- Pediatric Surgery Division, Department of Surgery, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital, Yogyakarta, Indonesia
Background: Hirschsprung-associated enterocolitis (HAEC) is a major contributor in the mortality of Hirschsprung disease (HSCR) patients that can occur both preoperatively and post-operatively. Several cut-off values of HAEC score have been used, i.e., ≥10 and ≥4. Here, we compared the HAEC frequency after transanal endorectal pull-through (TEPT) using two cut-offs of scoring system and associated them with the risk factors.
Methods: Cross-sectional analysis was conducted using medical records of HSCR patients who were aged ≤18 years old and underwent TEPT at our institution, Indonesia between 2009 and 2016. HAEC was determined using the scoring system with cut-off values of ≥10 and ≥4.
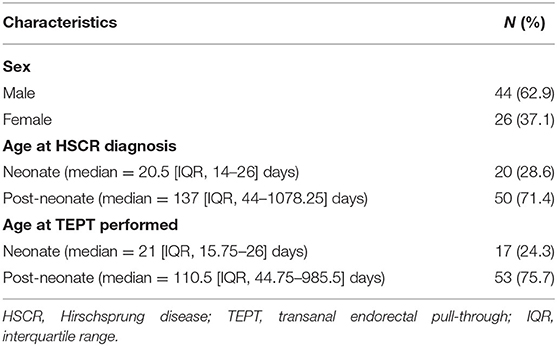
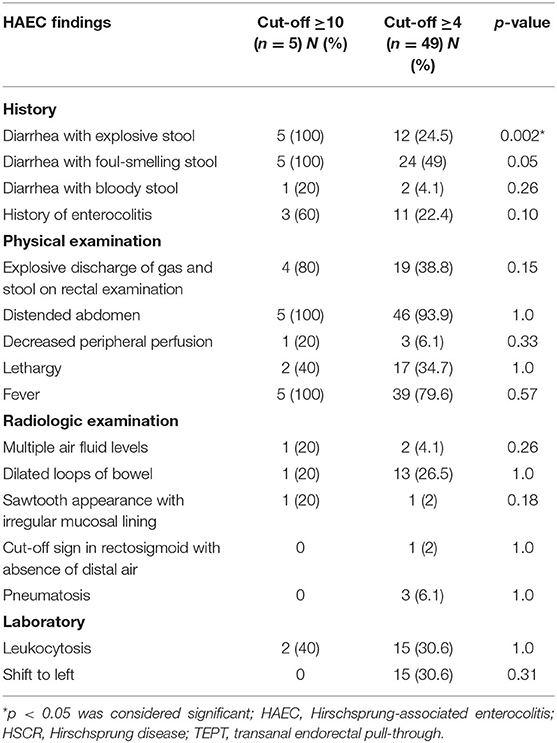
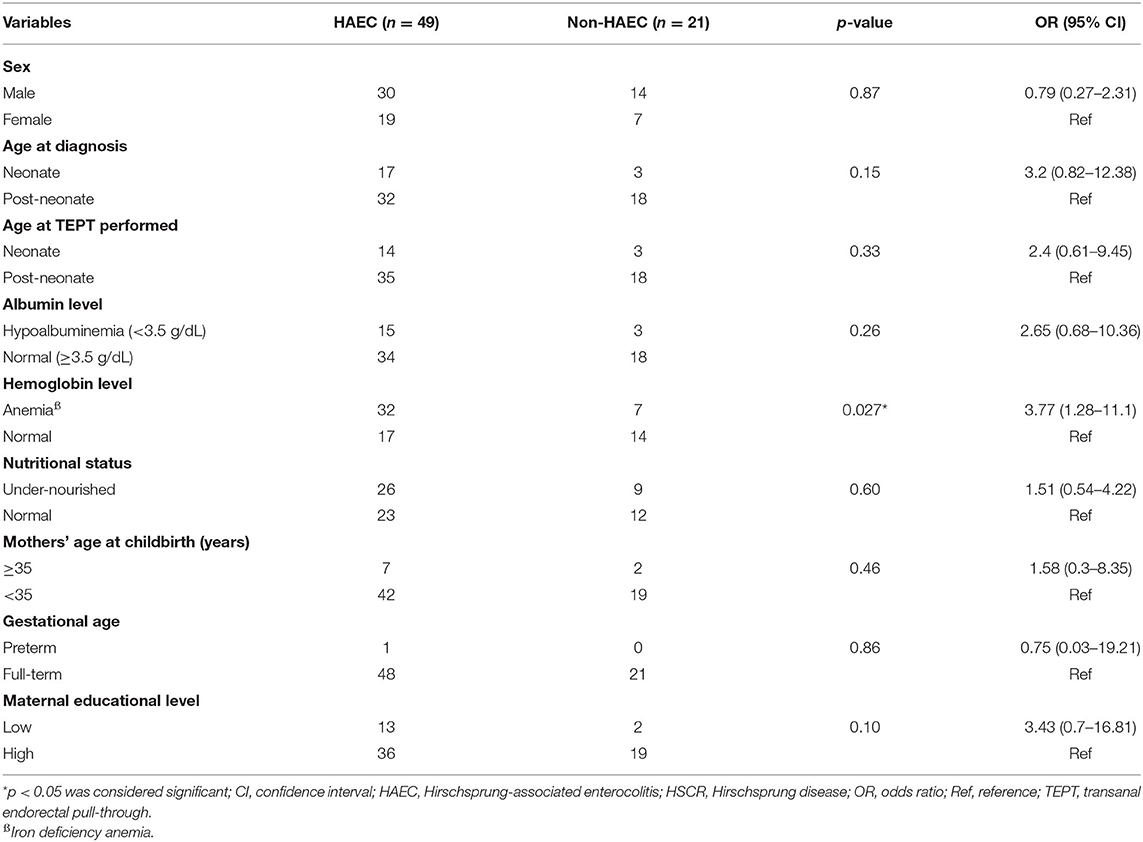
Results: Seventy subjects were used in the final analysis, consisting of 44 males and 26 females. There was a significant difference in one HAEC finding between the ≥10 and ≥4 cut-off groups; diarrhea with explosive stools (p = 0.002). The HAEC frequency was 5/70 (7.1%) and 49/70 (70%) patients using cut-off values of ≥10 and ≥4 (p < 0.0001), respectively. We found that patients with anemia (i.e., iron deficiency anemia) had a higher risk of HAEC after TEPT than patients with normal hemoglobin level with OR of 3.77 (95% CI = 1.28–11.1; p = 0.027), while no associations were found between other variables, including sex, age at diagnosis, age at definitive therapy, albumin level, and nutritional status and HAEC following TEPT (p = 0.87, 0.15, 0.33, 0.26, and 0.60, respectively). Also, no associations were observed between maternal education level, mother's age at pregnancy and gestational age and HAEC after definitive surgery (p = 0.10, 0.46, and 0.86, respectively).
Conclusions: This report is the first study comparing two different cut-off values of scoring system to evaluate the HAEC frequency after TEPT and results suggest further using cut-off of ≥4 to expand the diagnosis of HAEC. Moreover, we also show for the first time that hemoglobin level is a strong risk factor for the HAEC development after TEPT.
Introduction
Hirschsprung-associated enterocolitis (HAEC) is a major contributor of mortality for patients with Hirschsprung disease (HSCR) (1, 2). It can occur both preoperatively and post-operatively (3, 4).
The objective of surgical treatment for HSCR is to remove the aganglionic colon and to anastomose the ganglionic colon above the dentate line (1, 5). Transanal endorectal pull-through (TEPT) is the most current popular procedure in the surgical correction of HSCR since it is less invasive and has many advantages compared with the transabdominal approaches (5–9).
Various risk factors of HAEC after TEPT have been reported, such as sex, age at HSCR diagnosis and pull-through performed, however, they are still limited (10, 11). Moreover, several cut-off values of HAEC score have been used, i.e., ≥10 and ≥4 (12–15). Originally, the HAEC scoring criteria was determined using a Delphi-based consensus of experts that concluded that a score ≥10 was diagnostic of HAEC (12). Frykman et al. (16) performed a critical univariate and multivariate application on those scoring criteria, clustered some of the criteria and concluded that a score ≥4 optimized sensitivity and specificity. Therefore, they proposed a score ≥4 as diagnostic of HAEC (16). Several papers confirmed that the HAEC scoring system with cut-off of 4 increase the sensitivity, which is better for screening purposes (15, 17). Here, we compared the HAEC frequency after TEPT using two cut-offs of scoring system and associated them with the risk factors, including sex, age at HSCR diagnosis and TEPT performed, nutritional status, hemoglobin and albumin level, gestational age, mother's age during pregnancy, and maternal education level.
Materials and Methods
Subjects
We conducted a cross-sectional analysis using medical records of patients with HSCR who were aged ≤18 years old and underwent TEPT at our institution, Indonesia. Our institution is an academic and tertiary referral hospital for patients from two provinces, i.e., Special Region of Yogyakarta and southern part of Central Java, Indonesia. Medical records with incomplete data were excluded from analysis.
This study received prior approval from the Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital (KE/FK/786/EC/2015, KE/FK/713/EC/2015, and KE/FK/1356/EC/2015).
TEPT
The surgical procedure was conducted based on previous reports (6, 7, 18). First, the anal mucosa was exposed by performing everting sutures throughout the anus. Subsequently, the mucosa about 0.5 cm above the dentate line was incised circumferentially. A submucosal dissection was conducted proximally about 1–2 cm, followed by full thickness of rectal wall conversion until the transition zone was determined. Frozen section was conducted to confirm the presence of the ganglion cells in the colon. Once the ganglion cells identified, as a minimum an additional 5 cm of colon was resected to make sure that the transition zone was removed along with the aganglionic colon. Final step was a colo-anal anastomosis.
HAEC Scoring System
Diagnosis of HAEC was determined by scoring system, and included patient history, physical examination, radiologic, and laboratory findings (12–15). We used scores of ≥4 and ≥10 as cut-off values for diagnosis of HAEC (13–17).
Risk Factors
We gathered data on sex, age at HSCR diagnosis, age at TEPT performed, weight and height before TEPT, preoperative albumin, and hemoglobin level.
We categorized hemoglobin and albumin level into anemia (i.e., iron deficiency anemia) and normal (19), and hypoalbuminemia and normal, respectively. The weight and height data were used to measure the nutritional status. Nutritional status of children younger than 5 years old was expressed as weight-for-age z scores (WAZ), while for children over 5 years old, it was expressed as BMI-for-age in relation to growth standards of the age and gender according to the WHO growth chart. We used WHO anthro-application to calculate the nutritional status. The scores gained were then classified as underweight-severely underweight (z < -2) and normal (+2> z >-2) (20, 21).
We also collected the gestational age, mother's age during pregnancy and maternal education level. For maternal education level, we categorized it into low (no education—elementary) and high (junior—high school—bachelor), while for gestational age and mother's age during pregnancy, we classified them into preterm and full-term and ≤35 and >35 years, respectively.
Statistical Analysis
Sample size estimation was determined using an anticipated incidence of HAEC cut-off value of ≥10 and ≥4 of 30 and 70%, respectively, a confidence level of 95% and a power of 80%. The calculated minimum size required was 46 samples. Data were presented as number and percentage for categorical variables. Univariate analysis using Chi-squared or Fisher's Exact tests were performed for each categorical variable to evaluate the association between risk factors and HAEC incidence.
Results
Baseline Characteristics
We collected 75 medical records which fulfilled the inclusion criteria and excluded 5 medical records due to incomplete data. Thus, data from 70 subjects were used in the final analysis, consisting of 44 males and 26 females. Most of our patients were diagnosed (71.4%) and operated on (75.7%) at the post-neonatal period (Table 1). All patients had short-aganglionosis, non-syndromic, and underwent one-stage TEPT without any prior colostomy. The median of follow-up was 131 [interquartile range (IQR), 50.5–342.5] days after TEPT.
Comparison of HAEC Frequency After TEPT Using Two Different Cut-Offs Scoring System
There was a significant difference in one HAEC finding between the ≥10 and ≥4 cut-off groups; diarrhea with explosive stools (p = 0.002, Table 2).
Next, we compared the HAEC incidence after TEPT using two cut-offs of scoring system. The HAEC frequency was 5/70 (7.1%) and 49/70 (70%) patients using cut-off of ≥10 and ≥4 (p < 0.0001), respectively.
Association Between Risk Factors and HAEC After TEPT
We found that patients with anemia had a higher risk of HAEC after pull-through than patients with normal hemoglobin level with OR of 3.77 (95% CI = 1.28–11.1; p = 0.027) when we used cut-off value of ≥4 (Table 3). Apart from that, there were no associations between gender, albumin level, and nutritional status with HAEC incidence following TEPT procedure (p = 0.87, 0.26, and 0.60, respectively).
We also observed no associations between maternal education level, mother's age at childbirth and gestational age and HAEC occurrence after TEPT (p = 0.10, 0.46, and 0.86, respectively).
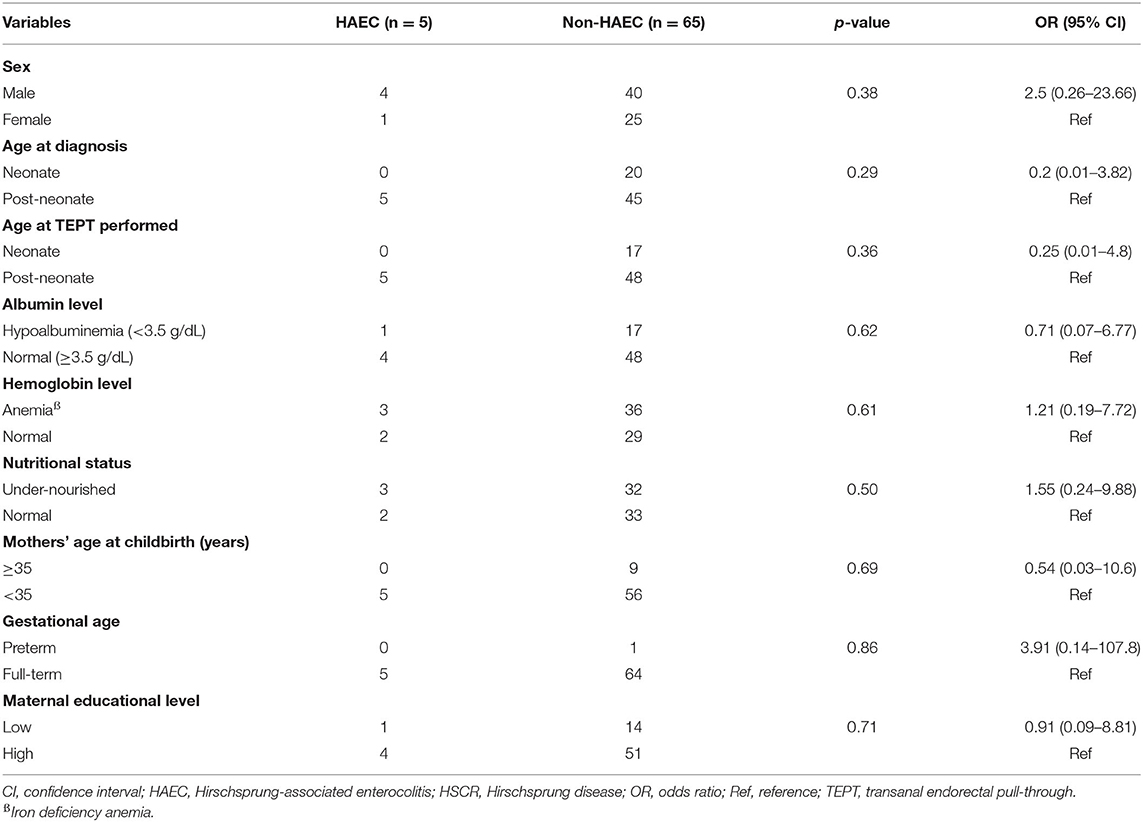
However, none of the risk factors were associated with HAEC following pull-through when we used cut-off value of ≥10 (Table 4).
Discussion
Comparison of HAEC Frequency After TEPT Using Two Different Cut-Offs Scoring System
HAEC has remained a serious complication for patients with HSCR despite the various advancements in diagnosis and therapy. Its pathogenesis and etiology continue to be enigmatic (22). Previous studies about definitive treatment and associated risk factors of HAEC have obtained inconsistent findings (8, 9, 13). Moreover, several cut-offs of scoring system for HAEC diagnosis have been proposed (12, 13, 15–17). Our study intended to compare the HAEC frequency after TEPT between two cut-offs scoring system and identify any associated risk factors. Here, we found that the HAEC frequency after TEPT procedure in our institution was 10-times higher when using cut-off value of ≥4 (~70%) than when using cut-off value of ≥10 (~7%). These findings support our previous study that cut-off value of ≥4 increases the possibility to diagnose HAEC, particularly the mild one (15). However, at least two novelties were noted in our current study: (1) we compared two cut-off values of HAEC scoring system in patients following transanal-approach pull-through [vs. transabdominal Soave and Duhamel pull-through (15)]; and (2) our study provided a new evidence supporting the use of cut-off value of ≥4 to increase the possibility to diagnose HAEC after TEPT from a developing country setting [vs. developed countries (16, 17)].
Frykman et al. suggested that the HAEC scoring system with cut-off of 4 [sensitivity 83.7%; specificity 98.6%, AUC 0.910 (0.85–0.97)] should be used rather than 10 [sensitivity 41.9%; specificity 100%, AUC 0.744 (0.669–0.820)] to prevent under-diagnosing patients with HAEC (16). Thus, lowering the cut-off score could double the incidence of HAEC. Here, we got the average Delphi score of 6.59 ± 2.081, which means many patients would be underdiagnosed if we had used 10 as our cut-off point.
Association Between Risk Factors and HAEC After TEPT
Interestingly, the hemoglobin level was significantly associated with HAEC incidence after TEPT (p = 0.027, Table 3). Anemia might induce dysbiosis in infant intestinal lumen which may contribute to the development of HAEC together with alterations in the intestinal barrier and impaired GI mucosal immunity (22, 23). Patients might have better nutrition and oxygen transport during an operation in a non-anemic condition which would eventually improve the surgery outcome. To the best of our knowledge, our study is the first report showing the effect of iron deficiency anemia on the incidence of HAEC after pull-through.
Our results show that the incident of HAEC after TEPT was not associated either with age at HSCR diagnosis nor age at TEPT performed (p = 0.15 and 0.33, respectively, Table 3). Previous study also reported no significant increase in post-operative HAEC following pull-through with delayed diagnosis or timing of surgery (24, 25). Another study showed that HAEC admissions decreased by 30% with each doubling of age at diagnosis. Early initial management might be more important than definitive surgery itself, for example, colonic irrigation to reduce the size of distended colon and giving probiotics to maintain intestinal integrity, and controlling cytokines' regulatory effect, which would affect the maturation and development of gut immunity (26–28).
One study showed that patients with underweight status have higher proportion of colitis/enteritis after colorectal resection when compared to normal or obese patients (29). Although not statistically significant as a risk factor, malnutrition might contribute to HAEC incidence by its correlation with low hemoglobin and albumin level (29, 30).
We found that hypoalbuminemia during TEPT procedure did not correlate with HAEC incidence after TEPT procedure (Table 3). However, a previous study found that any decrease of albumin level from its normal level has serious outcomes for each 1 g/dl albumin level drop in colorectal surgical patients (31). This discrepancy might be due to different outcomes being measured, while we specifically looked for incident of HAEC in after TEPT procedure. Despite its insignificance as a prognostic factor for HAEC after TEPT, the level of albumin >3.5 g/dl before doing a surgery is important to be considered due to its relation to recovery time, length of stay, morbidity, and mortality in colorectal surgery (31).
Another novelty of our study is we evaluated maternal variables as possible risk factors of HAEC [vs. patient risk factors, i.e., sex, length of aganglionosis, and presence of other major anomalies (10, 11)]. Although, mothers with higher education level may have easier understanding about the acceptance of diagnostic measures, procedures, and risks, we did not find any difference in HAEC incidence between low and high education level (32).
Previous studies suggested that infants born preterm have looser tight junction, less goblet cell secretion, lesser Paneth cells number and secretory function, and lack of IgA secretion and this lack of immunity can lead to higher risk of developing HAEC (33). But, we also failed to prove the correlation and this insignificancy might be associated with the low number of preterm HAEC infants in this study, i.e., one case.
Infants born from mothers with age more than 35 years old have higher tendency to have trisomy 21, while trisomy 21 might increase the incidence of HAEC (34). It might be associated with the fact that trisomy 21 infants show less effective immune response and poorer wound healing (34). However, our study failed to show the association between mothers' age at childbirth and HAEC after TEPT.
Limitations of this study include its retrospective nature and data were subjected to different confounding variables such as variation of follow-up times and various surgeons performing TEPT. Since the medical records were used, our study might bias toward patients with post-operative HAEC and exclude patients with fewer HAEC criteria. Moreover, further prospective multicenter study is necessary and important to clarify and confirm our results.
Conclusions
This report is the first study comparing two different cut-off values to evaluate the frequency of HAEC after TEPT and results suggest further using cut-off of ≥4 to expand the diagnosis of HAEC. Moreover, we also show for the first time that hemoglobin level is a strong risk factor for the HAEC development after TEPT.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Medical and Health Research Ethics Committee of the Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada/Dr. Sardjito Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
G, RL, BA, and FA conceived the study. RL, SK, BA, FA, RP, and G collected and analyzed the data. SK drafted the manuscript. GS, AR, GA, AD, AM, and G critically revised the manuscript for important intellectual content. G, AD, and AM facilitated all project-related tasks. All authors have read and approved the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was funded by the Ministry of Education, Culture, Research and Technology, Indonesia.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the staff and nursing team who were involved in the patient's care. Some results for the manuscript are from RL's, BA's, and FA's theses.
References
1. Tam PK. Hirschsprung's disease: a bridge for science and surgery. J Pediatr Surg. (2016) 51:18–22. doi: 10.1016/j.jpedsurg.2015.10.021
2. Lee CC, Lien R, Chian MC, Yang PH, Chu SM, Fu JH, et al. Clinical impacts of delayed diagnosis of Hirschsprung's disease in newborn infants. Pediatr Neonatol. (2012) 53:133–7. doi: 10.1016/j.pedneo.2012.01.011
3. Demeheri FR, Halawiesh IF, Coran AG, Teitelbaum DH. Hirschsprung-associated enterocolitis: pathogenesis, treatment and prevention. Pediatr Surg Int. (2013) 29:873–81. doi: 10.1007/s00383-013-3353-1
4. Gosain A, Frykman PK, Cowles RA, Horton J, Levitt M, Rothstein DH, et al. Guidelines for the diagnosis and management of Hirschsprung-associated enterocolitis. Pediatr Surg Int. (2017) 33:517–21. doi: 10.1007/s00383-017-4065-8
5. Kyrklund K, Sloots CEJ, de Blaauw I, Bjørnland K, Rolle U, Cavalieri D, et al. ERNICA guidelines for the management of rectosigmoid Hirschsprung's disease. Orphanet J Rare Dis. (2020) 15:164. doi: 10.1186/s13023-020-01362-3
6. De la Torre-Mondragón L, Ortega-Salgado JA. Transanal endorectal pull-through for Hirschsprung's disease. J Pediatr Surg. (1998) 33:1283–6. doi: 10.1016/s0022-3468(98)90169-5
7. Langer JC, Minkes RK, Mazziotti MV, Skinner MA, Winthrop AL. Transanal one-stage Soave procedure for infants with Hirschsprung's disease. J Pediatr Surg. (1999) 34:148–51. doi: 10.1016/s0022-3468(99)90246-4
8. Mao YZ, Tang ST, Li S. Duhamel operation vs. transanal endorectal pull-through procedure for Hirschsprung disease: a systematic review and meta-analysis. J Pediatr Surg. (2018) 53:1710–5. doi: 10.1016/j.jpedsurg.2017.10.047
9. Chen Y, Nah SA, Laksmi NK, Ong CC, Chua JH, Jacobsen A, et al. Transanal endorectal pull-through versus transabdominal approach for Hirschsprung's disease: a systematic review and meta-analysis. J Pediatr Surg. (2013) 48:642–51. doi: 10.1016/j.jpedsurg.2012.12.036
10. Le-Nguyen A, Righini-Grunder F, Piché N, Faure C, Aspirot A. Factors influencing the incidence of Hirschsprung associated enterocolitis (HAEC). J Pediatr Surg. (2019) 54:959–63. doi: 10.1016/j.jpedsurg.2019.01.026
11. Chung PHY, Yu MON, Wong KKY, Tam PKH. Risk factors for the development of post-operative enterocolitis in short segment Hirschsprung's disease. Pediatr Surg Int. (2019) 35:187–91. doi: 10.1007/s00383-018-4393-3
12. Pastor AC, Osman F, Teitelbaum DH, Caty MG, Langer JC. Development of a standardized definition for Hirschsprung's-associated enterocolitis: a Delphi analysis. J Pediatr Surg. (2008) 44:251–6. doi: 10.1016/j.jpedsurg.2008.10.052
13. Parahita IG, Makhmudi A, Gunadi. Comparison of Hirschsprung-associated enterocolitis following Soave and Duhamel procedures. J Pediatr Surg. (2018) 53:1351–4. doi: 10.1016/j.jpedsurg.2017.07.010
14. Yulianda D, Sati AI, Makhmudi A, Gunadi. Risk factors of preoperative Hirschsprung-associated enterocolitis. BMC Proc. (2019) 13(Suppl. 11):18. doi: 10.1186/s12919-019-0172-y
15. Gunadi, Sukarelawanto AVR, Ritana A, Balela N, Putri WJK, Sirait DN, et al. Postoperative enterocolitis assessment using two different cut-off values in the HAEC score in Hirschsprung patients undergoing Duhamel and Soave pull-through. BMC Pediatr. (2020) 20:457. doi: 10.1186/s12887-020-02360-x
16. Frykman PK, Kim S, Wester T, Nordenskjöld A, Kawaguchi A, Hui TT, et al. HAEC Collaborative Research Group: critical evaluation of the Hirschsprung-associated enterocolitis (HAEC) score: a multicenter study of 116 children with Hirschsprung disease. J Pediatr Surg. (2018) 53:708–17. doi: 10.1016/j.jpedsurg.2017.07.009
17. Dore M, Sanchez AV, Junco PT, Barrena S, De Ceano-Vivas M, Gomez JJ, et al. Reliability of the Hirschsprung-associated enterocolitis score in clinical practice. Eur J Pediatr Surg. (2019) 29:132–7. doi: 10.1055/s-0038-1677046
18. Gunadi, Ivana G, Mursalin DA, Pitaka RT, Zain MW, Puspitarani DA, et al. Functional outcomes of patients with short-segment Hirschsprung disease after transanal endorectal pull-through. BMC Gastroenterol. (2021) 21:85. doi: 10.1186/s12876-021-01668-x
19. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. (WHO/NMH/NHD/MNM/11.1) (2011). Available online at: http://www.who.int/vmnis/indicators/haemoglobin.pdf (accessed November 16, 2020).
20. De Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes. (2010) 5:458–60. doi: 10.3109/17477161003615583
21. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr Suppl. (2006) 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x
22. Gosain A, Brinkman AS. Hirschsprung's associated enterocolitis. Curr Opin Pediatr. (2015) 27:364–9. doi: 10.1097/MOP.0000000000000210
23. Ho TTB, Kumar A, Louis-Jacques AF, Dishaw LJ, Yee AL, Groer MW. The development of intestinal dysbiosis in anemic preterm infants. J Perinatol. (2020) 40:1066–74. doi: 10.1038/s41372-020-0599-z
24. Hackam DJ, Filler RM, Pearl RH. Enterocolitis after the surgical treatment of Hirschsprung's disease: risk factors and financial impact. J Pediatr Surg. (1998) 33:830–3. doi: 10.1016/s0022-3468(98)90652-2
25. Ruttenstock E, Puri P. Systematic review and meta-analysis of enterocolitis after one-stage transanal pull-through procedure for Hirschsprung's disease. Pediatr Surg Int. (2010) 26:1101–5. doi: 10.1007/s00383-010-2695-1
26. Langer JC, Durrant AC, de la Torre L, Teitelbaum DH, Minkes RK, Caty MG, et al. One-stage transanal Soave pullthrough for Hirschsprung disease: a multicenter experience with 141 children. Ann Surg. (2003) 238:569–83; discussion: 583–5. doi: 10.1097/01.sla.0000089854.00436.cd
27. Holschneider AM, Puri P editors. Hirschsprung's Disease and Allied Disorders. New York, NY: Springer Berlin Heidelberg (2008). doi: 10.1007/978-3-540-33935-9
28. Wang X, Li Z, Xu Z, Wang Z, Feng J. Probiotics prevent Hirschsprung's disease-associated enterocolitis: a prospective multicenter randomized controlled trial. Int J Colorectal Dis. (2015) 30:105–10. doi: 10.1007/s00384-014-2054-0
29. Rhee R, Miyagaki H, Yan X, Kumara S, Njoh L, Cekic V, et al. Morbidity and outcomes of colorectal surgery in the underweight population: results from the American college of surgeons national surgical quality improvement program (ACSNSQIP) database. Gastroenterology. (2013) 144:S793–4. doi: 10.1016/S0016-5085(13)62930-3
30. Rahman MS, Mushfiquee M, Masud MS, Howlader T. Association between malnutrition and anemia in under-five children and women of reproductive age: evidence from Bangladesh Demographic and Health Survey 2011. PLoS ONE. (2019) 14:e0219170. doi: 10.1371/journal.pone.0219170
31. Truong A, Hanna MH, Moghadamyeghaneh Z, Stamos MJ. Implications of preoperative hypoalbuminemia in colorectal surgery. World J Gastrointest Surg. (2016) 8:353–62. doi: 10.4240/wjgs.v8.i5.353
32. Dzurova D, Pikhart H. Down syndrome, paternal age and education: comparison of California and the Czech Republic. BMC Public Health. (2005) 5:69. doi: 10.1186/1471-2458-5-69
33. Halpern MD, Denning PW. The role of intestinal epithelial barrier function in the development of NEC. Tissue Barriers. (2015) 3:e1000707. doi: 10.1080/21688370.2014.1000707
Keywords: HAEC following pull-through, developing country, HAEC scoring system, transanal endorectal pull through, Hirschsprung disease, children
Citation: Gunadi, Luzman RA, Kencana SMS, Arthana BD, Ahmad F, Sulaksmono G, Rastaputra AS, Arini GP, Pitaka RT, Dwihantoro A and Makhmudi A (2021) Comparison of Two Different Cut-Off Values of Scoring System for Diagnosis of Hirschsprung-Associated Enterocolitis After Transanal Endorectal Pull-Through. Front. Pediatr. 9:705663. doi: 10.3389/fped.2021.705663
Received: 05 May 2021; Accepted: 26 July 2021;
Published: 16 August 2021.
Edited by:
Jürgen Schleef, Institute for Maternal and Child Health Burlo Garofolo (IRCCS), ItalyReviewed by:
Tadaharu Okazaki, Juntendo University, JapanEinar Olafur Arnbjornsson, Skåne University Hospital, Sweden
Copyright © 2021 Gunadi, Luzman, Kencana, Arthana, Ahmad, Sulaksmono, Rastaputra, Arini, Pitaka, Dwihantoro and Makhmudi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gunadi, ZHJndW5hZGlAdWdtLmFjLmlk
 Gunadi
Gunadi Raedi Ardlo Luzman
Raedi Ardlo Luzman