
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 01 July 2021
Sec. Pediatric Neurology
Volume 9 - 2021 | https://doi.org/10.3389/fped.2021.678890
 Marady Hun1
Marady Hun1 Min Xie1
Min Xie1 Zhou She1
Zhou She1 Amin S. Abdirahman1
Amin S. Abdirahman1 Cuifang Li1
Cuifang Li1 Feifeng Wu1
Feifeng Wu1 Senlin Luo1
Senlin Luo1 Phanna Han2
Phanna Han2 Rithea Phorn3
Rithea Phorn3 Pan Wu4
Pan Wu4 Haiyan Luo4
Haiyan Luo4 Keke Chen5
Keke Chen5 Jidong Tian1
Jidong Tian1 Wuqing Wan1
Wuqing Wan1 Chuan Wen1*
Chuan Wen1*This study investigated the management and clinical outcomes along with associated factors of posterior reversible encephalopathy syndrome (PRES) in childhood hematologic/oncologic diseases. We present data from children with hematologic/oncologic diseases who developed PRES after treatment of the primary disease with chemotherapy and hematopoietic stem cell transplantation (HSCT) at 3 medical centers in Changsha, China from 2015 to 2020, and review all previously reported cases with the aim of determining whether this neurologic manifestation affects the disease prognosis. In the clinical cohort of 58 PRES patients, hypertension [pooled odds ratio (OR) = 4.941, 95% confidence interval (CI): 1.390, 17.570; P = 0.001] and blood transfusion (OR = 14.259, 95% CI: 3.273, 62.131; P = 0.001) were significantly associated with PRES. Elevated platelet (OR = 0.988, 95% CI: 0.982, 0.995; P < 0.001), hemoglobin (OR = 0.924, 95% CI: 0.890, 0.995; P < 0.001), and blood sodium (OR = 0.905, 95% CI: 0.860, 0.953; P < 0.001), potassium (OR = 0.599, 95% CI: 0.360, 0.995; P = 0.048), and magnesium (OR = 0.093, 95% CI: 0.016, 0.539; P = 0.008) were protective factors against PRES. Data for 440 pediatric PRES patients with hematologic/oncologic diseases in 21 articles retrieved from PubMed, Web of Science, and Embase databases and the 20 PRES patients from our study were analyzed. The median age at presentation was 7.9 years. The most common primary diagnosis was leukemia (62.3%), followed by solid tumor (7.7%) and lymphoma (7.5%). Most patients (65.0%) received chemotherapy, including non-induction (55.2%) and induction (44.8%) regimens; and 86.5% used corticosteroids before the onset of PRES. Although 21.0% of patients died during follow-up, in most cases (93.2%) this was not attributable to PRES but to severe infection (27.3%), underlying disease (26.1%), graft-vs.-host disease (14.8%), multiple organ dysfunction syndrome (8.0%), and respiratory failure (3.4%). PRES was more common with HSCT compared to chemotherapy and had a nearly 2 times higher mortality rate in patients with oncologic/hematologic diseases than in those with other types of disease. Monitoring neurologic signs and symptoms in the former group is therefore critical for ensuring good clinical outcomes following treatment of the primary malignancy.
Approximately 70,000 new cases of oncologic disease diagnosed annually are among adolescents and young adults (1, 2). Over the past decades, the 5-year survival rate for pediatric cancer improved from 58% in the period from 1975 to 1977 to 83% from 2005 to 2015 and 84% from 2010 to 2016 (3–7). Acute lymphoblastic leukemia (ALL) is the most common childhood malignancy—accounting for 20% of all cancers occurring before 20 years of age (8, 9)—and has good prognosis: the current 5-year overall survival rate of childhood ALL is 90% (8). This is mainly due to the reduction of risk and adverse reactions associated with cytotoxic therapies including hematopoietic stem cell transplantation (HSCT) and chemotherapy. Posterior reversible encephalopathy syndrome (PRES), a severe neurologic complication and adverse reaction in pediatric oncologic/hematologic patients following chemotherapy and HSCT treatment (10–16), is a clinical syndrome characterized by headache, seizures, mental and visual impairment, and vomiting accompanied by reversible vasogenic edema observed by magnetic resonance imaging (MRI) that impacts the subcortical white matter of supratentorial lobes, especially in the parieto-occipital lobes (17–19).
PRES was first described in 1996 in adults with various primary diagnoses (20), and occurs less frequently in children (21). Nephrotic syndrome is a major primary cause of PRES in children (21–23). However, PRES has recently been reported in single- or multi-center studies of pediatric oncologic/hematologic diseases such as leukemia, lymphoma, solid tumors, and non-malignant disease after chemotherapy and HSCT, with high morbidity and mortality rates ranging from 2.4 to 22.6% (11, 12, 15, 18, 22, 24–28). In patients with oncologic/hematologic diseases, the main causes of death were underlying diseases, severe infection, multiple organ dysfunction syndrome (MODS), respiratory failure, graft-vs.-host disease (GVHD), and severe organ toxicity (15, 18, 25–32); and several studies found that the deaths were directly attributable to PRES (12, 18, 24, 28, 30).
Despite these recent findings, most studies to date on PRES have had small sample sizes and are case reports or series; thus, a comprehensive view of PRES in a large sample is lacking. To address this issue, in this study we investigated the features, management, and clinical outcomes of PRES in a large sample of pediatric patients with oncologic/hematologic diseases with the aim of determining whether this neurologic manifestation affects the prognosis of the primary disease.
The multicenter cohort comprised pediatric patients treated between January 2015 and December 2020 at The Second Xiangya Hospital, Hunan Children's Hospital, and Hunan Provincial People's Hospital (all in Changsha, China). We used a retrospective matched case–control study design to analyze data for patients who developed PRES—which was diagnosed according to established clinical and neuroimaging criteria (17, 33)—after HSCT or chemotherapy for oncologic/hematologic diseases and non-HSCT chemotherapy for non-oncologic/hematologic diseases. PRES was suspected when patients experienced abrupt onset of 1 of the following symptoms: headache, seizures, visual disturbances, confusion, and radiologic findings (focal regions of brain vasogenic edema). We analyzed clinical symptoms, laboratory parameters, neuroimaging findings, treatment strategies, and outcomes from the time of diagnosis of oncologic/hematologic and non-oncologic/hematologic diseases to the onset of PRES (Tables 1, 2).
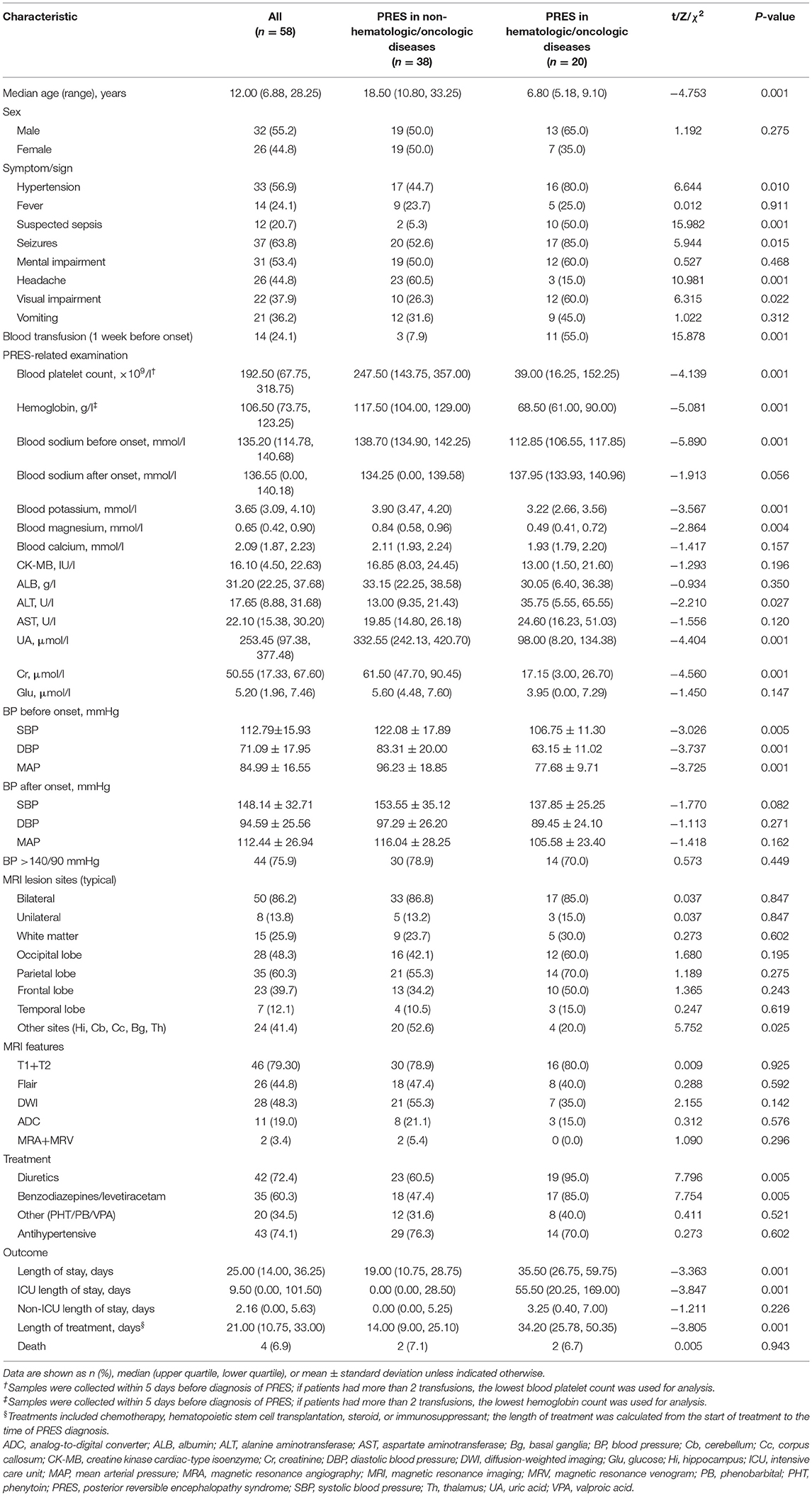
Table 1. Clinical and epidemiologic characteristics of patients with PRES in oncologic/hematologic diseases or non-oncologic/hematologic diseases.
For the systematic review of previously reported cases of PRES, we searched the PubMed, Web of Science, and Embase databases for articles published in English using the following terms: (posterior leukoencephalopathy syndrome OR posterior reversible encephalopathy syndrome OR reversible posterior leukoencephalopathy syndrome OR PRES OR RPLS) AND (child OR children OR childhood). We also included 1 of the following terms to identify case reports and series of children (range 0–18 years of age) with oncologic/hematologic disease and PRES: oncologic/hematologic disease, HSCT, or chemotherapy. We manually searched the reference list of each article and selected all relevant publications from 2015 to 2020 (Supplementary Table 1). Two investigators (M. Hun and M. Xie) independently reviewed the titles and abstracts of the articles for related publications; any discrepancies were resolved by a third investigator (C. Wen). The inclusion criteria for the studies were as follows: (1) case reports, case series, or retrospective studies providing sufficient data on pediatric patients (<20 years of age) with oncologic/hematologic diseases and PRES; (2) studies estimating the relationships between PRES-related factors including primary oncologic/hematologic diseases, clinical etiology, symptoms, imaging findings, and clinical outcome in children; (3) published in English; and (4) used a self-designed table to extract data from all included literature including last name of the first author, year of publication, country, sample size, age, sex ratio, primary diagnosis, oncologic treatment at PRES onset, PRES related to treatment (anti-epileptic+anti-hypertensive), electroencephalogram (EEG) findings, symptoms/signs, neuroimaging data related to initial lesion sites, follow-up findings (follow-up times and outcome), and clinical outcome (Figure 1). Duplicated publications and studies with incomplete data, unclear outcomes, or on non-pediatric PRES were excluded.
Data for the multicenter cohort were analyzed using SAS v9.4 software (SAS Institute, Cary, NC, USA). Quantitative data conforming to a normal distribution are described as means and standard deviations and were analyzed with the independent-samples t-test. For non-normally distributed data, the median with upper and lower quartiles are presented and the non-parametric test was used to evaluate differences between groups. With the occurrence of oncologic/hematologic diseases as the dependent variable, statistically significant variables with physiologic and biochemical significance in the single-factor analysis were entered into the logistic regression model with a stepwise screening method (forward selection with entry standard = 0.05 and elimination standard = 0.10). Odds ratio (OR) was used as the risk assessment parameter. All tests were 2-sided and P < 0.05 was considered statistically significant.
For the systematic review, statistical analyses were performed using Excel v16.43.1 (Microsoft, Redmond, WA, USA) and SAS v9.4. Continuous variables are presented as mean ± standard deviation and categorical variables are reported as numbers and percentages in the comparison of PRES related to chemotherapy vs. HSCT in pediatric oncologic/hematologic diseases.
For the meta-analysis, we used Review Manager v5.4.1 (http://www.cochrane.org) software for statistical analyses of the included data. Between-group differences with a P < 0.05 were considered statistically significant, and forest plots were generated to display related factors. The quality of included studies was evaluated based on Newcastle–Ottawa Scale (NOS) score; the full score is 9 stars, and scores of 1–3, 4–6, and 7–9 stars represent low-, medium-, and high-quality studies, respectively (34).
The multicenter cohort comprised 58 pediatric PRES patients; 7 with oncologic/hematologic diseases and 38 (including 18 adults) with non-oncologic/hematologic diseases were enrolled at The Second Xiangya Hospital; and 8 and 5 PRES patients with oncologic/hematologic diseases were enrolled at Hunan Children's Hospital and Hunan Provincial People's Hospital, respectively, between 2015 and 2020. The 58 PRES patients were classified into oncologic/hematologic disease (n = 20) and non-oncologic/hematologic disease (n = 38) groups; baseline characteristics are shown in Table 1. Compared to PRES patients with non-oncologic/hematologic diseases, those with oncologic/hematologic diseases had higher rates of hypertension (P = 0.01), suspected sepsis (P = 0.001), seizures (P = 0.015), headaches (0.001), and blood transfusions (P = 0.01); higher blood platelet count (P = 0.001), hemoglobin (P = 0.001), blood sodium level at disease onset (P = 0.001), blood potassium (0.001), and blood magnesium (P = 0.004); lower systolic blood pressure (P = 0.005), diastolic blood pressure (P = 0.001), and mean arterial blood pressure (P = 0.001) before disease onset; more frequently used diuretics (P = 0.005) and benzodiazepines/levetiracetam (P = 0.005); had longer stays at the hospital (P = 0.001) and intensive care unit (P = 0.001); and had a longer latency from the initiation of treatment (chemotherapy/HSCT/steroid/immunosuppressive) to PRES diagnosis (P = 0.001) (Tables 1, 2).
The univariate analysis showed that hypertension and blood transfusion were significantly associated with the development of PRES in both groups. Variables with significant associations (P ≤ 0.05) were included in the competing risk regression analysis. In the final model, the pooled OR was 4.941 (95% CI: 1.390, 17.570; P = 0.001) for hypertension and 14.259 (95% CI: 3.273, 62.131; P = 0.001), for blood transfusion. Protective factors against PRES were elevated platelet count (OR = 0.988, 95% CI: 0.982, 0.995; P < 0.001), hemoglobin count (OR = 0.924, 95% CI: 0.890, 0.995; P < 0.001), blood sodium (OR = 0.905, 95% CI: 0.860, 0.953; P < 0.001), blood potassium (OR = 0.599, 95% CI: 0.360, 0.995; P = 0.048), and blood magnesium (OR = 0.093, 95% CI: 0.016, 0.539; P = 0.008) (Table 2).
We carried out a meta-analysis of 3 studies including the present investigation and studies by Thavamani et al. (NOS score = 9) (22) and Gaziev et al. (NOS score = 7) (15) to determine whether blood transfusion is a risk factor for PRES. The results of the heterogeneity test [χ2 = 39.08, df = 2, I2 = 95%, P = 0.00001 (Q-test)] indicated low homogeneity between the 3 studies according to Cochrane criteria (35). We examined the funnel plot for asymmetry but found that it was within the acceptable range (Supplementary Figure 1). The mixed-effect or pooled hazard ratio of the 3 studies (1.24, 95% CI: 1.04, 1.48) was significant (Z = 2.36, P = 0.02), indicating that blood transfusion had a significant effect on the occurrence of PRES in pediatric oncologic/hematologic diseases (Figure 2).
The review of the literature ultimately yielded 21 PRES articles (11, 12, 15, 18, 24–26, 28, 31, 36–46) comprising a total of 440 pediatric PRES patients with hematologic/oncologic diseases, which were included in the meta-analysis along with the data of the 20 PRES patients from the present study (Supplementary Table 1 and Figure 1).
The median age at PRES presentation was ~7.9 years; 93 patients (38.1%) were between 10 and 19 years old, and 237 (56.6%) were male (Table 3). The most common primary diagnosis was leukemia (62.3%), followed by solid tumors (7.7%) and lymphoma (7.5%); 22.5% of patients had non-malignant disease. Chemotherapy was the most common treatment (65.0%) and the majority of patients (55.2%) were treated with a non-induction regimen, with an induction regimen used in 44.8% of cases. Additionally, 86.5% of patients used corticosteroids before the onset of PRES. Benzodiazepam (54.9%), diazepam (47.1%), and levetiracetam (47.1%) were the commonly used anti-epileptics, and 74% of patients used anti-hypertensive agents. In terms of imaging features, abnormal signals were observed in the following brain regions: occipital cortex (73.8%), parietal cortex (58.0%), frontal cortex (38.7%), and temporal cortex (26.5%). In the follow-up, there was complete resolution in 76.1% of cases (≤30 days, 83.3% vs. >30 days, 70.7%), partial resolution in 7.0% (≤30 days, 5.6% vs. >30 days, 12.2%), and residual lesions in 16.9% (≤30 days, 11.1% vs. >30 days, 17.1%). EEG revealed focal slowing in 66.0%, diffuse slowing in 37.9%, and periodic lateralized epileptiform discharge in 7.4%, with abnormal activity in the occipital (66.7%), temporal (22.2%), parietal (16.7%), and frontal (1.8%) cortices. A total of 88 patients (21.0%) died during follow-up; however, most of the deaths (93.2%) were not attributable to PRES but to severe infection (27.3%), underlying disease (26.1%), GVHD (14.8%), MODS (8.0%), and respiratory failure (3.4%), with only 6 deaths (6.8%) resulting from PRES. The cause of death was unknown or multifactorial in 13.6% of cases (Table 3).
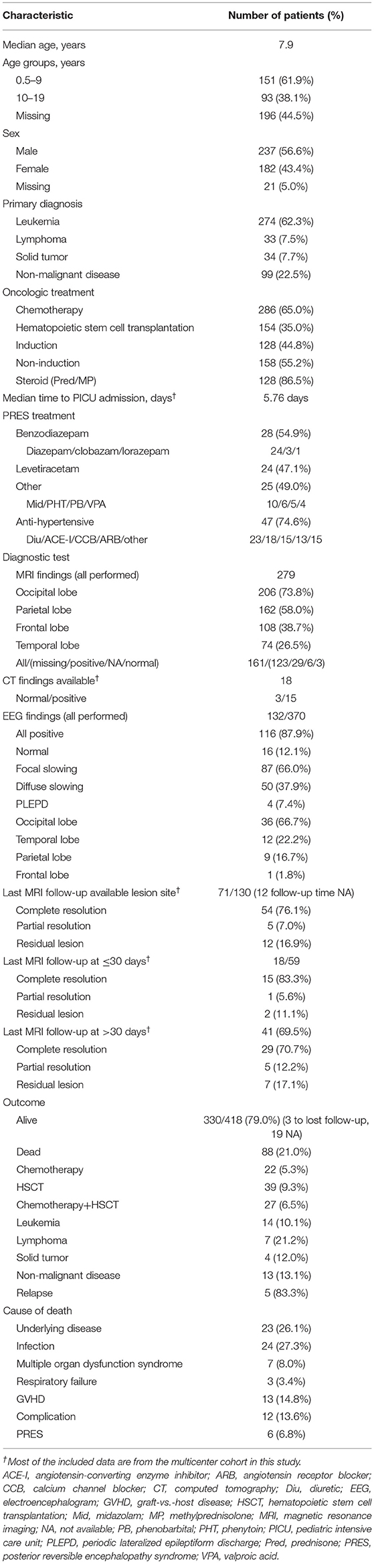
Table 3. Demographic and clinical characteristics and outcomes of PRES patients with pediatric oncologic/hematologic diseases.
The median age at presentation of PRES in patients with oncologic/hematologic diseases was 5.7 years for those treated with chemotherapy and 8.9 years for those treated by HSCT (Table 4). The demographic profile of the chemotherapy and HSCT treatment groups also differed: 27.2 and 40.3%, respectively, were between 10 and 19 years, and 57.0 and 64.5%, respectively, were male. Of the 274 patients with leukemia (62.3%), 78.0% received chemotherapy and 33.1% underwent HSCT. Among the 33 lymphoma patients (7.5%), 32 (11.2%) received chemotherapy and 1 (0.7%) was treated by HSCT; the proportions were 4.5 and 13.6%, respectively, for the 34 solid tumor patients (7.7%) and 6.3 and 52.6%, respectively, for the 99 patients with non-malignant diseases (22.5%).
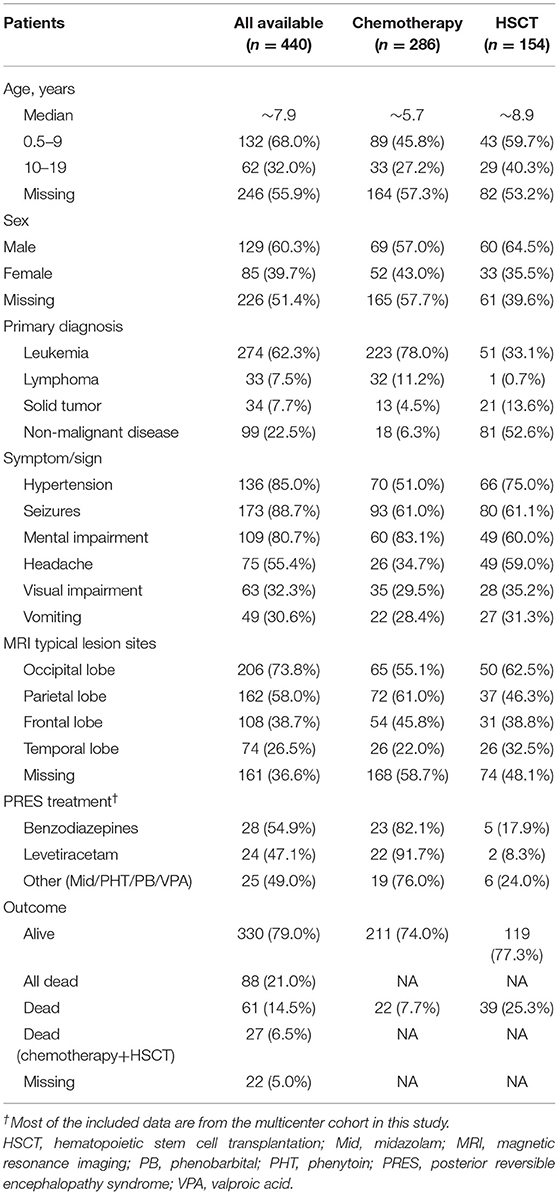
Table 4. Comparison of demographic and clinical characteristics and outcomes of PRES patients with pediatric oncologic/hematologic diseases treated with chemotherapy vs. HSCT.
The incidence of hypertension was higher after HSCT than after chemotherapy (66/88, 75.0% vs. 70/139, 51.0%). The rate of seizures was similar between patients treated with chemotherapy and those treated by HSCT (61.0 vs. 61.1%); meanwhile, the chemotherapy group had higher rates of mental impairment (83.1 vs. 60.0%) and headache (34.7 vs. 59.0%) and lower rates of visual impairment (29.5 vs. 35.2%) and vomiting (28.4 vs. 31.3%) than the HSCT group. Significant differences were also observed by MRI between the chemotherapy and HSCT groups in terms of the affected brain areas including the occipital (55.1 vs. 62.5%), parietal (61.0 vs. 46.3%), frontal (45.8 vs. 38.8%), and temporal (22.0 vs. 32.5%) lobes. The mortality due to PRES was higher for patients treated by HSCT than in those receiving chemotherapy (25.3 vs. 7.7%). Comparison of our data with those from a previous study (12) (NOS score = 7) confirmed that chemotherapy was safer than HSCT for the treatment of pediatric oncologic/hematologic diseases with PRES (mixed-effect or pooled risk ratio = 0.35, 95% CI: 0.24, 0.50, Z = 5.77, P = 0.00001) (Figure 3), with no obvious heterogeneity between the 2 studies [χ2 = 0.88, df = 1, I2 = 0%, P = 0.35 (Q-test)].
Our retrospective analysis of the multicenter cohort of pediatric patients with PRES with oncologic/hematologic diseases revealed significant differences compared to those with non-oncologic/hematologic diseases. Because of the small number of pediatric PRES cases, some adults were included in the latter group.
Many aspects of PRES in pediatric oncologic/hematologic diseases—i.e., clinical features, prognostic factors and outcome, and management—remain unclear. Diagnostic criteria for PRES have been proposed by previous studies (17, 19, 33, 47). PRES is a neurotoxic state that manifests during oncologic/hematologic treatment (10, 11, 15, 18, 24, 25, 27, 32, 48–54); higher single and cumulative doses of chemotherapy and HSCT and longer treatment duration are associated with greater neurotoxicity to both central and peripheral nervous systems (CNS and PNS, respectively) (52, 54). Cytotoxic therapy may contribute to PRES by directly acting on the vascular endothelium and causing capillary leakage and destruction of the blood–brain barrier (33, 55). In ALL patients, PRES mainly occurred during the induction phase of chemotherapy with methotrexate, prednisolone, vincristine, doxorubicin, and asparaginase (11, 12, 24, 26, 36, 46, 56, 57). Vincristine was been linked to peripheral neuropathy and may be a causative factor in PRES (43, 51–54, 58). Additionally, asparaginase, methotrexate, cytarabine, and intrathecal chemotherapy are known to be neurotoxic to the CNS and PNS (52, 59). High-dose chemotherapy and drugs used to prevent GVHD in HSCT (59) including cyclosporine, and tacrolimus can lead to PRES (52, 59) possibly by promoting hypertension (60), similar to the cytotoxic steroid-based drugs that are typically included in chemotherapy/HSCT regimens (61–64). Recent studies indicate that steroids promote PRES in patients with oncologic/hematologic diseases (11, 15, 52, 64, 65) (Figure 4).
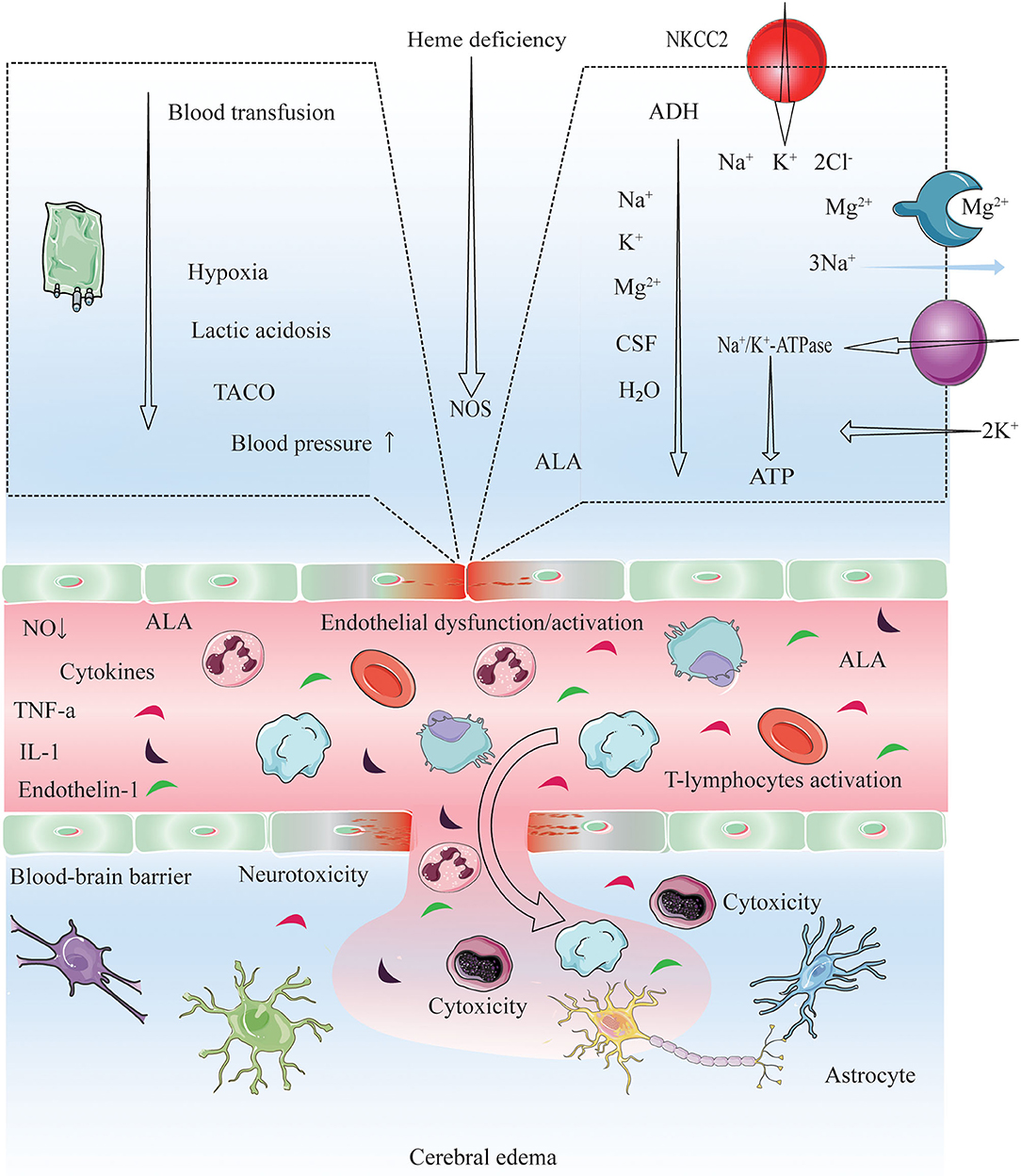
Figure 4. Proposed pathogenic model for cerebral edema and CNS dysfunction after conducting chemotherapy, HSCT and immunosuppressive agents. Endothelial wall inflammation disrupts the tight junctions and increase the permeability of the BBB due to high levels of circulating cytokines (TNF-α, IL-1, endothelin-1) and activating leucocytes (autoreactive T-cells). Consequently, enhanced fluid and cell diapedesis, and interstitial edema formation ensues. PRES manifestation and the dysfunction of microvasculature may be driven by the presence of checkpoint inhibitors (HSCT, chemotherapy, and immunosuppressive agent), by interactions with autoantibodies and autoreactive T-cells, and via abnormal secretion of angiogenic growth factors (VEGF) and proangiogenic cytokines (IL-8) (33, 66, 67), VEGF expression is increased, leading to increased vascular permeability and interstitial cerebral edema (33). Blood transfusion triggers a rapid increase in the hemoglobin, platelet, and viscosity levels, which is thought to trigger transfusion-associated circulatory overload (TACO) (68–71). Elevated blood pressure, acute hypoxia, anemia, and lactic acidosis are all risk factors for TACO (69, 72); on the other hand, acute hypoxia may decrease cerebrospinal fluid (CSF) volume, increase cerebral blood volume (CBV), and increase brain parenchyma perfusion as an early responses to hypoxia (within 40 min) (73, 74). This increase could induce acute vascular endothelium dysfunction and an elevation of vascular resistance, leading to extravasation of macromolecules into the brain. Also, the velocity of brain blood flow is shown to increase after transfusion (70, 75). Cytokines induce the expression of adhesion molecules (ICAM-1, VCAM-1), which interact with leukocytes and potentiate ROS production. ROS and ALA might cause direct endothelial cell injuries, increasing the expression of VEGF and vascular permeability. A low ATP supply impairs energy-dependent processes, such as NA+/K+ ATPase function. While an ADH excess causes ALA neurotoxicity and the effect of IL-6 in the hypothalamus might lead to an increment in ADH secretion. ADH inhibits NA+/K+ ATPase and induces NKCC2 and AQP4 in astrocytes, leading to increase ion/water influx and swelling (76). ADH excess may also lead to electrolyte disorders (hyponatremia, hypocalcemia, hypomagnesemia) (22, 63, 77–79). NO deficiency: PTX3, heme deficiency and ROS might impair NOS function, thus decreasing NO synthesis and causing endothelial dysfunction. PEPT2 dysfunction: The PEPT2*2 variant has a lower affinity for ALA than PEPT2*1, which might cause a diminished ALA efflux in the choroid plexus and a more significant ALA neurotoxicity in the brain (80). Electrolyte disorders (hyponatremia, hypocalcemia, hypomagnesemia), low CSF (81), and lack of ATP might also reduce PEPT2 function. These cascades lead to vasogenic cerebral edema, and certain precipitants are probably necessary to cause PRES and CNS dysfunction. PRES, posterior reversible encephalopathy syndrome; TACO, transfusion-associated circulatory overload; ADH, Antidiuretic hormone; ALA, 5-Aminolevulinic acid; ALAS1, 5-Aminolevulinic acid synthase-1; AQP4, Aquaporin-4; BBB, Blood-brain barrier; ICAM1, Intracellular adhesion molecule-1; VCAM-1, vascular cell adhesion molecule 1; IL, Interleukin; NKCC1, Na+ K+ 2Cl− Cotransporter 1; NO, Nitric oxide; NOS, Nitric oxide synthase; PEPT2, Peptide transporter-2; PTX3, Pentraxin-3; ROS, Reactive oxygen species; TCA, Tricarboxylic acid cycle; TNF-α,Tumor necrosis factor-α; VCAM1, Vascular cell adhesion protein-1; VEGF, Vascular endothelial growth factor.
Electrolyte disorders are common in cancer patients—occurring in as many as one-third—and may worsen prognosis (82–86). The manifestations of acute hyponatremia vary from non-specific symptoms (e.g., headache, nausea, vomiting, and muscle cramps) to life-threatening conditions such as bradycardia, hypertension, impaired thermoregulation, cerebral herniation, convulsions, and coma (82, 83, 87). HSCT and chemotherapy-induced febrile neutropenia—which is associated with decline in blood electrolyte (sodium, potassium and magnesium) levels—have a potentially fatal outcome. Thus, it is critical to monitor electrolyte balance in cancer patients (88, 89). On the other hand, electrolyte abnormalities are useful prognostic indicators in palliative care (90). PRES-related electrolyte disorders are rare, although there is increasing evidence that hyponatremia contributes to the pathogenesis of PRES (27, 42, 91, 92); the mechanism may involve interference by aquaporins with the regulation of osmotic pressure in the brain (93–95). Hyponatremia was observed in 70.5% of ALL patients with PRES treated with chemotherapy along with hypocalcemia (41.9%) and abnormal magnesium (25.6%) and glucose (35.7%) (11), as well as in 38% of patients who underwent HSCT (96). A case of ALL with PRES secondary to hyponatremia has also been reported (42). In our pediatric cohort with oncologic/hematologic diseases, elevated blood sodium, potassium, and magnesium levels were protective factors against PRES, implying that interventions that increase blood electrolyte concentrations can be beneficial in this group (Figure 4).
Although the exact cause of PRES is not known, it is thought to be related to the production of toxins induced by HSCT and chemotherapy that target capillary endothelial cells, leading to the failure of cerebral blood pressure autoregulation, endothelial dysfunction, and vasogenic edema (52, 97). PRES is usually observed in the context of acute hypertension (sometimes treatment-induced) (10–12, 15, 17–19, 22–28, 32, 33, 44, 46, 47, 57, 65, 98–105), and may be overlooked in patients with near-normal blood pressure at symptom onset (98–100). Hypertension is more common in children than in adults with PRES (106). Between 67 and 100% of patients with PRES have hypertension after undergoing HSCT or receiving chemotherapy in pediatric oncologic/hematological diseases (10–13, 15, 24–28, 36, 40, 46, 56, 57, 105, 107, 108). In our systematic review, the rate of hypertension was higher after HSCT than after chemotherapy (75.0 vs. 51.0%) in pediatric patients with oncologic/hematologic diseases and PRES; we previously showed that hypertension leads to a poor outcome in this group (10) (Figure 4).
Adverse events associated with blood transfusion in cancer patients following chemotherapy/HSCT include febrile non-hemolytic transfusion, allergic, and delayed hemolytic transfusion reactions; acute hemolytic transfusion reactions (AHTRs); transfusion-associated circulatory overload or acute lung injury (TACO and TRALI, respectively), GVHD; dyspnea; immunomodulation; red blood cell alloimmunization; iron overload; and microbial infection. TRALI, TACO, and AHTRs are potentially fatal complications (68). TACO is characterized by respiratory distress, pulmonary edema, left or right heart failure, elevated central venous pressure, or hypertension, which occur within 2 h or up to 6 h after the start of transfusion (109). Elevated blood pressure is a risk factor for TACO (69). The rapid increases in hemoglobin level and blood viscosity after transfusion are thought to cause PRES by inducing acute vascular endothelial dysfunction and increasing vascular resistance, resulting in extravascular leakage of fluid and macromolecules in the brain (70). There have been several reports of blood transfusion-related PRES, with symptoms lasting from 2 h to over 1 month (70, 75). Only a few studies have investigated risk factors for PRES related to transfusion in children; these involved patients with sickle cell disease (SCD) (110) or thalassemia (15). Ours is the first report of blood transfusion-related PRES in pediatric oncologic/hematologic diseases (Figure 4).
There is no specific intervention for PRES, but the condition is reversible by addressing the etiology. Clinical management involves a combination of symptomatic life-supporting treatments and control of causative factors. A previous retrospective study found that mechanical ventilation was required in 71% of adult patients with severe PRES; the mortality rate attributed to PRES was 5.7%, with toxicity (44%) and hypertensive encephalopathy (41%) being the main causes of death (47). In pediatric patients with oncologic/hematologic diseases, early diagnosis of PRES is critical for avoiding neurologic sequelae and death (10–12, 15, 17, 19, 24, 32, 105, 111). PRES in children is more common in hematologic diseases compared to other malignancies and is associated with hypertension, infection, and steroid use; seizures are the most common acute manifestation. Most MRI changes resolve, but persistent imaging abnormalities and epilepsy can develop (44). We previously demonstrated that female sex, age >10 years old, acute GVHD, hypertension, immunodeficiency, SCD, T cell leukemia, and CNS leukemia/involvement are linked to poor outcome in children with oncologic/hematologic diseases and PRES (10); in this population, the main causes of death are underlying diseases, severe infection, MODS, respiratory failure, GVHD, and severe organ toxicity (15, 18, 25–32), although in some cases mortality was a direct result of PRES (12, 18, 24, 28, 30). In our systematic review, only 6.8% of the deaths were attributable to PRES, and the mortality rate was higher following HSCT than chemotherapy (25.3 vs. 7.7%), indicating that the latter is a safer treatment option for pediatric patients with oncologic/hematologic diseases who develop PRES. Based on the above findings, we recommend the following protocol for the management of PRES in pediatric patients with oncologic/hematologic diseases treated with chemotherapy or HSCT: treatment of specific symptoms including seizures, lowering of blood pressure, and eliminating or reducing causative factors/medications (111–114) (Table 5).
Our systematic review of 21 studies including 1,213 participants and our 20 cases provides the most comprehensive analysis to date of PRES in children with oncologic/hematologic diseases. However, there were several limitations to the current work. (1) Selection bias could not be ruled out in our comparative analysis of factors related to PRES in oncologic/hematologic and non-oncologic/hematologic diseases, as we included adults in the latter group because of the scarcity of pediatric patients. (2) Given the observational study design, we could not exclude the possibility of confounding factors, although there was consistency between the primary and propensity factor-matched analyses. Nevertheless, we were unable to establish a cause-and-effect relationship between PRES and oncologic/hematologic diseases as some of our patients were lost follow-up and there was no radiologic follow-up. (3) There may have been publication bias in our meta-analysis because of restrictions on the year of publication. (4) Some of the included case series had insufficient patient information, corresponding to a low level of evidence. (5) In our previous study, we identified several factors associated with a poor outcome for PRES in pediatric oncologic/hematologic diseases (10); however, the random-effects model in the present study identified only 2 of these factors (hypertension and blood transfusion) as being significantly associated with PRES, indicating low concordance between the findings of the 2 studies.
The results of our study identified hypertension; blood transfusion; and severe decreases in blood sodium, potassium, and magnesium as risk factors for PRES in pediatric patients with oncologic/hematologic diseases. Neurotoxicity related to chemotherapy and HSCT was related to a longer treatment duration. PRES was more common with HSCT compared to chemotherapy and had a nearly 2 times higher mortality rate in patients with oncologic/hematologic diseases than in those with other types of disease. Knowing the risk factors and protective factors based on the characteristics of the individual patient can help to prevent neurological complications or improve their management.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Second Xiangya Hospital, Hunan Children's Hospital, Hunan Provincial People's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
MH, MX, JT, and CW: study conception and design and manuscript writing. MH, PH, MX, HL, and RP: development of methodology and statistical analysis. JT, ZS, FW, CL, AA, HL, PW, KC, and WW: data collection. All authors manuscript formatting and editing.
This work was supported by grants from the National Natural Science Foundation of China (No. 82070758), Hunan Provincial Key R&D Program Project (No. 2020SK2084), and Natural Science Foundation of Hunan Province in China (No. 2019JJ40413).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2021.678890/full#supplementary-material
1. Close AG, Dreyzin A, Miller KD, Seynnaeve BKN, Rapkin LB. Adolescent and young adult oncology-past, present, and future. CA Cancer J Clin. (2019) 69:485–96. doi: 10.3322/caac.21585
2. North American Association of Central Cancer Registries (NAACCR) Incidence Data-Cancer in North America (CiNA) Analytic File 1995-2015 [Internet]. Public Use (Which Includes Data From the Center for Disease Control and Prevention's National Program of Cancer Registries [NPCR], the Canadian Council of Cancer Registry's [CCCR's] Provincial and Territorial Registries, and the National Cancer Institute's [NCI's] SEER Registries) (2016).
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
4. Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS. Challenging issues in pediatric oncology. Nat Rev Clin Oncol. (2011) 8:540–9. doi: 10.1038/nrclinonc.2011.95
5. O'Leary M, Krailo M, Anderson JR, Reaman GH, Children's Oncology G. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Semin Oncol. (2008) 35:484–93. doi: 10.1053/j.seminoncol.2008.07.008
6. Bernsen EC, Hagleitner MM, Kouwenberg TW, Hanff LM. Pharmacogenomics as a tool to limit acute and long-term adverse effects of chemotherapeutics: an update in pediatric oncology. Front Pharmacol. (2020) 11:1184. doi: 10.3389/fphar.2020.01184
7. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. (2021) 71:7–33. doi: 10.3322/caac.21654
8. Dixon S, Chen Y, Yasui Y, Pui C, Hunger S, Silverman L, et al. Reduced morbidity and mortality in survivors of childhood acute lymphoblastic leukemia: a report from the childhood cancer survivor study. J Clin Oncol. (2020) 38, 3418–29. doi: 10.1200/jco.20.00493
9. Howlader NNAKrapcho M, et al. SEER Cancer Statistics Review, 1975-2014, (ed April 2017). Bethesda, MD: National Cancer Institute (2017).
10. Hun M, Tian J, Xie M, She Z, Abdirahman AS, Han P, et al. Analysis of risk factors associated with poor outcome in posterior reversible encephalopathy syndrome after treatment in children: systematic review and meta-analysis. Front Neurol. (2020) 11:938. doi: 10.3389/fneur.2020.00938
11. Anastasopoulou S, Eriksson MA, Heyman M, Wang C, Niinimaki R, Mikkel S, et al. Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia: clinical characteristics, risk factors, course, and outcome of disease. Pediatr Blood Cancer. (2019) 66:e27594. doi: 10.1002/pbc.27594
12. Zama D, Gasperini P, Berger M, Petris M, De Pasquale MD, Cesaro S, et al. A survey on hematology-oncology pediatric AIEOP centres: the challenge of posterior reversible encephalopathy syndrome. Eur J Haematol. (2018) 100:75–82. doi: 10.1111/ejh.12984
13. Shash HA, Aldaama S, Al-Hawaj GA, Alfareed AM, Alafghani SA. Posterior reversible encephalopathy syndrome in pediatric oncology and post bone Marrow transplant: single center experience and systematic review. Blood. (2018) 132:5694. doi: 10.1182/blood-2018-99-115808
14. Young G. How I treat pediatric venous thromboembolism. Blood. (2017) 130:1402–8. doi: 10.1182/blood-2017-04-742320
15. Gaziev J, Marziali S, Paciaroni K, Isgro A, Di Giuliano F, Rossi G, et al. Posterior reversible encephalopathy syndrome after hematopoietic cell transplantation in children with hemoglobinopathies. Biol Blood Marrow Transplant. (2017) 23:1531–40. doi: 10.1016/j.bbmt.2017.05.033
16. Shenoy S, Eapen M, Panepinto JA, Logan BR, Wu J, Abraham A, et al. A trial of unrelated donor marrow transplantation for children with severe sickle cell disease. Blood. (2016) 128:2561–7. doi: 10.1182/blood-2016-05-715870
17. Schmiegelow K, Attarbaschi A, Barzilai S, Escherich G, Frandsen TL, Halsey C, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. (2016) 17:e231–9. doi: 10.1016/S1470-2045(16)30035-3
18. Chen Q, Zhao X, Fu HX, Chen YH, Zhang YY, Wang JZ, et al. Posterior reversible encephalopathy syndrome (PRES) after haploidentical haematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant. (2020) 55:2035–42. doi: 10.1038/s41409-020-0894-5
19. Gao B, Lyu C, Lerner A, McKinney AM. Controversy of posterior reversible encephalopathy syndrome: what have we learnt in the last 20 years? J Neurol Neurosurg Psychiatry. (2018) 89:14–20. doi: 10.1136/jnnp-2017-316225
20. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. (1996) 334:494–500. doi: 10.1056/NEJM199602223340803
21. Gupta V, Bhatia V, Khandelwal N, Singh P, Singhi P. Imaging findings in pediatric posterior reversible encephalopathy syndrome (PRES): 5 years of experience from a Tertiary Care Center in India. J Child Neurol. (2016) 31:1166–73. doi: 10.1177/0883073816643409
22. Thavamani A, Umapathi KK, Puliyel M, Super D, Allareddy V, Ghori A. Epidemiology, comorbidities, and outcomes of posterior reversible encephalopathy syndrome in children in the United States. Pediatr Neurol. (2019) 103, 21–6. doi: 10.1016/j.pediatrneurol.2019.07.007
23. Raj S, Overby P, Erdfarb A, Ushay HM. Posterior reversible encephalopathy syndrome: incidence and associated factors in a pediatric critical care population. Pediatr Neurol. (2013) 49:335–9. doi: 10.1016/j.pediatrneurol.2013.06.007
24. Hafez HA, Ragab I, Sedky M, Shams M, Youssef A, Refaat A, et al. Patterns, risk factors and outcome predictors of posterior reversible encephalopathy syndrome in pediatric cancer patients. Leukemia Lymphoma. (2020) 62, 462–8. doi: 10.1080/10428194.2020.1832658
25. Li XY, Huang K, Zhou DH, Li Y, Xu HG, Weng WJ, et al. Severe hypertension is an independent risk factor for posterior reversible encephalopathy syndrome post-hematopoietic cell transplantation in children with thalassemia major. Clin Transplant. (2019) 33:e13459. doi: 10.1111/ctr.13459
26. Danhofer P, Tomeckova M, Cerna D, Zapletalova D, Horak O, Aulicka S, et al. Prognostic factors and seizure outcome in posterior reversible encephalopathy syndrome (PRES) in children with hematological malignancies and bone marrow failure: a retrospective monocentric study. Seizure. (2019) 72:1–10. doi: 10.1016/j.seizure.2019.08.007
27. Banerjee JS, Heyman M, Palomäki M, Lähteenmäki P, Arola M, Riikonen PV, et al. Posterior reversible encephalopathy syndrome: risk factors and impact on the outcome in children with acute lymphoblastic leukemia treated with nordic protocols. J Pediatric Hematol Oncol. (2018) 40:e13–8. doi: 10.1097/MPH.0000000000001009
28. Tambasco N, Mastrodicasa E, Salvatori C, Mancini G, Romoli M, Caniglia M, et al. Prognostic factors in children with PRES and hematologic diseases. Acta Neurol Scand. (2016) 134:474–83. doi: 10.1111/ane.12570
29. Habetz K, Ramakrishnaiah R, Raina SK, Fitzgerald RT, Hinduja A. Posterior reversible encephalopathy syndrome: a comparative study of pediatric versus adult patients. Pediatr Neurol. (2016) 65:45–51. doi: 10.1016/j.pediatrneurol.2016.09.001
30. Behfar M, Babaei M, Radmard AR, Kooraki S, Farajifard H, Naji P, et al. Posterior reversible encephalopathy syndrome after allogeneic stem cell transplantation in pediatric patients with fanconi anemia, a prospective study. Biol Blood Marrow Transplant. (2020) 26:e316–21. doi: 10.1016/j.bbmt.2020.08.021
31. Shkalim-Zemer V, Konen O, Levinsky Y, Michaeli O, Yahel A, Krauss A, et al. Calcineurin inhibitor-free strategies for prophylaxis and treatment of GVHD in children with posterior reversible encephalopathy syndrome after stem cell transplantation. Pediatr Blood Cancer. (2017) 64:e26531. doi: 10.1002/pbc.26531
32. de Laat P, Te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink MM. Posterior reversible encephalopathy syndrome in childhood cancer. Ann Oncol. (2011) 22:472–8. doi: 10.1093/annonc/mdq382
33. Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. (2015) 14:914–25. doi: 10.1016/S1474-4422(15)00111-8
34. Wells GA, Shea B, O'Connell DPJ, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Health Research Institute (2011). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
35. Deeks JHJ, Altman D. Identifying and measuring heterogeneity. The Cochrane Handbook for Systematic Reviews of Interventions Version. (2011) 5.
36. Lin W, Xie J, Zhang J, Cheng H, Cui H, Zhang Y, et al. Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia during remission induction chemotherapy: a single-center retrospective study. Minerva Pediatrica. (2019). doi: 10.23736/s0026-4946.19.05675-5
37. Kapoor R, Simalti A, Kumar R, Yanamandra U, Das S, Singh J, et al. PRES in pediatric HSCT: a single-center experience. J Pediatr Hematol Oncol. (2018) 40, 433–7. doi: 10.1097/MPH.0000000000001190
38. Musiol K, Waz S, Boron M, Kwiatek M, Machnikowska-Sokolowska M, Gruszczynska K, et al. PRES in the course of hemato-oncological treatment in children. Childs Nerv Syst. (2018) 34:691–9. doi: 10.1007/s00381-017-3664-y
39. Aureli V, Giammattei L, Maduri R, Daniel RT, Messerer M. Posterior reversible encephalopathy syndrome (PRES) due to neuroblastoma in a child presenting with acute hydrocephalus. Childs Nerv Syst. (2018) 34:15–7. doi: 10.1007/s00381-017-3640-6
40. Khan SJ, Arshad AA, Fayyaz MB, Ud Din Mirza I. Posterior reversible encephalopathy syndrome in pediatric cancer clinical and radiologic findings. J Global Oncol. (2017) 4, 1–8. doi: 10.1200/JGO.17.00089
41. Fraint E, Miller R, Walter A. Posterior reversible encephalopathy syndrome and cerebral sinus thrombosis in a case of pediatric B-cell all. J Pediatr Hematol Oncol. (2017) 39:e71–3. doi: 10.1097/MPH.0000000000000728
42. Eroglu N, Bahadir A, Erduran E. A case of all developing posterior reversible encephalopathy secondary to hyponatremia. J Pediatr Hematol Oncol. (2017) 39:e476–8. doi: 10.1097/MPH.0000000000000827
43. Pavlidou E, Pavlou E, Anastasiou A, Pana Z, Tsotoulidou V, Kinali M, et al. Posterior reversible encephalopathy syndrome after intrathecal methotrexate infusion: a case report and literature update. Quant Imaging Med Surg. (2016) 6:605–11. doi: 10.21037/qims.2016.10.07
44. Khan RB, Sadighi ZS, Zabrowski J, Gajjar A, Jeha S. Imaging patterns and outcome of posterior reversible encephalopathy syndrome during childhood cancer treatment. Pediatr Blood Cancer. (2016) 63:523–6. doi: 10.1002/pbc.25790
45. Tavil B, Isgandarova F, Bayhan T, Unal S, Kuskonmaz B, Gumruk F, et al. Sorafenib-induced posterior reversible encephalopathy syndrome in a child with FLT3-ITD-positive acute myeloid leukemia. J Pediatr Hematol Oncol. (2016) 38:240–2. doi: 10.1097/MPH.0000000000000521
46. Cifci Sunamak E, Ozdemir N, Celkan T. Posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia: experience of a single center using BFM protocols. Pediatric Blood Cancer. (2019) 66:e27711. doi: 10.1002/pbc.27711
47. Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, Coquet I, et al. Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS ONE. (2012) 7:e44534. doi: 10.1371/journal.pone.0044534
48. Juan Cintrón-García AKG. Management of CNS toxicity of chemotherapy and targeted agents: 2020. Am J Cancer Res. (2020) 10:2617–20.
49. Baytan B, Evim MS, Guler S, Gunes AM, Okan M. Acute central nervous system complications in pediatric acute lymphoblastic leukemia. Pediatr Neurol. (2015) 53:312–8. doi: 10.1016/j.pediatrneurol.2015.03.006
50. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin. (2020) 70:86–104. doi: 10.3322/caac.21596
51. Argyriou AA, Bruna J, Genazzani AA, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity: management informed by pharmacogenetics. Nat Rev Neurol. (2017) 13:492–504. doi: 10.1038/nrneurol.2017.88
52. Stone JB, DeAngelis LM. Cancer-treatment-induced neurotoxicity–focus on newer treatments. Nat Rev Clin Oncol. (2016) 13:92–105. doi: 10.1038/nrclinonc.2015.152
53. Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev. (2016) 50:118–28. doi: 10.1016/j.ctrv.2016.09.005
54. Park S, Goldstein D, Krishnan A, Lin C, Friedlander M, Cassidy J, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA Cancer J Clin. (2013) 63:419–37. doi: 10.3322/caac.21204
55. Granata G, Greco A, Iannella G, Granata M, Manno A, Savastano E, et al. Posterior reversible encephalopathy syndrome–Insight into pathogenesis, clinical variants and treatment approaches. Autoimmun Rev. (2015) 14:830–6. doi: 10.1016/j.autrev.2015.05.006
56. Banerjee J, Niinimaki R, Lahteenmaki P, Hed Myrberg I, Arola M, Riikonen P, et al. The spectrum of acute central nervous system symptoms during the treatment of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. (2020) 67:e27999. doi: 10.1002/pbc.27999
57. Tang JH, Tian JM, Sheng M, Hu SY, Li Y, Zhang LY, et al. Study of posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia after induction chemotherapy. J Child Neurol. (2016) 31:279–84. doi: 10.1177/0883073815589758
58. Peddi P, Peddi S, Santos E, Morgensztern D. Central nervous system toxicities of chemotherapeutic agents. Expert Rev Anticancer Ther. (2014) 14:857–63. doi: 10.1586/14737140.2014.911089
59. Magge RS, DeAngelis LM. The double-edged sword: neurotoxicity of chemotherapy. Blood Rev. (2015) 29:93–100. doi: 10.1016/j.blre.2014.09.012
60. Tse S, Saunders E, Silverman E, Vajsar J, Becker L, Meaney B. Myasthenia gravis and polymyositis as manifestations of chronic graft-versus-host-disease. Bone Marrow Transplantat. (1999) 23:397–9. doi: 10.1038/sj.bmt.1701575
61. Irvin W, MacDonald G, Smith J, Kim W. Dexamethasone-induced posterior reversible encephalopathy syndrome. J Clin Oncol. (2007) 25:2484–6. doi: 10.1200/JCO.2007.10.9991
62. Zaccara G, Perucca E. Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord. (2014) 16:409–31. doi: 10.1684/epd.2014.0714
63. Floeter A, Patel A, Tran M, Chamberlain M, Hendrie P, Gopal A, et al. Posterior reversible encephalopathy syndrome associated with dose-adjusted EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) chemotherapy. Clin Lymphoma Myeloma Leukemia. (2017) 17:225–30. doi: 10.1016/j.clml.2016.12.004
64. Parikh NS, Schweitzer AD, Young RJ, Giambrone AE, Lyo J, Karimi S, et al. Corticosteroid therapy and severity of vasogenic edema in posterior reversible encephalopathy syndrome. J Neurol Sci. (2017) 380:11–5. doi: 10.1016/j.jns.2017.06.044
65. Chen TH. Childhood posterior reversible encephalopathy syndrome: clinicoradiological characteristics, managements, and outcome. Front Pediatr. (2020) 8:585. doi: 10.3389/fped.2020.00585
66. Stefanou MI, Gepfner-Tuma I, Brendle C, Kowarik M, Meiwes A, Eigentler T, et al. Posterior reversible encephalopathy syndrome in a melanoma patient with dabrafenib and trametinib treatment following immunotherapy. J Dtsch Dermatol Ges. (2020) 18:136–9. doi: 10.1111/ddg.13991
67. Masetti R, Cordelli DM, Zama D, Vendemini F, Biagi C, Franzoni E, et al. PRES in children undergoing hematopoietic stem cell or solid organ transplantation. Pediatrics. (2015) 135:890–901. doi: 10.1542/peds.2014-2325
68. Radhika Dasararaju M, Marisa B. Marques MD. Adverse Effects of Transfusion. Cancer Control. (2015) 22:16–25. doi: 10.1177/107327481502200104
69. Roubinian NH, Hendrickson JE, Triulzi DJ, Gottschall JL, Michalkiewicz M, Chowdhury D, et al. Contemporary risk factors and outcomes of transfusion-associated circulatory overload. Crit Care Med. (2018) 46:577–85. doi: 10.1097/CCM.0000000000002948
70. Ito Y, Niwa H, Iida T, Nagamatsu M, Yasuda T, Yanagi T, et al. Post-transfusion reversible posterior leukoencephalopathy syndrome with cerebral vasoconstriction. Neurology. (1997) 49:1174–5. doi: 10.1212/WNL.49.4.1174
71. Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. N Engl J Med. (2017) 377:1261–72. doi: 10.1056/NEJMra1612789
72. Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA. (2015) 314:2514–23. doi: 10.1001/jama.2015.13977
73. Mestre H, Du T, Sweeney AM, Liu G, Samson AJ, Peng W, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. (2020) 367. doi: 10.1126/science.aax7171
74. Dubowitz DJ, Dyer EA, Theilmann RJ, Buxton RB, Hopkins SR. Early brain swelling in acute hypoxia. J Appl Physiol. (2009) 107:244–52. doi: 10.1152/japplphysiol.90349.2008
75. Nakamura Y, Sugino M, Tsukahara A, Nakazawa H, Yamamoto N, Arawaka S. Posterior reversible encephalopathy syndrome with extensive cytotoxic edema after blood transfusion: a case report and literature review. BMC Neurol. (2018) 18:190. doi: 10.1186/s12883-018-1194-1
76. Lykke K, Assentoft M, Horlyck S, Helms HC, Stoica A, Toft-Bertelsen TL, et al. Evaluating the involvement of cerebral microvascular endothelial Na(+)/K(+)-ATPase and Na(+)-K(+)-2Cl(-) co-transporter in electrolyte fluxes in an in vitro blood-brain barrier model of dehydration. J Cereb Blood Flow Metab. (2019) 39:497–512. doi: 10.1177/0271678X17736715
77. Zappia F, Verzicco I, Simoni R, Ferrari M, Coghi P, Bozzetti F, et al. Posterior reversible encephalopathy syndrome in an oncological normotensive patient: evidence for a pathogenic role of concomitant low magnesium serum levels and chemotherapy treatment. Acta Biomed. (2020) 91:365–72. doi: 10.23750/abm.v91i2.8685
78. Hokkoku K, Furukawa Y, Yamamoto J, Uchida Y, Sonoo M. Reversible cerebral vasoconstriction syndrome accompanied by hypomagnesemia. Neurol Sci. (2018) 39:1141–2. doi: 10.1007/s10072-018-3281-x
79. Kaplinsky C, Alon US. Magnesium homeostasis and hypomagnesemia in children with malignancy. Pediatr Blood Cancer. (2013) 60:734–40. doi: 10.1002/pbc.24460
80. Jonathan Moss AW. Opening the floodgates to the brain. Science. (2020) 367:1195. doi: 10.1126/science.aba8801
81. Zheng YY, Weng XP, Fu FW, Cao YG, Li Y, Zheng GQ, et al. Cerebrospinal fluid hypovolemia and posterior reversible encephalopathy syndrome. Front Neurol. (2020) 11:591. doi: 10.3389/fneur.2020.00591
82. Fiordoliva I, Meletani T, Baleani MG, Rinaldi S, Savini A, Di Pietro Paolo M, et al. Managing hyponatremia in lung cancer: latest evidence and clinical implications. Ther Adv Med Oncol. (2017) 9:711–9. doi: 10.1177/1758834017736210
83. Berardi R, Rinaldi S, Caramanti M, Grohe C, Santoni M, Morgese F, et al. Hyponatremia in cancer patients: time for a new approach. Crit Rev Oncol Hematol. (2016) 102:15–25. doi: 10.1016/j.critrevonc.2016.03.010
84. Kopec I, Groeger J. Life-threatening fluid and electrolyte abnormalities associated with cancer. Crit Care Clin. (1988) 4:81–105. doi: 10.1016/S0749-0704(18)30506-2
85. Lewis MA, Hendrickson AW, Moynihan TJ. Oncologic emergencies: pathophysiology, presentation, diagnosis, and treatment. CA Cancer J Clin. (2011) 61:287–314. doi: 10.3322/caac.20124
86. Vassilopoulou-Sellin R, Newman B, Taylor S, Guinee V. Incidence of hypercalcemia in patients with malignancy referred to a comprehensive cancer center. Cancer. (1993) 71:1309–12. doi: 10.1002/1097-0142(19930215)71:4<1309::AID-CNCR2820710423>3.0.CO;2-M
87. Kutz A, Ebrahimi F, Aghlmandi S, Wagner U, Bromley M, Illigens B, et al. Risk of adverse clinical outcomes in hyponatremic adult patients hospitalized for acute medical conditions: a population-based Cohort study. J Clin Endocrinol Metab. (2020) 105:3428–36. doi: 10.1210/clinem/dgaa547
88. Shaikh A, Bawany S, Masood N, Khan A, Abbasi A, Niamutullah S, et al. Incidence and impact of baseline electrolyte abnormalities in patients admitted with chemotherapy induced febrile neutropenia. J Cancer. (2011) 2:62–6. doi: 10.7150/jca.2.62
89. Philibert D, Desmeules S, Filion A, Poirier M, Agharazii M. Incidence and severity of early electrolyte abnormalities following autologous haematopoietic stem cell transplantation. Nephrol Dialysis Transplant. (2008) 23:359–63. doi: 10.1093/ndt/gfm571
90. Alsirafy S, Sroor M, Al-Shahri M. Predictive impact of electrolyte abnormalities on the admission outcome and survival of palliative care cancer referrals. J Palliative Med. (2009) 12:177–80. doi: 10.1089/jpm.2008.0200
91. Aulakh P, Fatakhov E, Koch C, Kapil S. Posterior reversible encephalopathy syndrome with documented hyponatraemia. BMJ Case Rep. (2013). doi: 10.1136/bcr-2013-009311
92. Jeon J, Park S, Seo J. Posterior reversible encephalopathy syndrome due to hyponatremia. J Epilepsy Res. (2014) 4:31–3. doi: 10.14581/jer.14008
93. Papadopoulos M, Verkman A. Aquaporin-4 and brain edema. Pediatric Nephrol. (2007) 22:778–84. doi: 10.1007/s00467-006-0411-0
94. Boulard G, Marguinaud E, Sesay M. Osmotic cerebral oedema: the role of plasma osmolarity and blood brain barrier. Ann francaises d'anesthesie et de reanimation. (2003) 22:215–9. doi: 10.1016/S0750-7658(03)00009-1
95. Jaramillo-Calle DA, Solano JM, Rabinstein AA, Bonkovsky HL. Porphyria-induced posterior reversible encephalopathy syndrome and central nervous system dysfunction. Mol Genet Metab. (2019) 128:242–53. doi: 10.1016/j.ymgme.2019.10.011
96. Grioni D, Pavan F, Prunotto G, Canonico F, Grandi C, Rovelli A. Should posterior reversible encephalopathy syndrome be mainly considered an epileptic disorder? Results of a sequential neurophysiological study in a pediatric Cohort. Neuropediatrics. (2017) 48:72–8. doi: 10.1055/s-0037-1598251
97. Sul J, Deangelis L. Neurologic complications of cancer chemotherapy. Semin Oncol. (2006) 33:324–32. doi: 10.1053/j.seminoncol.2006.03.006
98. Savvidou M, Hingorani A, Tsikas D, Frölich J, Vallance P, Nicolaides K. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. (2003) 361:1511–7. doi: 10.1016/S0140-6736(03)13177-7
99. Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses. (2014) 82:619–22. doi: 10.1016/j.mehy.2014.02.022
100. Staykov D, Schwab S. Posterior reversible encephalopathy syndrome. J Intensive Care Med. (2012) 27:11–24. doi: 10.1177/0885066610393634
101. Li K, Yang Y, Guo D, Sun D, Li C. Clinical and MRI features of posterior reversible encephalopathy syndrome with atypical regions: a descriptive study with a large sample size. Front Neurol. (2020) 11:194. doi: 10.3389/fneur.2020.00194
102. Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: a review with emphasis on neuroimaging characteristics. J Neurol Sci. (2019) 404:72–9. doi: 10.1016/j.jns.2019.07.018
103. Ghali MGZ, Davanzo J, Leo M, Rizk E. Posterior reversible encephalopathy syndrome in pediatric patients: pathophysiology, diagnosis, and management. Leuk Lymphoma. (2019) 60:2365–72. doi: 10.1080/10428194.2019.1594210
104. Chen Z, Zhang G, Lerner A, Wang AH, Gao B, Liu J. Risk factors for poor outcome in posterior reversible encephalopathy syndrome: systematic review and meta-analysis. Quant Imaging Med Surg. (2018) 8:421–32. doi: 10.21037/qims.2018.05.07
105. Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA. Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc. (2010) 85:427–32. doi: 10.4065/mcp.2009.0590
106. Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke. (2010) 41:1957–62. doi: 10.1161/STROKEAHA.109.575167
107. Ghali MGZ, Styler MJ. Etiologies, cerebral vasomotion, and endothelial dysfunction in the pathophysiology of posterior reversible encephalopathy syndrome in pediatric patients. J Pediatric Neurol. (2020) 18:55–78. doi: 10.1055/s-0040-1702934
108. Straathof K, Anoop P, Allwood Z, Silva J, Nikolajeva O, Chiesa R, et al. Long-term outcome following cyclosporine-related neurotoxicity in paediatric allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. (2017) 52:159–62. doi: 10.1038/bmt.2016.232
109. Andrzejewski C, Casey M, Popovsky M. How we view and approach transfusion-associated circulatory overload: pathogenesis, diagnosis, management, mitigation, and prevention. Transfusion. (2013) 53:3037–47. doi: 10.1111/trf.12454
110. Kolovou V, Zampakis P, Ginopoulou A, Varvarigou A, Kaleyias J. Reversible posterior leukoencephalopathy syndrome after blood transfusion in a pediatric patient with sickle cell disease. Pediatr Neurol. (2013) 49:213–7. doi: 10.1016/j.pediatrneurol.2013.04.024
111. Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, et al. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med. (2007) 33:230–6. doi: 10.1007/s00134-006-0459-0
112. Brady TM, Stefani-Glucksberg A, Simonetti GD. Management of high blood pressure in children: similarities and differences between US and European guidelines. Pediatr Nephrol. (2019) 34:405–12. doi: 10.1007/s00467-018-3946-y
113. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. doi: 10.1097/HJH.0000000000001039
114. Vasquez A, Gainza-Lein M, Sanchez Fernandez I, Abend NS, Anderson A, Brenton JN, et al. Hospital emergency treatment of convulsive status epilepticus: comparison of pathways from ten pediatric research centers. Pediatr Neurol. (2018) 86:33–41. doi: 10.1016/j.pediatrneurol.2018.06.004
115. Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. (2017) 5:95. doi: 10.1186/s40425-017-0300-z
Keywords: posterior reversible encephalopathy syndrome, chemotherapy, hematopoietic stem cell transplantation, children, oncologic/hematologic diseases, neurotoxicity, management
Citation: Hun M, Xie M, She Z, Abdirahman AS, Li C, Wu F, Luo S, Han P, Phorn R, Wu P, Luo H, Chen K, Tian J, Wan W and Wen C (2021) Management and Clinical Outcome of Posterior Reversible Encephalopathy Syndrome in Pediatric Oncologic/Hematologic Diseases: A PRES Subgroup Analysis With a Large Sample Size. Front. Pediatr. 9:678890. doi: 10.3389/fped.2021.678890
Received: 10 March 2021; Accepted: 02 June 2021;
Published: 01 July 2021.
Edited by:
Brahim Tabarki Melaiki, University of Sousse, TunisiaReviewed by:
Angelo Lavano, University of Magna Graecia, ItalyCopyright © 2021 Hun, Xie, She, Abdirahman, Li, Wu, Luo, Han, Phorn, Wu, Luo, Chen, Tian, Wan and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Wen, Y2h1YW53ZW5AY3N1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.