
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pediatr. , 29 June 2021
Sec. Pediatric Immunology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.687538
 Suleiman Al-Hammadi1,2*†
Suleiman Al-Hammadi1,2*† Amal M. Yahya3†
Amal M. Yahya3† Abdulla Al-Amri3
Abdulla Al-Amri3 Amar Shibli3
Amar Shibli3 Ghazala B. Balhaj4
Ghazala B. Balhaj4 Mohamed I. Tawil5†
Mohamed I. Tawil5† Ranjit Vijayan6†
Ranjit Vijayan6† Abdul-Kader Souid2†
Abdul-Kader Souid2†In the United Arab Emirates, BCG (Bacillus Calmette-Guérin) is administered to all newborns. We present here a young infant with an inborn error of immunity (IEI) who developed fatal adverse events to this live-attenuated vaccine. This male infant received BCG (Serum Institute of India Pvt., Ltd., India) on Day 11 of life. On Day 25, he developed fever, followed by cervical lymphadenitis and bilateral otitis media with fluid drainage. On Day 118, he was admitted with severe hemophagocytic lymphohistiocytosis (HLH), and passed away on Day 145. The diagnostic exome sequencing test identified a hemizygous nonsense variant, NM_000397.3(CYBB):c.676C>T, p.Arg226* (rs137854592). Pathogenic variants of CYBB [cytochrome b(-245), beta subunit; Mendelian Inheritance in Man [MIM] accession code, 300481] are known to cause “immunodeficiency 34, mycobacteriosis, X-linked” (IMD34, MIM#300645) and “chronic granulomatous disease, X-linked” (CGDX, MIM#306400). The natural history of his illness is consistent with “X-linked recessive Mendelian susceptibility to mycobacterial disease (MSMD).” This entity is responsible for his BCG disease and is a likely trigger of his HLH. This disastrous event underlines the importance of developing worldwide policies that target BCG disease prevention, especially in communities with high prevalence of IEI. Moreover, screening for genetic causes of MSMD in the community could pave the way, at least partially, for scale-up of tuberculosis (TB) prevention.
Bacillus Calmette-Guérin, a live vaccine derived from attenuated Mycobacterium bovis cultures, is administered to all newborns in the United Arab Emirates (UAE) since 2005 (1). This practice has resulted in serious adverse events in our tribal communities, especially in children with unrevealed inborn error of immunity (IEI) (2, 3). Moreover, the BCG strains may also cause serious (often fatal) complications in “Mendelian susceptibility to mycobacterial diseases” (MSMD) (4). MSMD embraces inherited entities that result from both “virulent” and “faintly virulent” mycobacteria, including BCG vaccines (5, 6). An example of an MSMD-causing autosomal variant in our tribal communities is IFNGR2 (interferon-gamma receptor 2; MIM#147569):c.123C>G, p.Tyr41*, which results in “immunodeficiency 28, mycobacteriosis” (IMD28; MIM#614889) (7). Examples of MSMD-causing X-linked pathogenic variants include CYBB:Gln231Pro (rs151344498), CYBB:Thr178Pro (rs151344497), and NM_000397.3(CYBB):c.1662dup, p.Glu555* (rs1453468510) (8–11). This case report describes serious adverse events of the BCG vaccine in a young infant with X-linked MSMD. Its main aim is to provide further justifications for establishing safe rules for BCG vaccination.
This male infant, an offspring of first-cousin parents, was born at 35 weeks' gestation by Cesarean delivery due to premature contractions. The mother was gravida 4, para 3. Her pregnancy was complicated by gestational diabetes (managed with insulin) and systemic lupus erythematosus (SLE, treated with hydroxychloroquine). She was on prophylactic enoxaparin during the pregnancy. Otherwise, the family health history (parents, siblings, grandparents, uncles, and aunts, and first-degree cousins) was unremarkable, including negative exposure to known tuberculosis (TB) cases. His birth weight was 3,125 g. His postnatal period was complicated by respiratory distress that required neonatal intensive care supports for 11 days. BCG (0.05 ml, intradermal injection in the upper right arm [manufactured by Serum Institute of India Pvt. Ltd., India]) and hepatitis B pediatric [10 μg [0.5 ml] intramuscular injection] vaccines were then administered on Day 11 (the day of discharge from the neonatal intensive care unit, NICU). Thereafter, he developed unremitting fever (Figure 1A) and passed away on Day 145 (Table 1).
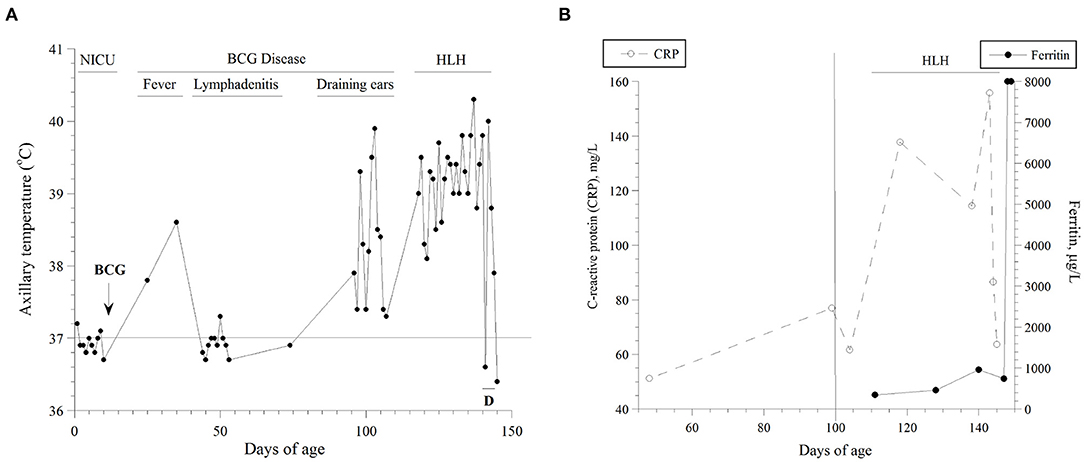
Figure 1. (A) Axillary temperatures as function of age. The highest value per day is shown. The natural course of his disease is also depicted. D, dexamethasone; NICU, neonatal intensive care unit; HLH, hemophagocytic lymphohistiocytosis. He was inpatient during the highlighted periods. (B) Serum levels of C-reactive protein (CRP, mg/l) and ferritin (μg/l) as functions of age. Estimations of the onset (vertical line on Day 100) and duration of his HLH are depicted.
Briefly, he was admitted on Day 45 with fever and bilateral cervical lymphadenitis (a tender left upper cervical lymph node measuring “2.3 × 1.7 × 1.5 cm” and a right cervical lymph node measuring “1.2 × 0.8 × 0.8 cm”; Figure 2A) and received empiric antibiotics for 10 days. On Day 96, he was readmitted for treatment of bilateral otitis media with yellow discharges and received again empiric antibiotics for 10 days. On Day 118, he was admitted with persistent high fever, massive/firm hepatosplenomegaly (spleen 10 cm below the left costal margin and liver down to the pelvis), skin rash, failure to gain weight, and an unhealed BCG injection site (Figure 3). Treatment for BCG disease was never received. On Day 140, he was started on dexamethasone (10 mg/m2/days, intravenously), as an empiric treatment of hemophagocytic lymphohistiocytosis (HLH). He passed away on Day 145 of multiorgan (hepatic, respiratory, and renal) failure and disseminated intravascular coagulation (DIC), Figure 2B.
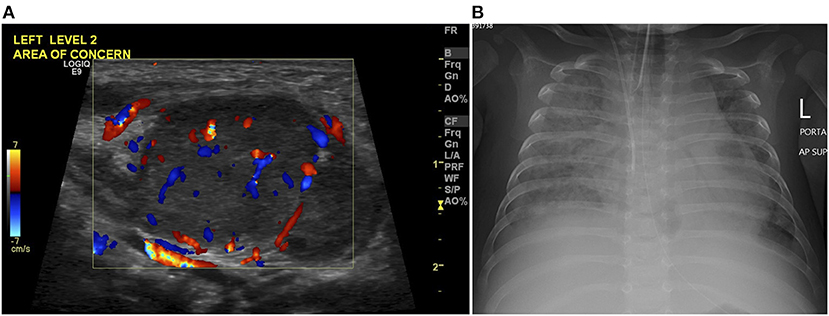
Figure 2. (A) Color Doppler images on neck ultrasound scan at age of 45 days demonstrates an enlarged left cervical lymph node of increased vascularity in keeping with lymphadenitis. (B) Chest radiograph at age of 145 days shows tube and line in position, and bilateral diffuse alveolar-interstitial opacification with right pleural effusion in keeping with pulmonary edema.
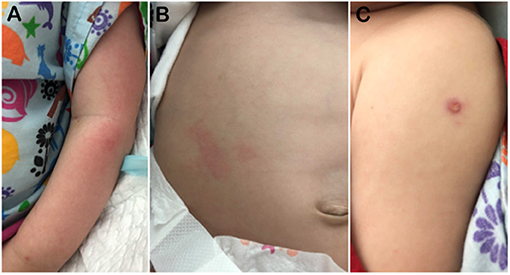
Figure 3. Photographs on Day 140 showing (A) his erythematous skin rash, (B) massive hepatomegaly, and (C) BCG site. The BCG vaccination site measured about 5 mm; it was a wet papule with central yellow crust.
During the last admission, his complete blood counts (CBC), blood film, lymphocyte subset, quantitative immunoglobulin, bone marrow aspirate and biopsy studies (morphology, cytogenetics, and flow cytometry; the results did not reveal active hemophagocytosis), skeletal scintigraphy, and echocardiography were unrevealing (Table 1). Epstein–Barr virus (EBV) and cytomegalovirus (CMV) real-time polymerase chain reaction (PCR) tests were negative. Neutrophil oxygen burst study was not done. Nasopharyngeal swabs for respiratory pathogens were negative by PCR, including coronavirus disease (COVID-19) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Initial neck ultrasound scan confirmed presence of a cervical lymphadenitis with no evidence of fluid collection. Computerized tomography (CT) of the chest, abdomen, and pelvis demonstrated hepatosplenomegaly, otherwise were unremarkable. Moderate ascites was demonstrated on follow-up ultrasound scan. Radiographically, he developed a progressive picture of acute respiratory syndrome.
Results of his serum ferritin and C-reactive protein (CRP) are summarized in Figure 1B. Between Days 120 and 145, his serum triglyceride was (mean ± SD) 1.77 ± 0.42 mmol/l (n = 5; normal 0.25–0.85), alanine transaminase (ALT) 202 unit/l (increased to 1,175 unit/l on Day 145; normal, ≤ 41), and ammonia 130 μmol/l (normal, 16–60). On Day 130, soluble interleukin-2 receptor (sIL-2R) was 5,386 pg/ml (reference values, 175–858) and interleukin six was 50.6 pg/ml (normal, ≤ 7.0). On Day 143, a blood culture grew Staphylococcus haemolyticus. On Day 144, he developed refractory coagulopathy, including hypofibrinogenemia. On Day 147 (two days after he passed away), diagnostic exome sequencing revealed the pathogenic hemizygous variant NM_000397.3(CYBB):c.676C>T, p.Arg226* (rs137854592).
This young infant has the hemizygous pathogenic variant CYBB:p.Arg226* (rs137854592) and has received the BCG vaccine. Shortly thereafter, he developed BCG disease (fever with lymphadenitis) and progressed to severe HLH (systemic inflammation with massive hepatosplenomegaly due to lymphocyte proliferation). Thus, his HLH is likely triggered by the BCG disease. His treatment must be urgent and directed toward both the BCG disease and HLH. Delaying effective therapies (anti-BCG disease as well as standard HLH management) until a molecular diagnosis is evident is imprudent. It is well to know that the minimum inhibitory concentrations of isoniazid, streptomycin, rifampicin, and ethambutol are included in the BCG vaccine Package Insert (Serum Institute of India Pvt. Ltd., Pune, India).
The term “Mendelian susceptibility to mycobacterial disease (MSMD)” labels inherited entities that result from both “virulent” and “faintly virulent” mycobacteria, including BCG vaccines (12–14). CYBB (MIM#300481; X-linked) encodes for the beta-subunit of phagocyte NADPH (nicotinamide adenine dinucleotide phosphate) oxidase. Pathogenic variants of CYBB such as Gln231Pro (rs151344498) and Thr178Pro (rs151344497) have been identified within this clinical cluster (8). Hemizygous males with these variants have reduced macrophage oxidative burst (a variant of chronic granulomatous disease, CGD), but normal respiratory function in the granulocytes and monocytes. They become symptomatic after exposure to mycobacteria, and their disease has been labeled: “X-linked recessive MSMD” (8).
Pathogenic variants of CYBB, including CYBB:p.Arg226* and NM_000397.3(CYBB):c.1662dup, p.Glu555* (rs1453468510) (15), are associated with “immunodeficiency 34, mycobacteriosis, X-linked” (IMD34; MIM#300645) and “chronic granulomatous disease, X-linked” (CGDX; MIM#306400) (10, 11). Arg226 is located near the C-terminus end of the fifth transmembrane helix of the protein, and a truncation (non-sense mutation) will delete the cytoplasmic dehydrogenase (DH) domain that consists of the FAD (flavin adenine dinucleotide) and NADPH-binding domains (Figure 4). This deletion will also remove the last transmembrane helix of the protein, likely making it unable to fold or function as expected.
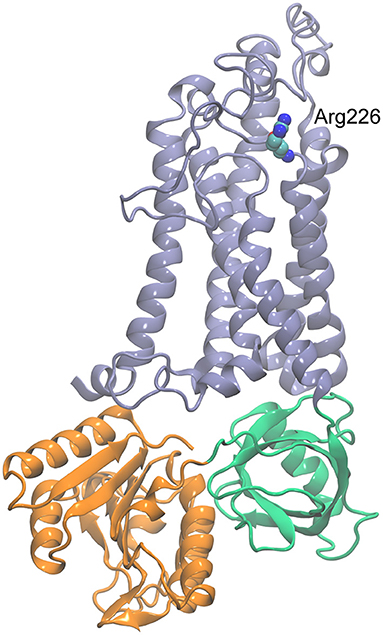
Figure 4. Homology model of CYBB generated with SWISS-MODEL (https://swissmodel.expasy.org/) using the dual oxidase 1 (DUOX1; Protein Data Bank ID: 7D3E) structure as the template. The transmembrane region is shown in blue, FAD-binding domain in green, and NADPH-binding domain in orange. Arg226 is shown in space-filling representation.
It is also important to point out that the T cell function in these CYBB variants is expected to be unaltered. Therefore, a widespread TB disease is unlikely. The problem, rather, is an uncontrolled systemic inflammation, as seen in HLH (16).
Studies are also needed to understand the contribution of IEI variants (i.e., MSMD) to TB disease in our community. Identifying and screening for such variants allow a safer BCG vaccination program and improve the TB screening in individuals at risk (17). Intense migration to UAE from TB endemic regions imposes high risk to the population, especially those with MSMD. In our population, the prevalence of latent TB infection (LTBI) in children is 0.45% and in adults 18.8% (8% among our medical students) (18–20). In addition, future studies are needed to determine whether the carrier state of CYBB:p.Arg226* could have contributed to the development of SLE in his mother.
This case report provides further justification for developing and implementing safe rules for BCG vaccination locally and internationally. It is the responsibility of health authorities to establish effective strategies that prevent improper vaccinations. This young infant emphasizes the need to take essential precautions before immunizing children and adults with live vaccines.
It is vital to emphasize the WHO position: “BCG vaccination is contraindicated for persons with congenital cell-mediated or severe combined immunodeficiency” (21). This statement, however, requires further clarification for risks associated with MSMD. Many of the severe forms of IEI manifest clinically in the first few months of life (22). Therefore, in some countries, the BCG vaccine is given to infants at a later age (23–25). Thus, we urge the authorities to refine their statement on BCG and include procedures that assure only healthy children receive the vaccine (26). A summary of the MSMD variants found in the UAE is shown in Table 2.
We conclude that “BCG vaccine should be deferred until required precautions are legitimately dismissed.” In addition, the “electronic health systems” should alert vaccination providers that “There should be no contraindication to the live vaccination before a dose can be requested.” More importantly, “parents should be well-informed and counseled that sharing critical information with the vaccination providers is essential.” These preliminary measures are easy to implement, while awaiting wise policies on BCG vaccination (27–29).
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Tawam Human Research Ethics Committee. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
A-KS, SA-H, RV, and GB: conceived, designed, and structured the report. RV: performed the variant analysis. MT: analyzed and interpreted the images. AY, AA-A, and AS: analyzed and interpreted the clinical data. A-KS, RV, and SA-H: wrote the paper. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are grateful to Tawam Hospital staff for their much appreciated care of this infant.
1. United Arab Emirates: WHO and UNICEF estimates of immunization coverage (2018) revision. Available online at: https://www.who.int/immunization/monitoring_surveillance/data/are.pdf (accessed January 05, 2021).
2. Al-Hammadi S, Alsuwaidi AR, Alshamsi ET, Ghatasheh GA, Souid AK. Disseminated Bacillus Calmette-Guérin (BCG) infections in infants with immunodeficiency. BMC Res Notes. (2017) 10:177. doi: 10.1186/s13104-017-2499-7
3. Al-Hammadi S, Alkuwaiti NS, Ghatasheh GA, Al Dhanhani H, Shendi HM, Elomami AS, et al. Adverse events of the BCG (Bacillus Calmette–Guérin) and rotavirus vaccines in a young infant with inborn error of immunity. Case Rep Immunol. (2020) 2020:8857152. doi: 10.1155/2020/8857152
4. Al-Muhsen S, Casanova JL. The genetic heterogeneity of Mendelian susceptibility to mycobacterial diseases. J Allergy Clin Immunol. (2008) 122:1043–51; quiz 1052-3. doi: 10.1016/j.jaci.2008.10.037
5. Wang LH, Yen CL, Chang TC, Liu CC, Shieh CC. Impact of molecular diagnosis on treating Mendelian susceptibility to mycobacterial diseases. J Microbiol Immunol Infect. (2012) 45:411–7. doi: 10.1016/j.jmii.2012.08.017
6. Bustamante J, Picard C, Boisson-Dupuis S, Abel L, Casanova JL. Genetic lessons learned from X-linked Mendelian susceptibility to mycobacterial diseases. Ann N Y Acad Sci. (2011) 1246:92–101. doi: 10.1111/j.1749-6632.2011.06273.x
7. Filipe-Santos O, Bustamante J, Chapgier A, Vogt G, de Beaucoudrey L, Feinberg J, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. (2006) 18:347–61. doi: 10.1016/j.smim.2006.07.010
8. Bustamante J, Arias AA, Vogt G, Picard C, Galicia LB, Prando C, et al. Germline CYBB mutations that selectively affect macrophages in kindreds with X-linked predisposition to tuberculous mycobacterial disease. Nat Immunol. (2011) 12:213–21. doi: 10.1038/ni.1992
9. Bustamante J, Picard C, Fieschi C, Filipe-Santos O, Feinberg J, Perronne C, et al. A novel X-linked recessive form of Mendelian susceptibility to mycobaterial disease. J Med Genet. (2007) 44:e65. doi: 10.1136/jmg.2006.043406
10. Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, Ariga T, et al. Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis. (2010) 45:246–65. doi: 10.1016/j.bcmd.2010.07.012
11. Aygun D, Koker MY, Nepesov S, Koker N, van Leeuwen K, de Boer M, et al. Genetic characteristics, infectious, and noninfectious manifestations of 32 patients with chronic granulomatous disease. Int Arch Allergy Immunol. (2020) 181:540–50. doi: 10.1159/000507366
12. Boisson-Dupuis S. The monogenic basis of human tuberculosis. Hum Genet. (2020) 139:1001–9. doi: 10.1007/s00439-020-02126-6
13. Ong RYL, Chan SB, Chew SJ, Liew WK, Thoon KC, Chong CY, et al. Disseminated Bacillus-Calmette-Guérin infections and primary immunodeficiency disorders in Singapore: a single center 15-year retrospective review. Int J Infect Dis. (2020) 97:117–25. doi: 10.1016/j.ijid.2020.05.117
14. Bustamante J. Mendelian susceptibility to mycobacterial disease: recent discoveries. Hum Genet. (2020) 139:993–1000. doi: 10.1007/s00439-020-02120-y
15. National Center for Biotechnology Information. ClinVar; [VCV000418153.2]. Available online at: https://www.ncbi.nlm.nih.gov/clinvar/variation/VCV000418153.2 (accessed January 7, 2021).
16. Bode SF, Ammann S, Al-Herz W, Bataneant M, Dvorak CC, Gehring S, et al. Inborn Errors Working Party of the EBMT. The syndrome of hemophagocytic lymphohistiocytosis in primary immunodeficiencies: implications for differential diagnosis and pathogenesis. Haematologica. (2015) 100:978–88. doi: 10.3324/haematol.2014.121608
17. Marciano BE, Huang CY, Joshi G, Rezaei N, Carvalho BC, Allwood Z, et al. BCG vaccination in patients with severe combined immunodeficiency: complications, risks, and vaccination policies. J Allergy Clin Immunol. (2014) 133:1134–41. doi: 10.1016/j.jaci.2014.02.028
18. Sheek-Hussein M, Hashmey R, Alsuwaidi AR, Al Maskari F, Amiri L, Souid AK. Seroprevalence of measles, mumps, rubella, varicella-zoster, HAV, HBV and HCV in Emirati medical students. BMC Public Health. (2012) 12:1047. doi: 10.1186/1471-2458-12-1047
19. Al Mekaini LA, Al Jabri ON, Narchi H, Kamal SM, Mabrook A, Al Kuwaiti MM, et al. The use of an interferon-gamma release assay to screen for pediatric latent tuberculosis infection in the eastern region of the Emirate of Abu Dhabi. Int J Infect Dis. (2014) 23:4–7. doi: 10.1016/j.ijid.2013.12.020
20. Almarzooqi F, Alkhemeiri A, Aljaberi A, Hashmey R, Zoubeidi T, Souid AK. Prospective cross-sectional study of tuberculosis screening in United Arab Emirates. Int J Infect Dis. (2018) 70:81–85. doi: 10.1016/j.ijid.2018.03.001
21. World Health Organization. BCG vaccine: WHO position paper, February 2018 - Recommendations. Vaccine. (2018) 36:3408–10. doi: 10.1016/j.vaccine.2018.03.009
22. Köker MY, Camcioglu Y, van Leeuwen K, Kiliç SS, Barlan I, Yilmaz M, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. (2013) 132:1156–63.e5. doi: 10.1016/j.jaci.2013.05.039
23. Kagina BM, Abel B, Bowmaker M, Scriba TJ, Gelderbloem S, Smit E, et al. Delaying BCG vaccination from birth to 10 weeks of age may result in an enhanced memory CD4 T cell response. Vaccine. (2009) 27:5488–95. doi: 10.1016/j.vaccine.2009.06.103
24. Al Jumaah S, Al Hajjar S, Al Mousa H. Bacille Calmette-Guérin vaccination in Saudi Arabia: benefits versus risks. Ann Saudi Med. (2012) 32:1–3. doi: 10.5144/0256-4947.2012.1
25. Alfawaz TS, Alshehri M, Alshahrani D. BCG related complications: a single center, prospective observational study. Int J Pediatr Adolesc Med. (2015) 2:75–78. doi: 10.1016/j.ijpam.2015.05.004
26. Available online at: https://www.who.int/immunization/policy/schedule.pdf (accessed January 7, 2021).
27. Bustamante J, Aksu G, Vogt G, de Beaucoudrey L, Genel F, Chapgier A, et al. BCG-osis and tuberculosis in a child with chronic granulomatous disease. J Allergy Clin Immunol. (2007) 120:32–8. doi: 10.1016/j.jaci.2007.04.034
28. Conti F, Lugo-Reyes SO, Blancas Galicia L, He J, Aksu G, de Oliveira EB, et al. Mycobacterial disease in patients with chronic granulomatous disease: a retrospective analysis of 71 cases. J Allergy Clin Immunol. (2016) 138:241–48.e3. doi: 10.1016/j.jaci.2015.11.041
Keywords: Bacillus Calmette-Guérin, BCG disease, vaccines, tuberculosis, immunodeficiency, inborn error of immunity, newborns, United Arab Emirates
Citation: Al-Hammadi S, Yahya AM, Al-Amri A, Shibli A, Balhaj GB, Tawil MI, Vijayan R and Souid A-K (2021) Case Report: BCG-Triggered Hemophagocytic Lymphohistiocytosis in an Infant With X-Linked Recessive Mendelian Susceptibility to Mycobacterial Disease Due to a Variant of Chronic Granulomatous Disease. Front. Pediatr. 9:687538. doi: 10.3389/fped.2021.687538
Received: 29 March 2021; Accepted: 24 May 2021;
Published: 29 June 2021.
Edited by:
Claudio Pignata, University of Naples Federico II, ItalyReviewed by:
Claudia Bracaglia, IRCCS Ospedale Pediatrico Bambino Gesù, ItalyCopyright © 2021 Al-Hammadi, Yahya, Al-Amri, Shibli, Balhaj, Tawil, Vijayan and Souid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suleiman Al-Hammadi, c3VsZWltYW4uYWxoYW1tYWRpQG1icnUuYWMuYWU=
†ORCID: Suleiman Al-Hammadi orcid.org/0000-0003-2664-8246
Amal M. Yahya orcid.org/0000-0002-765-40367
Mohamed I. Tawil orcid.org/0000-0003-3605-5495
Ranjit Vijayan orcid.org/0000-0002-3830-7409
Abdul-Kader Souid orcid.org/0000-0002-8562-4757
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.