tissue growth factor-β; TNFα, tumor necrosis factor-α.
1. Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res. (2016) 118:1294–312. doi: 10.1161/CIRCRESAHA.116.307509
2. Novella S, Pérez-Cremades D, Mompeón A, Hermenegildo C. Mechanisms underlying the influence of Oestrogen on cardiovascular physiology in women. J Physiol. (2019) 597:4873–86. doi: 10.1113/JP278063
3. Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. (2000) 101:1792–8. doi: 10.1161/01.cir.101.15.1792
4. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. (1997) 138:863–70. doi: 10.1210/endo.138.3.4979
5. Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. (2012) 135:54–70. doi: 10.1016/j.pharmthera.2012.03.007
6. Menazza S, Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. (2016) 118:994–1007. doi: 10.1161/CIRCRESAHA.115.305376
7. Yao Y, Chang X, Wang D, Ma H, Wang H, Zhang H, et al. Roles of ERK1/2 and PI3K/AKT signaling pathways in mitochondria-mediated apoptosis in testes of hypothyroid rats. Toxicol Res. (2018) 7:1214–24. doi: 10.1039/c8tx00122g
8. Prossnitz ER, Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol Cell Endocrinol. (2014) 389:71–83. doi: 10.1016/j.mce.2014.02.002
9. Fuentes N, Silveyra P. Estrogen receptor signaling mechanisms. Adv Protein Chem Struct Biol. (2019) 116:135–70. doi: 10.1016/bs.apcsb.2019.01.001
10. Crider A, Pillai A. Estrogen signaling as a therapeutic target in neurodevelopmental disorders. J Pharmacol Exp Ther. (2017) 360:48–58. doi: 10.1124/jpet.116.237412
11. Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism. (2014) 5:46. doi: 10.1186/2040-2392-5-46
12. Nordmeyer J, Eder S, Mahmoodzadeh S, Martus P, Fielitz J, Bass J, et al. Upregulation of myocardial estrogen receptors in human aortic stenosis. Circulation. (2004) 110:3270–5. doi: 10.1161/01.CIR.0000147610.41984.E8
13. Rouatbi H, Farhat N, Heying R, Gérard A, Vazquez-Jimenez JF, Seghaye MC. Right atrial myocardial remodeling in children with atrial septal defect involves inflammation, growth, fibrosis, and apoptosis. Front Pediatr. (2020) 8:40. doi: 10.3389/fped.2020.00040
14. Qing M, Schumacher K, Heise R, Wöltje M, Vazquez-Jimenez JF, Richter T, et al. Intramyocardial synthesis of pro- and anti-inflammatory cytokines in infants with congenital cardiac defects. J Am Coll Cardiol. (2003) 41:2266–74. doi: 10.1016/s0735-1097(03)00477-7
15. Liersch PN, Schwarz A, Sachweh J, Hermanns-Sachweh B, Heying R, Vázquez-Jimènez JF, et al. Gene expression of cytokines, growth factors and apoptosis regulators in a neonatal model of pulmonary stenosis. Future Cardiol. (2015) 11:297–307. doi: 10.2217/fca.15.25
16. Mir TS, Flato M, Falkenberg J, Haddad M, Budden R, Weil J, et al. Plasma concentrations of N-terminal brain natriuretic peptide in healthy children, adolescents, and young adults: effect of age and gender. Pediatr Cardiol. (2006) 27:73–7. doi: 10.1007/s00246-005-1022-4
17. Maffei S, Del Ry S, Prontera C, Clerico A. Increase in circulating levels of cardiac natriuretic peptides after hormone replacement therapy in postmenopausal women. Clin Sci. (2001) 101:447–53.
18. Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. (2011) 40:66–73. doi: 10.1007/s12016-010-8203-5
19. Tan IJ, Peeva E, Zandman-Goddard G. Hormonal modulation of the immune system—a spotlight on the role of progestogens. Autoimmun Rev. (2015) 14:536–42. doi: 10.1016/j.autrev.2015.02.004
20. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. (2007) 28:521–74. doi: 10.1210/er.2007-0001
21. Ito A, Bebo BF Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, et al. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. (2001) 167:542–52. doi: 10.4049/jimmunol.167.1.542
22. Chadwick CC, Chippari S, Matelan E, Borges-Marcucci L, Eckert AM, Keith JC Jr, et al. Identification of pathway-selective estrogen receptor ligands that inhibit NF-kappaB transcriptional activity. Proc Natl Acad Sci USA. (2005) 102:2543–8. doi: 10.1073/pnas.0405841102
23. Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. (1997) 25:2424–9. doi: 10.1093/nar/25.12.2424
24. Kurebayashi S, Miyashita Y, Hirose T, Kasayama S, Akira S, Kishimoto T. Characterization of mechanisms of interleukin-6 gene repression by estrogen receptor. J Steroid Biochem Mol Biol. (1997) 60:11–7. doi: 10.1016/s0960-0760(96)00175-6
25. McCracken SA, Drury CL, Lee HS, Morris JM. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J Reprod Immunol. (2003) 58:27–47. doi: 10.1016/s0165-0378(02)00081-5
26. Messingham KA, Heinrich SA, Kovacs EJ. Estrogen restores cellular immunity in injured male mice via suppression of interleukin-6 production. J Leukoc Biol. (2001) 70:887–95.
27. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. (2015) 294:63–9. doi: 10.1016/j.cellimm.2015.01.018
28. Zhao L, Mao Z, Brinton RD. A select combination of clinically relevant phytoestrogens enhances estrogen receptor beta-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology. (2009) 150:770–83. doi: 10.1210/en.2008-0715
29. Jankowski M, Rachelska G, Donghao W, McCann SM, Gutkowska J. Estrogen receptors activate atrial natriuretic peptide in the rat heart. Proc Natl Acad Sci U S A. (2001) 98:11765–70. doi: 10.1073/pnas.201394198
30. Schneider CP, Nickel EA, Samy TS, Schwacha MG, Cioffi WG, Bland KI, et al. The aromatase inhibitor, 4-hydroxyandrostenedione, restores immune responses following trauma-hemorrhage in males and decreases mortality from subsequent sepsis. Shock. (2000) 14:347–53. doi: 10.1097/00024382-200014030-00019
31. Tamir S, Izrael S, Vaya J. The effect of oxidative stress on ERalpha and ERbeta expression. J Steroid Biochem Mol Biol. (2002) 81:327–32. doi: 10.1016/s0960-0760(02)00115-2
32. Kurebayashi J, Otsuki T, Moriya T, Sonoo H. Hypoxia reduces hormone responsiveness of human breast cancer cells. Jpn J Cancer Res. (2001) 92:1093–101. doi: 10.1111/j.1349-7006.2001.tb01064.x
33. Frump AL, Selej M, Wood JA, Albrecht M, Yakubov B, Petrache I, et al. Hypoxia upregulates estrogen receptor β in pulmonary artery endothelial cells in a HIF-1α-dependent manner. Am J Respir Cell Mol Biol. (2018) 59:114–26. doi: 10.1165/rcmb.2017-0167OC
34. Ryu K, Park C, Lee Y. Hypoxia-inducible factor 1 alpha represses the transcription of the estrogen receptor alpha gene in human breast cancer cells. Biochem Biophys Res Commun. (2011) 407:831–6. doi: 10.1016/j.bbrc.2011.03.119
35. Qing M, Görlach A, Schumacher K, Wöltje M, Vazquez-Jimenez JF, Hess J, et al. The hypoxia-inducible factor HIF-1 promotes intramyocardial expression of VEGF in infants with congenital cardiac defects. Basic Res Cardiol. (2007) 102:224–32. doi: 10.1007/s00395-007-0639-2
36. Seghaye MC. The clinical implications of the systemic inflammatory reaction related to cardiac operations in children. Cardiol Young. (2003) 13:228–39. doi: 10.1017/s1047951103000465
37. Hövels-Gürich HH, Vazquez-Jimenez JF, Silvestri A, Schumacher K, Minkenberg R, Duchateau J, et al. Production of proinflammatory cytokines and myocardial dysfunction after arterial switch operation in neonates with transposition of the great arteries. J Thorac Cardiovasc Surg. (2002) 124:811–20. doi: 10.1067/mtc.2002.122308
38. Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. (2003) 75:S715–20. doi: 10.1016/s0003-4975(02)04701-x
39. Franke A, Lante W, Fackeldey V, Becker HP, Thode C, Kuhlmann WD, et al. Proinflammatory and antiinflammatory cytokines after cardiac operation: different cellular sources at different times. Ann Thorac Surg. (2002) 74:363–71. doi: 10.1016/s0003-4975(02)03658-5
40. Christaki E, Opal SM, Keith JC Jr, Kessinian N, Palardy JE, Parejo NA, et al. Estrogen receptor beta agonism increases survival in experimentally induced sepsis and ameliorates the genomic sepsis signature: a pharmacogenomic study. J Infect Dis. (2010) 201:1250–7. doi: 10.1086/651276
41. Wang M, Crisostomo P, Wairiuko GM, Meldrum DR. Estrogen receptor-alpha mediates acute myocardial protection in females. Am J Physiol Heart Circ Physiol. (2006) 290:H2204–9. doi: 10.1152/ajpheart.01219.2005
Keywords: ERα, ERβ, cytokines, myocardial expression, myocardial protection
Citation: Rouatbi H, Farhat N, Heying R, Vazquez-Jimenez JF, Parent A-S and Seghaye M-C (2021) Myocardial Expression of Estrogen Receptor-mRNA Is Associated With Lower Markers of Post-operative Organ Damage in Young Patients With Congenital Cardiac Defect. Front. Pediatr. 9:729198. doi: 10.3389/fped.2021.729198
Received: 22 June 2021; Accepted: 11 August 2021;
Published: 22 September 2021.
Edited by:
Diego Gallo, Politecnico di Torino, ItalyReviewed by:
Brian Rowan, Tulane University, United StatesCopyright © 2021 Rouatbi, Farhat, Heying, Vazquez-Jimenez, Parent and Seghaye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie-Christine Seghaye, bWNzZWdoYXllQGNodWxpZWdlLmJl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.