- 1Paediatric Education and Research Ladder, Department of Paediatrics and Child Health, University of the Witwatersrand, Johannesburg, South Africa
- 2Department of Paediatrics and Child Health, Division of Pulmonology, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa
- 3Department of Paeditrics, University of Pretoria, Pretoria, South Africa
- 4Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
- 5South African Medical Research Council Unit on Child and Adolescent Health, University of Cape Town, Cape Town, South Africa
- 6Department of Respiratory and Sleep Medicine, Queensland Children's Hospital, Queensland University of Technology, Brisbane, QLD, Australia
- 7Australian Centre for Health Services Innovation, Queensland University of Technology, Brisbane, QLD, Australia
- 8Child Health Division, Menzies School of Health Research, Charles Darwin University, Darwin, NT, Australia
Introduction
The substantial decline in the burden of childhood community acquired lower respiratory tract infections (LRTI) over the last decades is associated with improvements in immunization, nutrition, socioeconomic, and control of the HIV epidemic (1). However, LRTI remains the commonest cause of under-5 mortality outside the neonatal period (1). Although most children with LRTI fully recover, a proportion develop chronic respiratory symptoms and/or sequelae; reasons include host factors (immunosuppression, poor secretion clearance, airway abnormalities or genetic factors), infectious causes (TB or adenovirus), and/or adverse environmental factors. Early identification and management of children at-risk of respiratory sequelae may help to preserve long-term lung health. However, knowing who and when to investigate is challenging as there is little high-level evidence to support the timing and extent of investigations required.
In HIV and TB endemic settings, clinicians tend to attribute chronic respiratory symptoms or sequelae to childhood HIV and/or TB; however, improved prevention-of-mother-to-child-transmission (PMTCT) programs and access to antiretroviral therapy (ART) have reduced the burden of infections, including TB (2, 3). Once these infections are excluded, other conditions predisposing to chronic respiratory symptoms and sequelae must be considered (4, 5).
Prolonged, severe or recurrent infection, that may arise from an underlying predisposition, may trigger a vicious cycle of inflammation, impaired mucociliary clearance, and bacterial colonization leading to proteolytic lung damage and bronchiectasis (6, 7). In low- and middle-income countries, and socially disadvantaged communities in high-income countries, post-infectious bronchiectasis is the commonest sequelae of LRTI (8). In sub-Saharan Africa, the causes of childhood bronchiectasis remain poorly defined but the commonest causes are likely to be post-infectious or HIV-associated (9, 10).
This paper aims to provide guidance for healthcare providers to identify, investigate and manage a child with chronic respiratory symptoms (usually cough, wheeze, exercise intolerance) and identify who is at-risk of developing respiratory sequelae (bronchiectasis, post-infectious bronchiolitis obliterans, obstructive and/or restrictive lung function abnormalities, chronic hypoxia, or pulmonary hypertension) after a LRTI episode to minimize the risk of irreversible lung damage (11).
Diagnosis and Investigation
A single episode of LRTI in a child who fully recovers does not usually warrant further investigation. To decide if investigations are warranted, the SPUR acronym (Box 1) is used to alert the clinician to possible underlying problems (4). Children with “severe” clinical presentations (i.e., prolonged hospitalization, the need for ventilation or ICU admission, and/or multi-system involvement), or the “persistence” of symptoms (slow resolution of LRTI; >2 weeks), or residual symptoms of cough and/or wheeze require further investigation. In these circumstances, first consider: (i) alternate diagnoses, (ii) untreated or resistant pathogens, (iii) host immunosuppression, or (iv) complications such as empyema or abscess (12). In a small proportion of children with LRTI, the presence of “unusual clinical” findings or an “unusual comorbidity” at presentation should prompt further investigation and/or close monitoring. These include: a history of chronic cough or wheeze (generally >4 weeks), chest wall abnormalities, clubbing or pulmonary hypertension; or a comorbidity such as feeding difficulties, HIV, cardiac, or neurological diseases (4, 13, 14). Furthermore, “unusual CXR” or “unusual colony /microbiological findings” may warrant investigation. Lastly, “recurrent” LRTI (generally ≥2 episodes per year) requires further evaluation—an important consideration is whether the LRTI recurs in the same lobe, diffusely or in different areas.
Box 1. The SPUR acronym [adapted from (4)]. *Chronic cough is defined as a cough that is present for at least 4 weeks.
1. Severe
• Prolonged hospitalization
• Need for ventilation
• Multi-system involvement
2. Persistent
• Clinical – persistence of respiratory symptoms (cough* or wheeze)
• Radiological – failure of resolution
3. Unusual
■ Clinical
• Chronic upper (otitis-media, rhino-sinusitis) respiratory symptoms
• History of chronic respiratory symptoms (cough* or wheeze)
• Poor growth or failure to thrive
• Feeding difficulties
• Chest wall abnormalities
1. Asymmetry
2. Harrisons sulcus
3. Pectus excavatum
4. Pectus carinatum
5. Barrel shape
• Digital clubbing
• Features of pulmonary hypertension
■ Co-morbidities
• HIV-infection
• Primary or secondary immunodeficiency
• Malnutrition
• Neuromuscular disease
• Cerebral palsy
• Abnormal neonatal history (prematurity, broncho-pulmonary dysplasia, prolonged ventilation or oxygen dependence)
• Congenital heart lesion with increased pulmonary blood flow
■ CXR
• Homogenous air space opacification (white-out)
• Lobar/focal disease
• Differential transradiancy
• Airway compression
• Cystic lucencies
■ Colonies
• e.g.,: Pseudomonas aeruginosa, or other opportunistic infections
4. Recurrent
The presence of SPUR “red flags” should prompt the clinician to undertake a thorough history and examination, and consider relevant investigations (Supplementary Figure 1). The history may reveal patterns of symptoms that provide clues to the underlying diagnosis (Supplementary Figure 2).
Firstly, investigate for TB and HIV in high burden settings. TB is a common but often unrecognized cause of LRTI (15). Repeated lower respiratory samples may be needed for microbiological confirmation. Failure to confirm TB or an inadequate response to TB therapy (poor weight gain, and/or persistence or worsening of radiological features) should alert the clinician to consider drug resistant TB, non-adherence to therapy or an alternate diagnosis.
Next consider asthma (in a child with recurrent wheezing, exercise induced dyspnea or nocturnal cough) or protracted bacterial bronchitis (PBB) in an otherwise well-child with a chronic wet cough. In some settings, up to 40% of children with chronic cough may have PBB; untreated PBB increases bronchiectasis risk (16, 17). The definition of PBB is based on the presence of chronic wet cough (>4 weeks), an identified pathogen, and symptom resolution after administering appropriate oral antibiotics for 2 weeks (17).
Most asthma exacerbations are triggered by a viral respiratory tract infection (18). In young children, spirometry is challenging and diagnosing asthma based on bronchodilator responses is difficult. The following features may assist the clinician in diagnosing asthma in young children: (i) recurrent cough or wheezing with activity, or a nocturnal cough; (ii) a first degree relative with asthma; (iii) co-existing eczema or allergic rhinitis; (iv) a clinical response to a 3-month trial of controller therapy; or (v) elevated fractional exhaled Nitric Oxide (FeNO) for age (18). However, these features have a low probability and limited predictive value for asthma (19).
A follow-up chest x-ray is indicated in the presence of ongoing symptoms, lobar collapse, dense consolidation, or recurrence of radiological changes in the same lobe/segment—this may suggest the possibility of bronchial obstruction e.g., congenital airway abnormality, foreign body, lymph node or other cause of external compression. The availability of specialized imaging, lung function testing, a regional pulmonologist, or referral pathways for specialized testing would determine the next steps (Supplementary Figure 1).
Although the quality of evidence is low, the European Respiratory Society Clinical Practice Guideline for managing pediatric bronchiectasis recommended the following first line tests in children with suspected or confirmed bronchiectasis: full blood count, immunologlobulins, sweat test, CT, lung function tests, sampling the lower airway, and assessment for TB and HIV in endemic areas (20). Further immunodefeciency work-up, bronchoscopy, and identification of primary ciliary dyskinesia, gastro-esophageal reflux disease and airway aspiration may be warranted depending on clinical presentation (20).
We recommend using a checklist with an “outside” vs. “inside” lung approach (Table 1). Outside the lung, cardiac (L-R shunts), neuromuscular (weak cough, in-coordinated swallow, bulbar or pseudobulbar palsy), gastrointestinal (tracheo-esophageal fistula, gastro-esophogeal reflux disease), and primary or secondary immunodeficiency conditions should be considered (Table 1). Infants and young children may have up to ten upper respiratory infections per year, more so if they attend crèche or have older siblings (4, 13, 21, 22). Respiratory symptoms after a viral infection can persist for up to 4 weeks, but should get better over time and not be associated with a wet cough or sputum production (4).
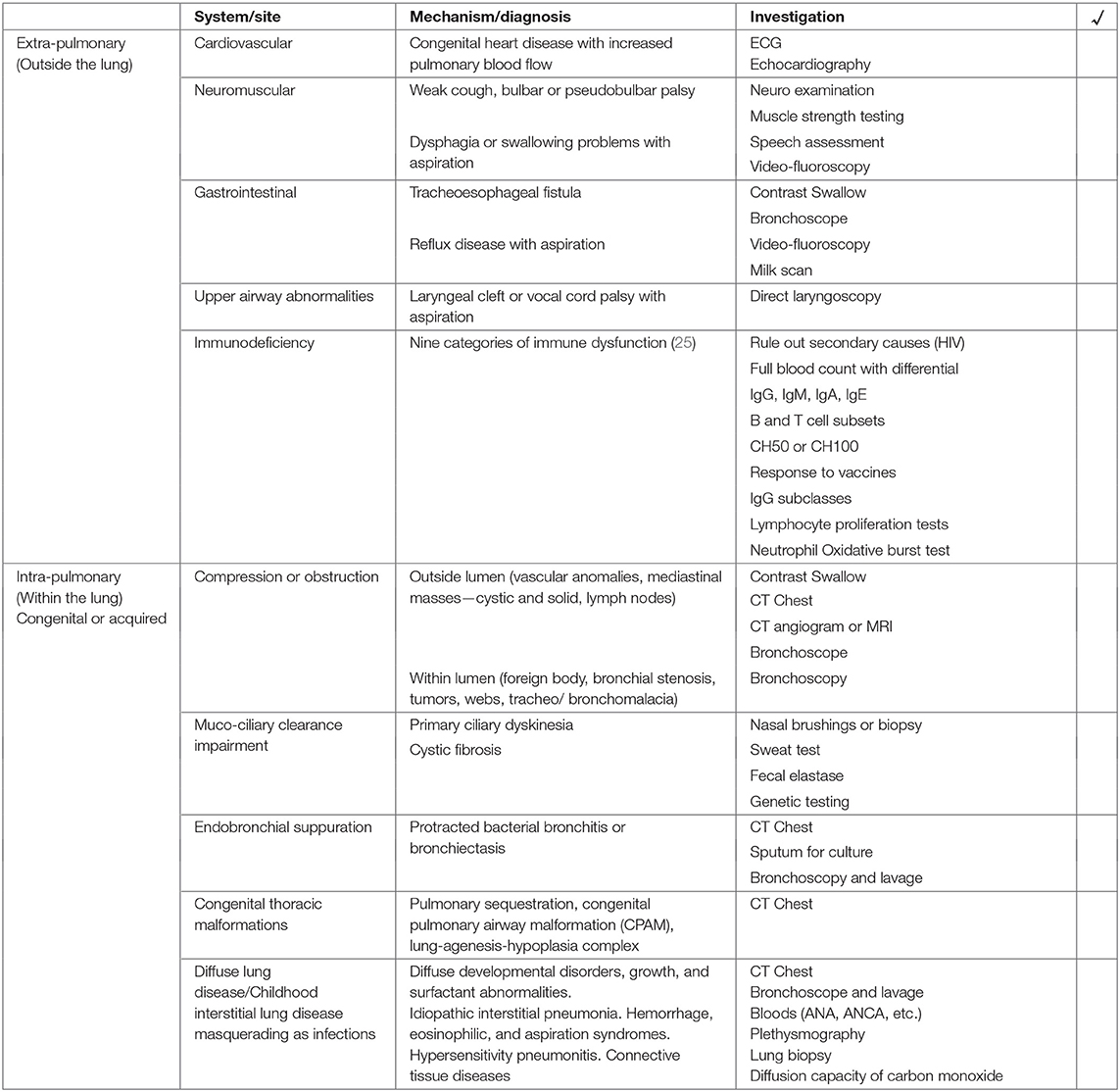
Table 1. A checklist approach to further investigate children at risk of respiratory sequelae following lower respiratory tract infection.
Within the lung, airway obstruction (outside the wall or within the lumen) or impaired muco-ciliary clearance mechanisms (primary ciliary dyskinesia or cystic fibrosis) result in stasis, bacterial colonization and recurrent inflammation (Supplementary Figure 3). Diffuse/childhood interstitial lung disease masquerading as infection, right middle lobe syndrome, or cystic parenchymal disease are other considerations (Table 1) (4).
Management
The aim of management is to prevent adverse pulmonary sequelae by early diagnosis and treatment which may interrupt progression to bronchiectasis and lung function impairment. Key management goals for those with bronchiectasis are: to improve quality of life (reduce cough, prevent exacerbations, improve effort tolerance), optimize lung growth (when possible), prevent further lung injury (treat inflammation, prevent exacerbations, treat underlying cause), and minimize complications (20, 23).
Management of at-risk children or those with bronchiectasis (suspected or confirmed) includes (Supplementary Figure 4):
1. General lung protective strategies
Reduce exposure to environmental insults (smoking or vaping, biomass and fossil gas combustion) that impair physiological processes such as ciliary function. In addition to ensuring that routine childhood immunizations are complete, consider annual influenza vaccination and booster pneumococcal vaccine in at-risk and immunosuppressed children. HIV-infected pregnant women and their infants should receive PMTCT. HIV-infected infants require early initiation of ART, and cotrimaxazole or isoniazid prophylaxis as indicated.
2. Treat the underlying cause
If identified, treat the underlying cause: eg, remove foreign body.
3. Interrupt the cycle of bacterial infection and inflammation
a. Airway clearance techniques:
Age-appropriate chest physiotherapy techniques are a cornerstone of therapy. Percussion of the chest by the caregiver is a feasible technique in younger children whereas self-initiated airway clearance techniques using assisted devices or maneuvers are more practical in older children (20).
Mucoactive agents such as recombinant-human DNAse, inhaled mannitol or hypertonic saline are not routinely recommended (20). Whilst the latter may be used selectively, recombinant-human DNAse is contraindicated in children without CF (20). Similarly, inhaled corticosteroids and long or short-acting beta2-agonists are not routinely recommended unless children have an asthma phenotype or eosinophilia (20).
b. Antibiotic therapy can be used for exacerbations, or for eradication and/or control:
• Exacerbations are deviations from baseline and may present with an increased cough frequency, increased sputum production or change of sputum color, and/or the presence of respiratory distress for more than 3 days (20). Antibiotics should be targeted at the organism identified on sputum culture or the colonizing organism identified prior to the exacerbation. Amoxicillin/clavulanic acid (14 days of therapy) is the preferred choice of treatment in children with chronic wet cough, PBB or bronchiectasis (17, 20, 24, 25).
• Eradication and/or control using intravenous, oral or inhaled therapy is necessary for organisms that colonize the airway and cause long-term deterioration in lung function, e.g., Pseudomonas aeruginosa (20).
c. Anti-inflammatory agents:
Azithromycin may be considered on alternate days for a minimum of 6 months (20). One RCT has shown that a long-term macrolide, azithromycin (10 mg per kg; max 500 mg), halves exacerbation rates in children with non-CF bronchiectasis (incidence rate ratio 0.50; 95% CI 0.35–0.71 compared to placebo) (26). The data in children is similar to the effect size described in meta-analyses of adults with bronchiectasis (27).
4. Monitoring for disease progression is essential. The following parameters should be monitored at least 6-monthly:
a. Clinical monitoring of cough, exercise intolerance, sputum color, frequency of exacerbations, co-morbidities and complications.
b. Microbiological monitoring of sputum to identify the colonizing organism is essential to tailor antimicrobial therapy during exacerbations, and eradicate/control unwanted colonizers such as Pseudomonas aeruginosa (20).
c. Spirometry or other lung function is required to document stability (20).
d. Radiological monitoring is usually done dependent on clinical features, and resource limitations (20). There is limited evidence to support baseline and repeat low radiation dose CT scans in resource limited settings.
5. Other supportive measures
Adequate nutrition is essential for lung growth in the first few years and important throughout life. There is no evidence to support the use of nutritional supplements except for Vitamin D supplements in deficient children (20). Concurrent gastro-esophageal reflux disease or asthma should be appropriately managed (18). Furthermore, traditional or cultural remedies that cause lipoid pneumonia should be specifically sought in setting where these practices are common (28). In children with severe disease and lung destruction, supplementary domiciliary oxygen and treatment of pulmonary hypertension and right heart failure may be required.
Conclusion
Clinicians should maintain a high level of suspicion for children at-risk of developing chronic respiratory symptoms or sequelae following LRTI. In endemic settings, TB and/ or HIV infection should be diagnosed or excluded promptly. Thereafter, other conditions that may lead to the development of chronic respiratory symptoms or sequelae should be considered. Early recognition of underlying disease and appropriate management can prevent disease progression, improve quality of life and optimize lung function.
Author Contributions
ZD prepared the first draft. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.708100/full#supplementary-material
Supplementary Figure 1. Pathway for children that require further investigation.
Supplementary Figure 2. Patterns of lower respiratory tract symptoms over a 12 month period [Adapted Penny ME. Pediatr Infect Dis. (1993) 12:762–3].
Supplementary Figure 3. Infographic demonstrating how airway obstruction by a foreign body (B) or impairment of muco-ciliary mechanism (C) result in stasis of secretions and inflammation as compared to normal (A).
Supplementary Figure 4. Managment steps of children with chronic respiratory symptoms or at-risk of respiratory sequelae.
References
1. Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SR. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. (2018) 18:1191–210. doi: 10.1016/S1473-3099(18)30310-4
2. Nanoo A, Izu A, Ismail NA, Ihekweazu C, Abubakar I, Mametja D, et al. Nationwide and regional incidence of microbiologically confirmed pulmonary tuberculosis in South Africa, 2004-12: a time series analysis. Lancet Infect Dis. (2015) 15:1066–76. doi: 10.1016/S1473-3099(15)00147-4
3. B-Lajoie MR, Drouin O, Bartlett G, Nguyen Q, Low A, Gavriilidis G, et al. Incidence and prevalence of opportunistic and other infections and the impact of antiretroviral therapy among HIV-infected children in low- and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. (2016) 62:1586–94. doi: 10.1093/cid/ciw139
4. Bush A. Recurrent respiratory infections. Pediatr Clin N Am. (2009) 56:67–100. doi: 10.1016/j.pcl.2008.10.004
5. Chang AB, Byrnes CA, Everard ML. Diagnosing and preventing chronic suppurative lung disease (CSLD) and bronchiectasis. Paediatr Respir Rev. (2011) 12:97–103. doi: 10.1016/j.prrv.2010.10.008
6. Cole P. The damaging role of bacteria in chronic lung infection. J Antimicrob Chemotherapy. (1997) 40(Suppl. A):5–10. doi: 10.1093/jac/40.suppl_1.5
7. Goyal V, Grimwood K, Marchant J, Masters IB, Chang AB. Pediatric bronchiectasis: no longer an orphan disease. Pediatr Pulmonol. (2016) 51:450–69. doi: 10.1002/ppul.23380
8. McCallum GB, Binks MJ. The epidemiology of chronic suppurative lung disease and bronchiectasis in children and adolescents. Front Pediatr. (2017) 5:27. doi: 10.3389/fped.2017.00027
9. Jeena PM, Coovadia HM, Thula SA, Blythe D, Buckels NJ, Chetty R. Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS. (1998) 12:1185–93. doi: 10.1097/00002030-199810000-00011
10. Verwey C, Velaphi S, Khan R. Bacteria isolated from the airways of paediatric patients with bronchiectasis according to HIV status. South Afr Med J. (2017) 107:435–9. doi: 10.7196/SAMJ.2017.v107i5.10692
11. Nathan AM, Teh CSJ, Eg KP, Jabar KA, Zaki R, Hng SY, et al. Respiratory sequelae and quality of life in children one-year after being admitted with a lower respiratory tract infection: a prospective cohort study from a developing country. Pediatr Pulmonol. (2020) 55:407–17. doi: 10.1002/ppul.24598
12. de Benedictis FM, Kerem E, Chang AB, Colin AA, Zar HJ, Bush A. Complicated pneumonia in children. Lancet. (2020) 396:786–98. doi: 10.1016/S0140-6736(20)31550-6
13. de Benedictis FM, Bush A. Recurrent lower respiratory tract infections in children. BMJ. (2018) 362:k2698. doi: 10.1136/bmj.k2698
14. Couriel J. Assessment of the child with recurrent chest infections. Br Med Bull. (2002) 61:115–32. doi: 10.1093/bmb/61.1.115
15. Martinez L, le Roux DM, Barnett W, Stadler A, Nicol MP, Zar HJ. Tuberculin skin test conversion and primary progressive tuberculosis disease in the first 5 years of life: a birth cohort study from Cape Town, South Africa. Lancet Child Adolesc Health. (2018) 2:46–55. doi: 10.1016/S2352-4642(17)30149-9
16. Chang AB, Robertson CF, Van Asperen PP, Glasgow NJ, Mellis CM, Masters IB, et al. A multicenter study on chronic cough in children : burden and etiologies based on a standardized management pathway. Chest. (2012) 142:943–50. doi: 10.1378/chest.11-2725
17. Chang AB, Upham JW, Masters IB, Redding GR, Gibson PG, Marchant JM, et al. Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr Pulmonol. (2016) 51:225–42. doi: 10.1002/ppul.23351
18. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Fontana, CA: National Heart, Lung, and Blood Institute; National Institutes of Health; World Health Organization (2020).
19. SIGN. British Guideline on the Management of Asthma: Quick Reference Guide. Scottish Intercollegiate Guidelines Network & British Thoracic Society (2019).
20. Chang AB, Fortescue R, Grimwood K, Alexopoulou E, Bell L, Boyd J, et al. Task Force report: European Respiratory Society guidelines for the management of children and adolescents with bronchiectasis. Eur Respir J. (2021). doi: 10.1183/13993003.02990-2020. [Epub ahead of print].
21. Grüber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatr Allergy Immunol. (2008) 19:505–12. doi: 10.1111/j.1399-3038.2007.00688.x
22. Lee AY, Gray PE. Evaluating for immunodeficiency in children with recurrent infection. Aust Fam Phys. (2014) 43:629–32.
23. Chang AB, Bush A, Grimwood K. Bronchiectasis in children: diagnosis and treatment. Lancet. (2018) 392:866–79. doi: 10.1016/S0140-6736(18)31554-X
24. Redding GJ. Update on treatment of childhood bronchiectasis unrelated to cystic-fibrosis. Paediatr Respir Rev. (2011) 12:119–23. doi: 10.1016/j.prrv.2010.10.012
25. Marchant J, Masters IB, Champion A, Petsky H, Chang AB. Randomised controlled trial of amoxycillin clavulanate in children with chronic wet cough. Thorax. (2012) 67:689–93. doi: 10.1136/thoraxjnl-2011-201506
26. Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, et al. Long-term azithromycin for indigenous children with non-cystic-fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. (2013) 1:610–20. doi: 10.1016/S2213-2600(13)70185-1
27. Wang D, Fu W, Dai J. Meta-analysis of macrolide maintenance therapy for prevention of disease exacerbations in patients with noncystic fibrosis bronchiectasis. Medicine. (2019) 98:e15285. doi: 10.1097/MD.0000000000015285
Keywords: LRTI, pneumonia, children, sequelae, lung disease
Citation: Dangor Z, Verwey C, Lala SG, Mabaso T, Mopeli K, Parris D, Gray DM, Chang AB and Zar HJ (2021) Lower Respiratory Tract Infection in Children: When Are Further Investigations Warranted? Front. Pediatr. 9:708100. doi: 10.3389/fped.2021.708100
Received: 11 May 2021; Accepted: 05 July 2021;
Published: 28 July 2021.
Edited by:
Albert Martin Li, The Chinese University of Hong Kong, ChinaReviewed by:
Saibal Das, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaCopyright © 2021 Dangor, Verwey, Lala, Mabaso, Mopeli, Parris, Gray, Chang and Zar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyaad Dangor, eml5YWFkLmRhbmdvckB3aXRzLmFjLnph
 Ziyaad Dangor
Ziyaad Dangor Charl Verwey
Charl Verwey Sanjay G. Lala
Sanjay G. Lala Theodore Mabaso
Theodore Mabaso Keketso Mopeli
Keketso Mopeli Denise Parris3
Denise Parris3 Diane M. Gray
Diane M. Gray Anne B. Chang
Anne B. Chang Heather J. Zar
Heather J. Zar