
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr., 05 August 2021
Sec. Pediatric Nephrology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.707560
Renal arterial infarction can present with hematuria, proteinuria, and hypertension, features often linked to glomerular disease. An aortic aneurysm is an extraordinarily rare complication of coarctation of the aorta. Acute renal infarction caused by emboli from the aortic aneurysm is a possible complication that has not been reported. We herein report a 10-year-old boy who presented with hematuria, proteinuria, hypertension, and skin rashes on both lower extremities mimicking acute glomerulonephritis but actually resulting from acute renal infarction caused by a coarcted aneurysm-associated thrombus. He was successfully treated with surgical excision of the coarcted aorta and aneurysm followed by subcutaneous low molecular weight heparin without recurrence.
Renal infarction is rare in the pediatric population (1–3) and has the potential for significant morbidity if recognition is delayed. Differential diagnoses include glomerulonephritis, pyelonephritis, gastroenteritis, or renal stones (4). Although cardioembolic disease, fibromuscular dysplasia, renal artery thrombosis, and hypercoagulable states are the most common causes of acute renal infarction, other etiologies that can predispose to emboli formation such as aortic aneurysm should be considered.
Herein, we report a case of acute renal infarction caused by an aneurysm-associated thrombus in a child with untreated aortic coarctation. He was managed successfully with surgical intervention and low molecular weight heparin.
A previously healthy 10-year-old boy presented with a 1-day history of fever, abdominal pain, and skin rashes on the lower extremities. The abdominal pain was described as burning and persistent on the left upper abdomen. There was no vomiting, diarrhea, trauma, dysuria, or abnormal urinary frequency. The family history was unremarkable. His blood pressure was 146/92 mmHg, heart rate 150 beats/min, respiratory rate 36 breaths/min, and body temperature 38.0°C. Physical examination revealed mild left upper quadrant abdominal tenderness with equivocal knocking pain on the left costovertebral region and non-blanchable rashes on bilateral lower extremities.
The laboratory studies showed leukocytosis, anemia, upper limit creatinine, elevated C-reactive protein, and lactate dehydrogenase. Urine analysis demonstrated the presence of microscopic hematuria and proteinuria, with pyuria (Table 1). His fever did not respond to empiric antibiotics, and both urine and blood cultures were negative. Abdominal computed tomography (CT) demonstrated renal infarction by the presence of wedge-shaped perfusion defects in the left kidney (Figure 1A). Hypercoagulatory, rheumatologic diseases investigations and an electrocardiogram did not reveal abnormalities. Echocardiogram and CT angiogram revealed coarctation of the descending aorta (Figure 1B) and a 35-mm aneurysm with an intra-aneurysmal mural thrombus distal to the coarctation (Figure 1C). His fever and rashes subsided after an uneventful surgical excision of the coarctation and aneurysm. The intraoperative specimen culture was negative. After surgery, his laboratory data, including white blood cells, C-reactive protein, lactate dehydrogenase, and abnormal urinary sediments returned to normal (Table 1). His renal infarction was treated with subcutaneous low molecular weight heparin followed by 6 months of oral anticoagulant therapy. At 5-year follow-up, he remained normotensive without recurrence.
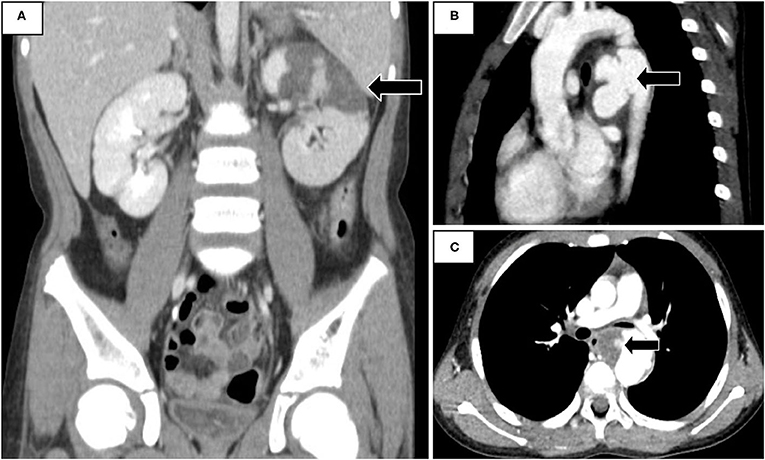
Figure 1. (A) Abdominal Computed tomography (CT) revealed infarction in left kidney. (B) CT angiogram demonstrated a coarcted aneurysm at the descending aorta. (C) Complicated intra-aneurysmal mural thrombus formation with ulceration.
To the best of our knowledge, our finding of a coarcted aneurysm-associated thrombus causing acute renal infarction has not been reported before. This 10-year-old boy presented with fever, rashes, and abdominal pain resulting from renal infarction caused by a coarcted aneurysm. The characteristics of hematuria, proteinuria, hypertension, and rashes on extremities mimic the features of Henoch–Schönlein purpura. Nevertheless, the left costovertebral knocking pain, fever, and elevated serum lactate dehydrogenase and C-reactive protein indicated the possibility of renal infarction (3, 5, 6). An abdominal CT confirmed the diagnosis. Besides thrombolytic or anticoagulant treatment, investigating the underlying etiology should be the focus in order to prevent recurrence of renal infarction. The most common causes of renal infarction are cardiogenic, including arrhythmia, cardiomyopathy, valvular heart diseases, and thrombi from the suprarenal aorta or left ventricle followed by renal artery injury and hypercoagulable state (6, 7). Diagnostic testings including intra-cardiac diseases, hypercoagulable conditions, rheumatologic diseases, and images for the vascular abnormalities are essential for uncovering the underlying etiology. Computed tomographic angiogram demonstrated a descending aortic coarctation-associated aneurysm and complicated intra-aneurysmal mural thrombus formation with ulceration.
Coarctation of the aorta (CoA) is one of the common congenital heart diseases, but only a few patients develop late complications, including hypertension, cardiac failure, coronary artery disease, and aneurysm (8). An aortic aneurysm is a lethal but potentially easily misdiagnosed sequela of corrected aortic coarctation. The prevalence of coarcted aneurysms is not rare, around 5.8%, but almost rises after surgical repair (9). Renal manifestation as the initial presentation of aortic aneurysm is very uncommon and carries diagnostic challenges in patients with previously unrecognized CoA (10). To our knowledge, coarctation-associated aneurysms have been reported in only two pediatric patients who had no prior repair (11, 12). The exact pathogenesis of aneurysms formation of untreated CoA remains unclear. Two hypotheses have been proposed to explain the development of a coarcted aneurysm: first, weaknesses of the aortic wall, caused by friable ductus tissue and coarctation-associated pressure gradient (8) and second, a mycotic aneurysm, also resulting from post stenotic pressure gradient, causing infectious destruction of the vascular wall.
In conclusion, our case highlights that acute renal infarction could present with features mimicking the characteristics of acute glomerulonephritis and intra-aortic thrombus caused by coarcted aorta should be considered as a possible origin of the renal emboli. Early diagnosis of the coarcted aneurysm with timely surgical intervention is essential to avoid catastrophic complications.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee on Human Studies at Chang Gung Memorial Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
M-HT and J-JD contributed to patient's care. Q-YZ, M-HT, and J-JD wrote the first draft of the manuscript. J-LH contributed to the final version of the manuscript. All the authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely acknowledge Tai-Wei Wu for the help in English writing.
1. Huang CC, Lo HC, Huang HH, Kao WF, Yen DHT, Wang LM, et al. ED presentations of acute renal infarction. Am J Emerg Med. (2007) 25:164–9. doi: 10.1016/j.ajem.2006.06.010
2. Nagasawa T, Matsuda K, Takeuchi Y, Fukami H, Sato H, Saito A, et al. A case series of acute renal infarction at a single center in Japan. Clin Exp Nephrol. (2016) 20:411–5. doi: 10.1007/s10157-015-1168-1
3. Bourgault M, Grimbert P, Verret C, Pourrat J, Herody M, Halimi JM, et al. Acute renal infarction: a case series. Clin J Am Soc Nephrol. (2013) 8:392–8. doi: 10.2215/CJN.05570612
4. Piccoli GB, Priola AM, Vigotti FN, Guzzo G, Veltri A. Renal infarction versus pyelonephritis in a woman presenting with fever and flank pain. Am J Kidney Dis. (2014) 64:311–4. doi: 10.1053/j.ajkd.2014.02.027
5. Lumerman JH, Hom D, Eiley D, Smith AD. Heightened suspicion and rapid evaluation with CT for early diagnosis of partial renal infarction. J Endourol. (1999) 13:209–14. doi: 10.1089/end.1999.13.209
6. Oh YK, Yang CW, Kim Y-L, Kang S-W, Park CW, Kim YS, et al. Clinical characteristics and outcomes of renal infarction. Am J Kidney Dis. (2016) 67:243–50. doi: 10.1053/j.ajkd.2015.09.019
7. Caravaca-Fontán F, Pampa Saico S, Elías Triviño S, Galeano Álvarez C, Gomis Couto A, Pecharromán de las Heras I, et al. Acute renal infarction: clinical characteristics and prognostic factors. Nefrol. (2016) 36:141–8. doi: 10.1016/j.nefroe.2016.04.003
8. Webb G. Treatment of coarctation and late complications in the adult. Semin Thorac Cardiovasc Surg. (2005) 17:139–42. doi: 10.1053/j.semtcvs.2005.03.001
9. Knyshov G V., Sitar LL, Glagola MD, Atamanyuk MY. Aortic aneurysms at the site of the repair of coarctation of the aorta: a review of 48 patients. Ann Thorac Surg. (1996) 61:935–9. doi: 10.1016/0003-4975(95)01189-7
10. Ito K, Ookawara S, Ueda Y, Miyazawa H, Tabei K, Morishita Y. Bilateral renal infarction mimicking rapidly progressive glomerulonephritis. Ren Fail. (2016) 38:484–5. doi: 10.3109/0886022X.2016.1138838
11. Pierce WS, Vincent WR, Fitzgerald E, Miller FJ. Coarctation of the abdominal aorta with multiple aneurysms: operative correction. Ann Thorac Surg. (1975) 20:687–63. doi: 10.1016/S0003-4975(10)65762-1
Keywords: renal infarction, coarcted aneurysm, pediatrics, thrombus, glomerulonephritis
Citation: Zhang Q-Y, Tseng M-H, Ding J-J and Huang J-L (2021) Case Report: Acute Renal Infarction in a Child With Coarctation of Aorta. Front. Pediatr. 9:707560. doi: 10.3389/fped.2021.707560
Received: 10 May 2021; Accepted: 28 June 2021;
Published: 05 August 2021.
Edited by:
Marina Vivarelli, Bambino Gesù Ospedale Pediatrico, ItalyReviewed by:
Evgenia Preka, Southampton Children's Hospital, United KingdomCopyright © 2021 Zhang, Tseng, Ding and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing-Long Huang, bG9uZ0BhZG0uY2dtaC5vcmcudHc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.