- 1PhD Program in Clinical and Experimental Medicine, University of Modena and Reggio Emilia, Modena, Italy
- 2Neonatal Intensive Care Unit, Women's and Children's Health Department, University Hospital of Modena, Modena, Italy
- 3Pediatrics, Women's, and Children's Health Department, University Hospital of Modena, Modena, Italy
- 4Pediatric Post-graduate School, University Hospital of Modena and Reggio Emilia, Modena, Italy
- 5Neonatal Intensive Care Unit, Santa Maria Nuova Hospital, Reggio Emilia, Italy
- 6Unit of Statistics, Department of Diagnostic, Clinical and Public Health Medicine, University of Modena and Reggio Emilia, Modena, Italy
Objective: This study aims to evaluate safety and success rates of lumbar puncture (LP) and to identify factors associated with adverse events or failure of LP in infants.
Methods: This two-center prospective observational study investigated infants younger than 90 days of age who underwent LP. Need for resuscitation oxygen desaturation (SpO2 < 90%), bradycardia and intraventricular hemorrhage were considered adverse events. LP failed if cerebrospinal spinal fluid was not collected or had traces of blood. Logistic regression analysis was used to evaluate whether corrected gestational age (GA), body weight at LP, position, and any respiratory support during LP affected SpO2 desaturation or failure of LP.
Results: Among 204 LPs, 134 were performed in full-term and 70 in pre-term born infants. SpO2 desaturations occurred during 45 (22.4%) LPs. At multivariate analysis, lower GA at LP (p < 0.001), non-invasive respiratory support (p 0.007) and mechanical ventilation (p 0.004) were associated with SpO2 desaturations. Transient, self-resolving bradycardia occurred in 7 (3.4%) infants. Two infants had intraventricular hemorrhage detected within 72 h of LP. No further adverse events were registered. Failure of LP occurred in 38.2% of cases and was not associated with any of the factors evaluated.
Conclusions: LP was safe in most infants. Body weight or GA at LP did not affect LP failure. These data are useful to clinicians, providing information on the safety of the procedure.
Introduction
The gold standard for the diagnosis of bacterial meningitis is a positive cerebrospinal fluid (CSF) culture, usually obtained through a lumbar puncture (LP) (1). The clinical suspicion of meningitis is greater in the presence of seizures, fever, bulging fontanel and abnormal consciousness, but in neonatal age the initial signs are often subtle, particularly in younger infants (2). Guidelines from the United States of America and United Kingdom recommend performing an LP in cases of suspected sepsis and meningitis in neonates and infants (3, 4).
However, LP is probably performed less frequently than it should be according to the recommendations. Clinicians sometimes may be reluctant to perform an LP because they fear adverse events, they consider a LP technically difficult or excessively invasive. The likelihood of performing an LP seems reduce with decreasing birth weight (5–7). Indeed, a recent study from the United States reported that even in confirmed early-onset sepsis only ~80% of full-term infants and ~40% of very pre-term infants undergo LP (5).
There are two main concerns regarding LP in infants: the safety and the success rates of the procedure. Although these aspects have been extensively investigated in adulthood and in children (8, 9), limited data is available in neonates with a lower gestational age (10).
Thus far, safety has been mainly evaluated in terms of vital sign stability and the occurrence of SpO2 desaturation and bradycardia during an LP. Five prospective studies included sick infants with variable gestational ages (11–15). Most of them evaluated only a few pre-mature infants (12–15), and only one study (11) investigated a larger population of pre-mature infants; however, the risk of complications according to gestational age and birth weight remains poorly defined (8, 9, 16). Failure rates in young infants (age, 0–90 days) have been addressed in seven prospective studies; most of them included full-term neonates, and some included infants up to 1 year of age (8, 9, 17–21). Risks of failure of LP seem higher in infants younger than 90 days of age (8, 9). The present study aimed to assess safety and failure rates of LP.
Materials and Methods
Aim of the Study
The primary aim of the study was to evaluate adverse events associated with LP. As a secondary aim, we assessed the failure rates of LP.
Study Design
This prospective observational study evaluated pre-term and full-term infants undergoing diagnostic LP in two tertiary level Italian hospitals (Azienda Policlinico, Modena; Azienda Santa Maria Nuova, Reggio Emilia). Infants younger than 90 days of age who were admitted to the Neonatal Intensive Care Units or to the Pediatric Departments between June 1, 2016 and December 31, 2019 were included. The study was approved by the local ethical committee (protocol 11/16), and written parental consent was obtained.
Procedure
As per practice in our centers, LP was usually included in the sepsis workup, but it was deferred in case of severe sepsis/septic shock (usually requiring catecholamine support) or in case of instable mechanically ventilated neonates. LPs were performed by physicians or residents. However, the decision regarding who had to start the procedure was left to the physician's discretion, usually depending on the severity of the infant's disease. Any procedure started by residents was performed under the supervision of physicians, who were ready to intervene if residents failed or the newborns worsened.
During the procedure, a nurse flexed the infant's hip and spine, avoiding neck flexion. The decision regarding the newborn's position during LP (sitting or lateral recumbent, both at maximal hip flexion) was made at the clinician's discretion. The procedure could be performed in one or more attempts. An attempt was defined as any single penetration of the needle into the skin; the duration of the procedure was defined as the time between the insertion of the first needle and the extraction of the last one.
A 22-gauge needle was used, and the stylet was always in place while advancing the needle. According to our protocol, a local anesthetic (EMLA) was applied on the spinal skin 30–60 min before performing the LP. Intravenous (midazolam 100 ug/kg) or oral (diazepam 0.2 mg/kg) sedatives or pain relievers (fentanyl 1 ug/kg ev) were given at the clinician's discretion. LP was not performed if the infant had congenital malformations of the spinal region, bleeding disorders, or cellulitis at the site of LP.
Vital signs were evaluated during the time between insertion of the first needle and removal of the last needle. Monitors (IntelliVue MP40 Neonatal or IntelliVue MP2; Philips) were used for vital signs monitoring.
Data Collection
A standardized form was ad-hoc developed for collecting the data. Information on infants undergoing LP was reported by a clinician involved in the procedure. The data form included patient-related factors (sex, gestational ages and body weights at birth and at LP, indication for LP, catecholamine or any respiratory support); who performed the LP (physician or resident); and factors related to the safety and success of LP (vital signs monitoring, need to start or increase oxygen or increase respiratory support during the procedure, need for resuscitation, patient position, number of attempts, the occurrence of any lesion at the skin site within 48 h after LP and evaluation of intraventricular hemorrhage).
Safety of LP was defined as the absence of adverse events. We defined as adverse events the need of intubation or cardiopulmonary resuscitation after LP; oxygen desaturation [oxygen saturation (SpO2) <90%] and transient bradycardia (heart rate <100 beats/min) were considered as secondary adverse events. We also evaluated the rate of intraventricular hemorrhage (IVH) assessed by ultrasound scans within 72 h of LP.
Failure of LP was defined as any of the following: inability to collect CSF or the occurrence of traumatic LP. An LP was considered traumatic if traces of blood could be seen at a visual threshold (22) or if the CSF was bloody.
Statistical Analyses
The characteristics of infants, clinicians, and procedure were summarized using descriptive statistics. Continuous variables were expressed as mean ± standard deviation or median and interquartile range, whereas categorical variables were expressed as frequencies. Adverse events or failure of LP and factors associated with both (i.e., position, who performed the LP, corrected gestational age, body weight at LP and respiratory support) were estimated at uni- and multi-variate logistic regression analysis. We used forward selection strategy for including variables in multivariable model. A p valued < 0.05 was considered significant. The odds ratio was used as a measure of association, and it was reported with its 95% confidence interval.
We estimated that a sample size of 220 LPs provided a two-sided 95% CI with a width equal to 0.13, when the sample proportion of LP failure is around 0.35 (35%). This proportion was estimated on the basis of previous published studies on this topic (17,18,20). Because no substantial information was available regarding SpO2 desaturations during LP, the sample size was estimated based on failure rates.
Results
Characteristics of the Population and Procedure
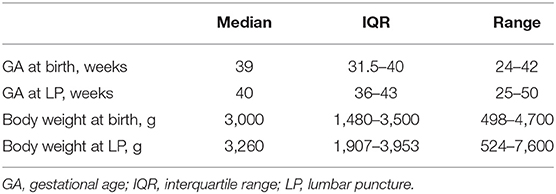
Among 204 LPs, 153 were performed in infants (75%) admitted to the neonatal intensive care units and 51 (25%) were performed in infants admitted to the pediatric ward (Tables 1, 2); 134 (65.7%) out of 204 were performed in full-term and 70 (34.3%) in pre-term infants. Forty LPs were performed in infants with a body weight <1,500 g at LP, of which 19 had a body weight <1,000 g.
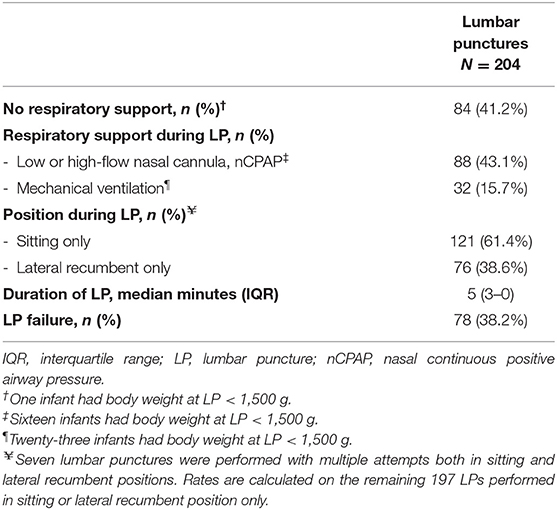
Table 2. Respiratory support, position during lumbar puncture and additional information on performing lumbar puncture.
Among 204 LPs, 108 were performed only by physicians and 96 by residents. LPs were more commonly performed by physicians alone in infants on mechanical ventilation (22 of 32 LPs) or those with a body weight <1,500 g at the time of LP (29 of 40 LPs).
Regarding the position 7 LPs were attempted in both sitting and lateral recumbent position. Among the remaining 197 LPs with a single position, sitting was the preferred position; however, all but 1 LPs in infants on mechanical ventilation (N = 32) were performed by placing the infants only in the lateral recumbent position only.
Safety of Lumbar Puncture
Cardiopulmonary Instability
No infants required intubation or cardiopulmonary resuscitation after LP.
Seven full-term infants received supplemental oxygen prior to LP in order to prevent SpO2 desaturation. No infants who were breathing in room air were given oxygen during LP. Oxygen was increased during LP in 13 infants (body weight <1,500 g, N = 8) who were on non-invasive respiratory support. Oxygen or inspiratory pressure were increased during LP in 10 of 32 (31.3%) infants on mechanical ventilation (body weight <1,500 g, N = 8).
Figure 1 shows the rates of SpO2 desaturations (SpO2 <90%) during 204 LPs performed in infants who had vital signs assessment. Forty-five LPs (22.1%) were performed in infants who had transient SpO2 desaturations during LP, although the value of SpO2 desaturation was not specified in 13 cases. SpO2 desaturations were significantly more common when LP was performed in infants with a lower body weight at LP (24 of 40 with a body weight <1,500 g vs. 21 of 164 with a body weight >1,500 g, p <0.001). Only five infants had SpO2 desaturation <60% during LP. The footnote of Figure 1 reports infants on catecholamine support and the percentage of SpO2 desaturations in these infants.
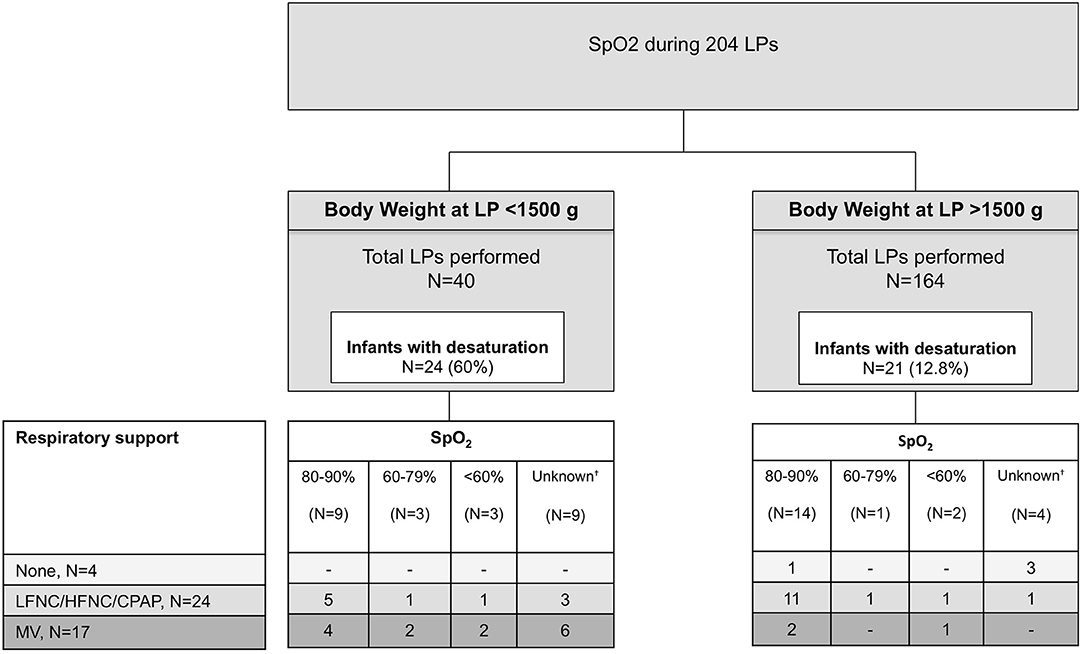
Figure 1. SpO2 desaturations (<90%) in 204 infants who had vital signs assessment during LP. SpO2 desaturations are divided according to their severity. HFNC, high-flow nasal cannula; MV, mechanical ventilation; LFNC, low-flow nasal cannula; LP, lumbar puncture; nCPAP, nasal continuous positive airway pressure.†Cases in which the standardized form indicates “desaturation” but without reporting the degree. Twenty of 204 (10%) of LPs were performed in infants on catecholamine support (dobutamine, dopamine or both). Among 20 LPs, 11 were performed in infants with body weight <1,500 g at the time of LP; 8 infants were on CPAP and 12 on mechanical ventilation. Desaturations occurred in 9 infants on catecholamine support.
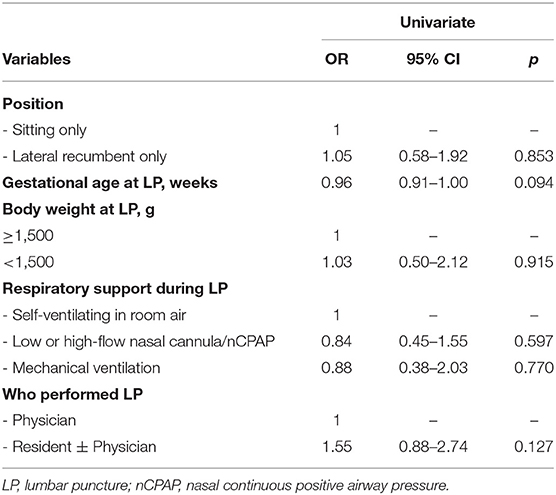
Lateral position, lower gestational age and body weight (<1,500 g) at LP and any respiratory support were significantly associated with SpO2 desaturation at univariate analysis (Table 3); at multivariate analysis, risks of SpO2 desaturations remained associated with lower gestational age at LP and use of any respiratory support. No differences were found when LP was performed by physicians alone or by residents.
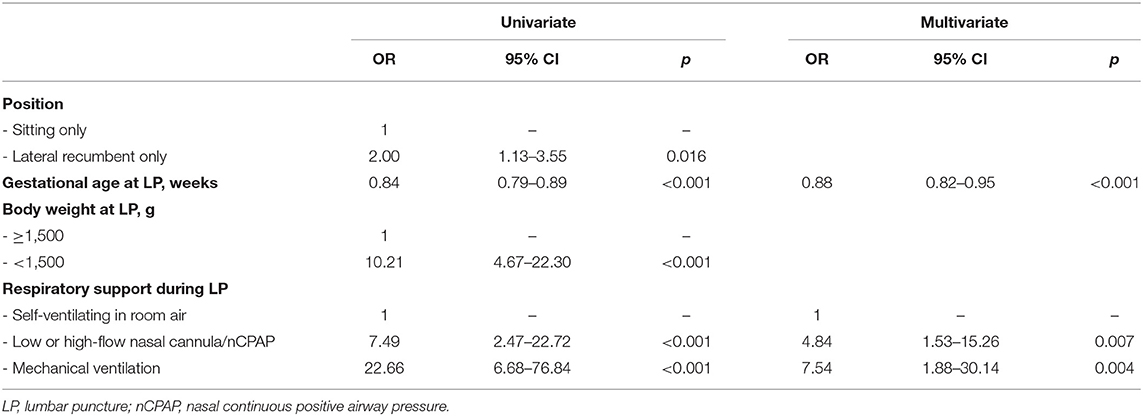
Table 3. Univariate and multivariate analyses of factors associated with SpO2 desaturations during lumbar puncture.
Transient, self-resolving bradycardia (heart rate over 70 beats/min) was observed during 7 LPs.
Further Complications
Among infants with body weight at LP <1,500 g, one had bilateral grade 1 IVH detected 24 h after LP and one had unilateral grade 2 IVH detected 72 h after LP, when a severe septic shock was diagnosed. No local complications at the LP site or further adverse events were observed.
Failure of Lumbar Puncture
The median of attempts performed was 1 (IQR 1–2, range 1–6). Among 204 LPs, 78 (38.2%) were unsuccessful: they were traumatic (mildly bloody: N = 47) or CSF was not obtained (N = 31). The remaining 126 (61.8%) were successful (Table 2); most of them (85, 67.5%) required only one attempt. At univariate analysis we did not find associations with LP failure (Table 4).
Discussion
This study aims to evaluate safety and success rates of LP in sick young infants aged 0 to 90 days. Our data shows that most LPs are safe, while the success rate is ~60%.
This is the largest prospective study investigating these aspects in infants; it includes about 20% of pre-term infants, who have the highest risk of meningitis (23). Adverse events and failure of LP have been evaluated according to different gestational ages and body weight, and information is provided in real-life settings, as suggested by others (24).
Since LP plays a pivotal role in diagnosing meningitis, clinicians should know if LP is associated with risks and, if so, the extent of the risks.
No infants required intubation after LP, cardiopulmonary resuscitation or drugs. Positive inspiratory pressure was increased after LP in a few infants who were mechanically ventilated.
In literature, the safety of LP in young infants has been mostly evaluated according to the severity of SpO2 desaturations (11, 13, 14). Unfortunately, these studies only concern a few newborns, and do not allow us to understand the true extent of the SpO2 desaturation. In order to better assess the actual risks, some have mimicked LP by positioning sick or healthy infants in different positions (24–26). These studies have shown that the lateral recumbent position is risky, particularly after maximal hip flexion (i.e., the “knee-chest” position). We found a strong association between the lateral recumbent position and SpO2 desaturations at univariate analysis; however, since all but one infant on mechanical ventilation were placed in the lateral recumbent position, we could not confirm this association by means of multivariate analysis.
It is unclear whether desaturations are related to the LP itself or sometimes to the positioning of the infants during the procedure (11, 26). Furthermore, during mechanical ventilation additional unintended risks may occur, since it is possible that the endotracheal tube dislocates. Therefore, we recommend the utmost care in preparing the infant to LP, paying attention to the correct position of the endotracheal tube. To date, only one study, which includes 7 pre-term and 9 full-term infants, has investigated the risks of SpO2 desaturation when LP is performed in neonates who suffer from respiratory disease (14). That study has found that the risk is significantly higher in pre-term neonates with respiratory distress syndrome. Consistent with that study, we found that the highest risk of SpO2 desaturation occurred in infants under mechanical ventilation.
Furthermore, we found that SpO2 desaturations were more common in infants with a lower gestational age at the time of LP. We emphasize the importance of using special care in the pre-term infant undergoing LP. Nevertheless, only a few infants (being all on respiratory support) experienced severe SpO2 desaturations or transient and self-resolving bradycardia. No infants had evidence of severe complications. Both infants who had intraventricular hemorrhage, had additional risks for bleeding (septic shock and low body weight), independently from the LP.
Approximately 38% of LPs failed, a rate that is consistent with the 23% to 41% reported in three studies regarding infants younger than 90 days of age (17, 18, 20). LP failure was not affected by a lower corrected gestational age and body weight at LP; these findings reassure clinicians who are less confident with LP in younger infants. Failure rates were not affected by the position, nor by the clinician who performed the LP (physicians or residents); these results support the training of young doctors in performing LP (17, 18, 20, 22). However, the failure rate is particularly high and additional techniques [i.e., ultrasound assisted LP (19)] will probably improve success rates in the future.
The present study has some potential limitations. Firstly, we were unable to calculate a sample size for the primary aim, since studies on the safety of LP are lacking. Secondly, an unquantified number of LPs were deferred, being a potential bias for the calculation of adverse events. However, this number seems low (<5–10% of total LPs), since we usually defer an LP only in septic shock (usually requiring catecholamine support) or in the case of instable, mechanically ventilated neonates. Additionally, the definition of traumatic LP was based only on visual assessment without obtaining a CSF red cell blood count. Further factors [such as inability to palpate the spinous process (8, 9) and the use of sedation or anesthetics] that were not investigated in this study could have affected the clinical worsening of neonates or the risk of an LP failure. Finally, SpO2 levels were not reported in all cases of desaturation, although missing values were only a few of the total LPs.
In conclusion, this study shows that LP was safe in most infants younger than 90 days of age. The risk of SpO2 desaturations was higher in infants with a lower gestational age at LP or those undergoing mechanical ventilation. However, only a few infants required escalating respiratory support, and none was intubated or resuscitated after LP. Pre-maturity was not associated with an increased risk of LP failure. By means of this information clinicians will be more confident while performing an LP, especially knowing which infants have the greatest risks associated with the procedure.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Comitato Etico dell'Area Vasta Emilia Nord (Protocol 11/16). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
LBe and ABe conceptualized and designed the study, coordinated and supervised data collection, drafted the initial manuscript, and reviewed and revised the manuscript. LM, ABa, FL, and LBa collected data and drafted the initial manuscript. LLug, LLuc, CR, MR, RD'A, and LI critically analyzed and interpreted data, and critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ku LC, Boggess KA, Cohen-Wolkowiez M. Bacterial meningitis in infants. Clin Perinatol. (2015) 42:29–45. doi: 10.1016/j.clp.2014.10.004
2. Berardi A, Lugli L, Rossi C, China MC, Vellani G, Contiero R, et al. Neonatal bacterial meningitis. Minerva Pediatr. (2010) 62(Suppl. 1):51–4.
3. National Collaborating Centre for Women's and Children's Health (UK). Bacterial Meningitis and Meningococcal Septicaemia: Management of Bacterial Meningitis and Meningococcal Septicaemia in Children and Young People Younger than 16 Years in Primary and Secondary Care. London: RCOG Press (2010). (National Institute for Health and Clinical Excellence: Guidance). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK83078/ (accessed December 4, 2020).
4. Polin RA Committee on Fetus and Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. (2012) 129:1006–15. doi: 10.1542/peds.2012-0541
5. Stoll BJ, Puopolo KM, Hansen NI, Sánchez PJ, Bell EF, Carlo WA, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. (2020) 174:e200593. doi: 10.1001/jamapediatrics.2020.0593
6. Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. (2011) 127:817–26. doi: 10.1542/peds.2010-2217
7. Berardi A, Baroni L, Bacchi Reggiani ML, Ambretti S, Biasucci G, Bolognesi S, et al. The burden of early-onset sepsis in Emilia-Romagna (Italy): a 4-year, population-based study. J Matern Fetal Neonatal Med. (2016) 29:3126–31. doi: 10.3109/14767058.2015.1114093
8. Baxter AL, Fisher RG, Burke BL, Goldblatt SS, Isaacman DJ, Lawson ML. Local anesthetic and stylet styles: factors associated with resident lumbar puncture success. Pediatrics. (2006) 117:876–81. doi: 10.1542/peds.2005-0519
9. Nigrovic LE, Kuppermann N, Neuman MI. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med. (2007) 49:762–71. doi: 10.1016/j.annemergmed.2006.10.018
10. Marshall ASJ, Sadarangani M, Scrivens A, Williams R, Yong J, Bowler U, et al. ‘The NeoCLEAR Collaborative Group'. Study protocol: NeoCLEAR: Neonatal Champagne Lumbar punctures Every time—An RCT: a multicentre, randomised controlled 2 ×2 factorial trial to investigate techniques to increase lumbar puncture success. BMC Pediatr. (2020) 20:165. doi: 10.1186/s12887-020-02050-8
11. Porter FL, Miller JP, Cole FS, Marshall RE. A controlled clinical trial of local anesthesia for lumbar punctures in newborns. Pediatrics. (1991) 88:663–9.
12. Kaur G, Gupta P, Kumar A. A randomized trial of eutectic mixture of local anesthetics during lumbar puncture in newborns. Arch Pediatr Adolesc Med. (2003) 157:1065. doi: 10.1001/archpedi.157.11.1065
13. Weisman LE, Merenstein GB, Steenbarger JR. The effect of lumbar puncture position in sick neonates. Am J Dis Child. (1983) 137:1077–9. doi: 10.1001/archpedi.1983.02140370037012
14. Sun S, Vangvanichyakorn K, Aranda Z, Ruiz N, Levine R. 1447 Harmful effect of lumbar puncture in newborn infants. Pediatric Res. (1981) 15:684. doi: 10.1203/00006450-198104001-01476
15. Fiser DH, Gober GA, Smith CE, Jackson DC, Walker W. Prevention of hypoxemia during lumbar puncture in infancy with preoxygenation. Pediatric Emergency Care. (1993) 9:81–3. doi: 10.1097/00006565-199304000-00005
16. Bedetti L, Marrozzini L, Baraldi A, Spezia E, Iughetti L, Lucaccioni L, et al. Pitfalls in the diagnosis of meningitis in neonates and young infants: the role of lumbar puncture. J Matern Fetal Neonatal Med. (2019) 32:4029–35. doi: 10.1080/14767058.2018.1481031
17. Pinheiro JM, Furdon S, Ochoa LF. Role of local anesthesia during lumbar puncture in neonates. Pediatrics. (1993) 91:379–82.
18. Neal JT, Kaplan SL, Woodford AL, Desai K, Zorc JJ, Chen AE. The effect of bedside ultrasonographic skin marking on infant lumbar puncture success: a randomized controlled trial. Ann Emerg Med. (2017) 69:610–9. doi: 10.1016/j.annemergmed.2016.09.014
19. Gorn M, Kunkov S, Crain EF. Prospective investigation of a novel ultrasound-assisted lumbar puncture technique on infants in the pediatric emergency department. Gerhardt RT, curatore. Acad Emerg Med. (2017) 24:6–12. doi: 10.1111/acem.13099
20. Hanson AL, Schunk JE, Corneli HM, Soprano JV. A randomized controlled trial of positioning for lumbar puncture in young infants. Pediatr Emerg Care. (2016) 32:504–7. doi: 10.1097/PEC.0000000000000469
21. Schreiner RL, Kleiman MB. Incidence and effect of traumatic lumbar puncture in the neonate. Dev Med Child Neurol. (1979) 21:483–7. doi: 10.1111/j.1469-8749.1979.tb01652.x
22. Shafer S, Rooney D, Schumacher R, House JB. Lumbar punctures at an academic level 4 NICU: indications for a new curriculum. Teach Learn Med. (2015) 27:205–7. doi: 10.1080/10401334.2014.979185
23. Berardi A, Sforza F, Baroni L, Spasa C, Ambretti S, Biasucci G, et al. Epidemiology and complications of late-onset sepsis: an Italian area-based study. PLoS ONE. (2019) 14:e0225407 doi: 10.1371/journal.pone.0225407
24. Öncel S, Günlemez A, Anik Y, Alvur M. Positioning of infants in the neonatal intensive care unit for lumbar puncture as determined by bedside ultrasonography. Arch Dis Child Fetal Neonatal Ed. (2013) 98:F133–5. doi: 10.1136/archdischild-2011-301475
25. Spahr RC. Knee-chest position and neonatal oxygenation and blood pressure. Arch Pediatr Adolesc Med. (1981) 135:79. doi: 10.1001/archpedi.1981.02130250065021
Keywords: lumbar puncture, meningitis, sepsis, newborn, pre-maturity, very low birth weight
Citation: Bedetti L, Lugli L, Marrozzini L, Baraldi A, Leone F, Baroni L, Lucaccioni L, Rossi C, Roversi MF, D'Amico R, Iughetti L and Berardi A (2021) Safety and Success of Lumbar Puncture in Young Infants: A Prospective Observational Study. Front. Pediatr. 9:692652. doi: 10.3389/fped.2021.692652
Received: 08 April 2021; Accepted: 17 May 2021;
Published: 14 June 2021.
Edited by:
Brenda M. Morrow, University of Cape Town, South AfricaReviewed by:
Corinna Binder-Heschl, Medical University of Graz, AustriaReinout A. Bem, Amsterdam University Medical Center, Netherlands
Copyright © 2021 Bedetti, Lugli, Marrozzini, Baraldi, Leone, Baroni, Lucaccioni, Rossi, Roversi, D'Amico, Iughetti and Berardi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Bedetti, bHVjYS5iZWRldHRpQHVuaW1vcmUuaXQ=
 Luca Bedetti
Luca Bedetti Licia Lugli2
Licia Lugli2 Lucia Marrozzini
Lucia Marrozzini Lorenzo Iughetti
Lorenzo Iughetti Alberto Berardi
Alberto Berardi